Immuno-Stimulating Activity of 1,25-Dihydroxyvitamin D in Blood Cells from Five Healthy People and in Blasts from Five Patients with Leukemias and Pre-Leukemic States
Abstract
1. Introduction
2. Results
2.1. Results of Whole Exon Sequencing (WES)
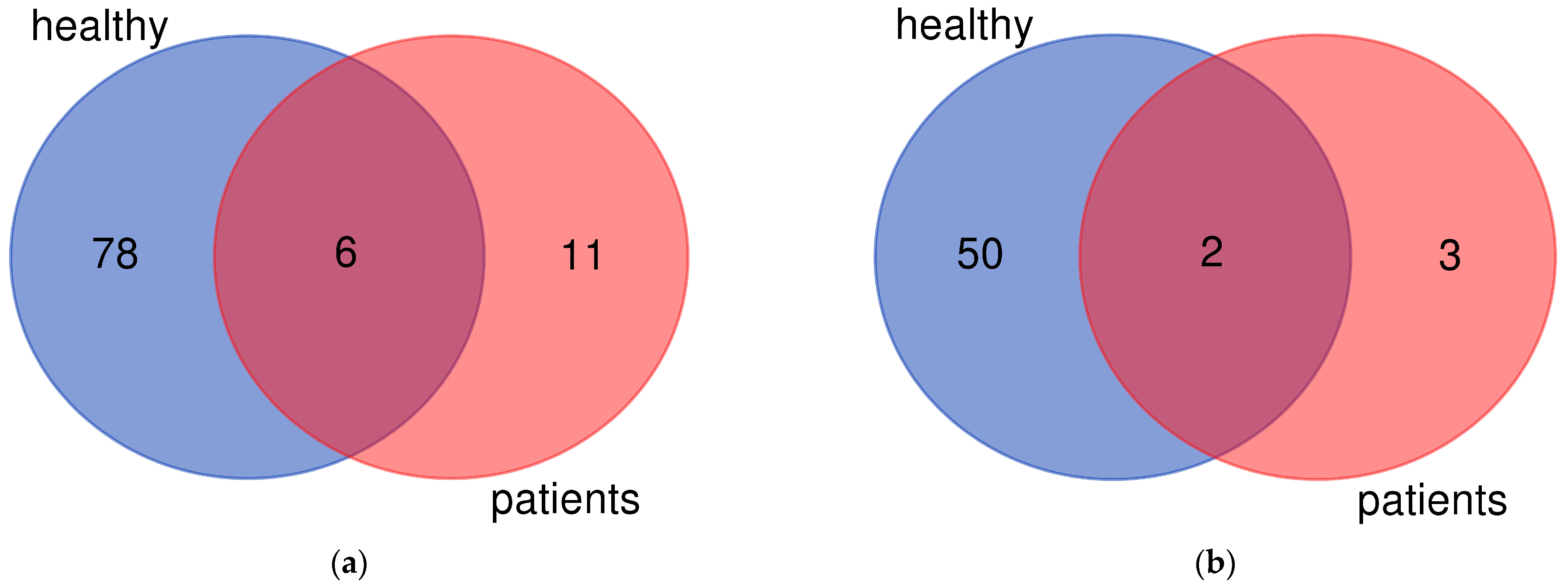
2.2. Results of RNA Sequencing (RNAseq)
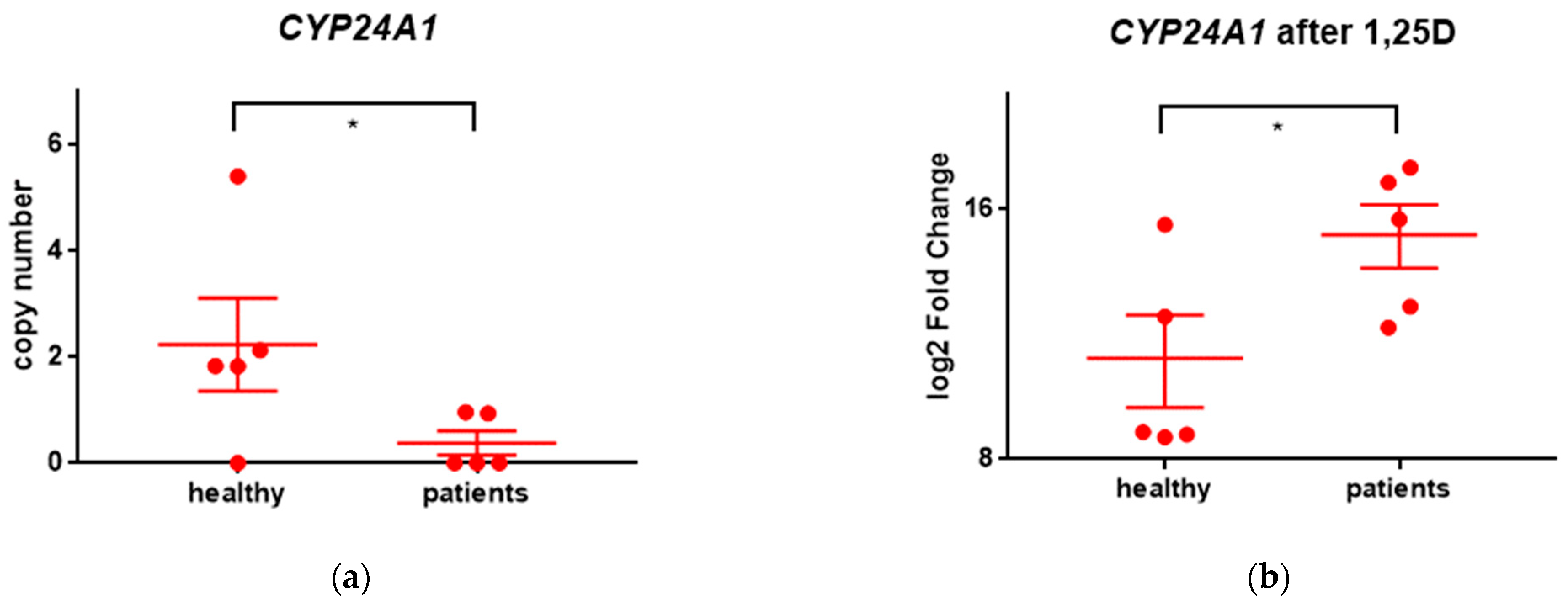
2.2.1. Genes Upregulated after Exposure to 1,25D
2.2.2. Genes Downregulated after Exposure to 1,25D
2.2.3. Cellular Processes Activated after Exposure of the Cells to 1,25D
3. Discussion
4. Materials and Methods
4.1. Isolation of Leukocytes
4.2. Isolation of DNA
4.3. Isolation of mRNA
4.4. Sequencing
4.5. WES Analysis
4.6. RNAseq Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Hanel, A.; Veldhuizen, C.; Carlberg, C. Gene-Regulatory Potential of 25-Hydroxyvitamin D(3) and D(2). Front. Nutr. 2022, 9, 910601. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Vitamin D and bone health. J. Nutr. 1996, 126 (Suppl. S4), 1159S–1164S. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.; Liu, P.; Modlin, R.; Adams, J.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Wang, T.-T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; Burton MacLeod, N.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-Scale in Silico and Microarray-Based Identification of Direct 1,25-Dihydroxyvitamin D3 Target Genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef]
- Hanel, A.; Carlberg, C. Time-Resolved Gene Expression Analysis Monitors the Regulation of Inflammatory Mediators and Attenuation of Adaptive Immune Response by Vitamin D. Int. J. Mol. Sci. 2022, 23, 911. [Google Scholar] [CrossRef]
- Ryynänen, J.; Seuter, S.; Campbell, M.; Carlberg, C. Gene regulatory scenarios of primary 1,25-dihydroxyvitamin D3 target genes in a human myeloid leukemia cell line. Cancers 2013, 5, 1221–1241. [Google Scholar] [CrossRef]
- Dusso, A.; Brown, A.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Dusso, A.; Finch, J.; Brown, A.; Ritter, C.; Delmez, J.; Schreiner, G.; Slatopolsky, E. Extrarenal production of calcitriol in normal and uremic humans. J. Clin. Endocrinol. Metab. 1991, 72, 157–164. [Google Scholar] [CrossRef]
- Grande, A.; Montanari, M.; Tagliafico, E.; Manfredini, R.; Zanocco Marani, T.; Siena, M.; Tenedini, E.; Gallinelli, A.; Ferrari, S. Physiological levels of 1alpha, 25 dihydroxyvitamin D3 induce the monocytic commitment of CD34+ hematopoietic progenitors. J. Leukoc. Biol. 2002, 71, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Luong, Q.T.; Koeffler, H.P. Vitamin D compounds: Activity against microbes and cancer. Anticancer. Res. 2006, 26, 2531–2542. [Google Scholar] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L.; Douvdevani, A.; Chaimovitz, C.; Shany, S. Regulation of TNF-α by 1α,25-dihydroxyvitamin D3 in human macrophages from CAPD patients. Kidney Int. 2001, 59, 69–75. [Google Scholar] [CrossRef]
- Prosser, D.; Jones, G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004, 29, 664–673. [Google Scholar] [CrossRef]
- Sandler, D.; Ross, J. Epidemiology of acute leukemia in children and adults. Semin. Oncol. 1997, 24, 3–16. [Google Scholar] [PubMed]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation, in 8th European Conference on Infections in Leukaemia. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Dodd, K.C.; Menon, M. Sex bias in lymphocytes: Implications for autoimmune diseases. Front. Immunol. 2022, 13, 945762. [Google Scholar] [CrossRef]
- Howard, C.M.; Zgheib, N.B.; Bush, S.; DeEulis, T.; Cortese, A.; Mollo, A.; Lirette, S.T.; Denning, K.; Valluri, J.; Claudio, P.P. Clinical relevance of cancer stem cell chemotherapeutic assay for recurrent ovarian cancer. Transl. Oncol. 2020, 13, 100860. [Google Scholar] [CrossRef]
- Yokota, T.; Kanakura, Y. Genetic abnormalities associated with acute lymphoblastic leukemia. Cancer Sci. 2016, 107, 721–725. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Chronic Myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S. Genetics of MDS. Blood 2019, 133, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Vaisanen, S.; Dunlop, T.; Sinkkonen, L.; Frank, C.; Carlberg, C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-dihydroxyvitamin D3. J. Mol. Biol. 2005, 350, 65–77. [Google Scholar] [CrossRef]
- Studzinski, G.P.; Harrison, J.S.; Wang, X.; Sarkar, S.; Kalia, V.; Danilenko, M. Vitamin D Control of Hematopoietic Cell Differentiation and Leukemia. J. Cell Biochem. 2016, 116, 1500–1512. [Google Scholar] [CrossRef]
- Harrison, J.; Bershadskiy, A. Clinical experience using vitamin D and analogs in the treatment of myelodysplasia and acute myeloid leukemia: A review of the literature. Leuk. Res. Treat. 2012, 125814, 8. [Google Scholar] [CrossRef]
- Marcinkowska, E. Vitamin D Derivatives in Acute Myeloid Leukemia: The Matter of Selecting the Right Targets. Nutrients 2022, 14, 2851. [Google Scholar] [CrossRef]
- Höbaus, J.; Hummel, D.; Thiem, U.; Fetahu, I.; Aggarwal, A.; Müllauer, L.; Heller, G.; Egger, G.; Mesteri, I.; Baumgartner-Parzer, S.; et al. Increased copy-number and not DNA hypomethylation causes overexpression of the candidate proto-oncogene CYP24A1 in colorectal cancer. Int. J. Cancer 2013, 133, 1380–1388. [Google Scholar] [CrossRef]
- Horváth, H.; Lakatos, P.; Kósa, J.; Bácsi, K.; Borka, K.; Bises, G.; Nittke, T.; Hershberger, P.; Speer, G.; Kállay, E. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J. Histochem. Cytochem. 2010, 58, 277–285. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance. Front. Microbiol. 2020, 11, 1902. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C. Ceruloplasmin and other copper binding components of blood plasma and their functions: An update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. JBIC J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- Claro da Silva, T.; Hiller, C.; Gai, Z.; Kullak-Ublick, G.A. Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2016, 163, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hong, Z.; Zhao, C.; Bi, Q.; Qiu, B. Associations between polymorphisms of the PDLIM4 gene and susceptibility to osteoporotic fracture in an elderly population of Han Chinese. Biosci. Rep. 2019, 39, BSR20181505. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, D.K.; Grossmann, M.E.; Cheville, J.C.; Gazi, M.H.; Gong, A.; Zhang, J.S.; Ajtai, K.; Burghardt, T.P.; Young, C.Y.F. PDLIM4, An Actin Binding Protein, Suppresses Prostate Cancer Cell Growth. Cancer Investig. 2009, 27, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Uversky, V.N. What Is Parvalbumin for? Biomolecules 2022, 12, 656. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Yang, Q.; Jiang, Y.; Lin, L.; Liu, X.; Wu, K. Vitelline Membrane Outer Layer 1 Homolog Interacts With Lysozyme C and Promotes the Stabilization of Tear Film. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6722–6727. [Google Scholar] [CrossRef]
- Ragland, S.; Criss, A. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Dyer, D.P. Understanding the mechanisms that facilitate specificity, not redundancy, of chemokine-mediated leukocyte recruitment. Immunology 2020, 160, 336–344. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation-A target for novel cancer therapy. Cancer Treat Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Gomes, I.M.; Maia, C.J.; Santos, C.R. STEAP proteins: From structure to applications in cancer therapy. Mol. Cancer Res. 2012, 10, 573–587. [Google Scholar] [CrossRef]
- Fischer, S.; Stegmann, F.; Gnanapragassam, V.S.; Lepenies, B. From structure to function-Ligand recognition by myeloid C-type lectin receptors. Comput. Struct. Biotechnol. J. 2022, 20, 5790–5812. [Google Scholar] [CrossRef]
- Xing, Q.; Feng, Y.; Sun, H.; Yang, S.; Sun, T.; Guo, X.; Ji, F.; Wu, B.; Zhou, D. Scavenger receptor MARCO contributes to macrophage phagocytosis and clearance of tumor cells. Exp. Cell Res. 2021, 408, 112862. [Google Scholar] [CrossRef]
- Tanaka, T.; Akira, S.; Yoshida, K.; Umemoto, M.; Yoneda, Y.; Shirafuji, N.; Fujiwara, H.; Suematsu, S.; Yoshida, N.; Kishimoto, T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 1995, 80, 353–361. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M. Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis. Mol. Immunol. 2022, 152, 172–182. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- The Pandas Development Team. Pandas-Dev/Pandas: Pandas (v1.5.2). Zenodo. 2022. Available online: https://zenodo.org/record/7344967#.ZCUH13ZByUk (accessed on 26 March 2023).
| Patient No | Diagnosis | Mutated Gene * | Fusion Gene ** |
|---|---|---|---|
| P1 | Acute lymphoblastic leukemia (ALL) | TRDV2-TRAC BCR-ABL1 MTAP-CDKN2B-AS1 | |
| P2 | Acute myeloid leukemia (AML) | NOP53-DHX34 | |
| P4 | Myelodysplastic syndrome (MDS) | TET2 | |
| P5 | Chronic myelomonocytic leukemia (CMML) | TET2, RUNX1 | |
| P6 | Acute myeloid leukemia (AML) | RUNX1-RUNX1T1 |
| Gene Name | Gene Description | Minimum Upregulation * | Maximum Upregulation * |
|---|---|---|---|
| CAMP | cathelicidin antimicrobial peptide | 1.3 | 9.5 |
| CP | ceruloplasmin | 1.5 | 17.5 |
| CYP24A1 | cytochrome P450 family 24 subfamily A member 1 | 8.5 | 18.0 |
| PDLIM4 | PDZ and LIM domain 4 | 1.0 | 11.5 |
| PVALB | parvalbumin | 1.7 | 14.2 |
| VMO1 | vitelline membrane outer layer 1 homolog | 1.0 | 5.9 |
| Gene Name | Gene Description | Minimum Downregulation * | Maximum Downregulation * |
|---|---|---|---|
| CXCL9 | C-X-C motif chemokine ligand 9 | −1.0 | −13.3 |
| STEAP1B | STEAP family member 1B | −1.2 | −8.6 |
| Patient | Sex | Age | Diagnosis | Blasts |
|---|---|---|---|---|
| P1 | F | 64 | Acute lymphoblastic leukemia (ALL) | 88.6% |
| P2 | F | 18 | Acute myeloid leukemia (AML) | 77% |
| P3 | F | 33 | Healthy | - |
| P4 | M | 74 | Myelodysplastic syndrome (MDS) * | 3% |
| P5 | M | 86 | Chronic myelomonocytic leukemia (CMML) | 2.6% ** |
| P6 | M | 37 | AML | 23.8% |
| H1 | F | 30 | Healthy | - |
| H2 | F | 38 | Healthy | - |
| H3 | M | 33 | Healthy | - |
| H4 | F | 32 | Healthy | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchwicka, A.; Nowak, K.; Satyr, A.; Wołowiec, D.; Marcinkowska, E. Immuno-Stimulating Activity of 1,25-Dihydroxyvitamin D in Blood Cells from Five Healthy People and in Blasts from Five Patients with Leukemias and Pre-Leukemic States. Int. J. Mol. Sci. 2023, 24, 6504. https://doi.org/10.3390/ijms24076504
Marchwicka A, Nowak K, Satyr A, Wołowiec D, Marcinkowska E. Immuno-Stimulating Activity of 1,25-Dihydroxyvitamin D in Blood Cells from Five Healthy People and in Blasts from Five Patients with Leukemias and Pre-Leukemic States. International Journal of Molecular Sciences. 2023; 24(7):6504. https://doi.org/10.3390/ijms24076504
Chicago/Turabian StyleMarchwicka, Aleksandra, Kuba Nowak, Anastasiia Satyr, Dariusz Wołowiec, and Ewa Marcinkowska. 2023. "Immuno-Stimulating Activity of 1,25-Dihydroxyvitamin D in Blood Cells from Five Healthy People and in Blasts from Five Patients with Leukemias and Pre-Leukemic States" International Journal of Molecular Sciences 24, no. 7: 6504. https://doi.org/10.3390/ijms24076504
APA StyleMarchwicka, A., Nowak, K., Satyr, A., Wołowiec, D., & Marcinkowska, E. (2023). Immuno-Stimulating Activity of 1,25-Dihydroxyvitamin D in Blood Cells from Five Healthy People and in Blasts from Five Patients with Leukemias and Pre-Leukemic States. International Journal of Molecular Sciences, 24(7), 6504. https://doi.org/10.3390/ijms24076504







