Effects of Follicle-Stimulating Hormone on Human Sperm Motility In Vitro
Abstract
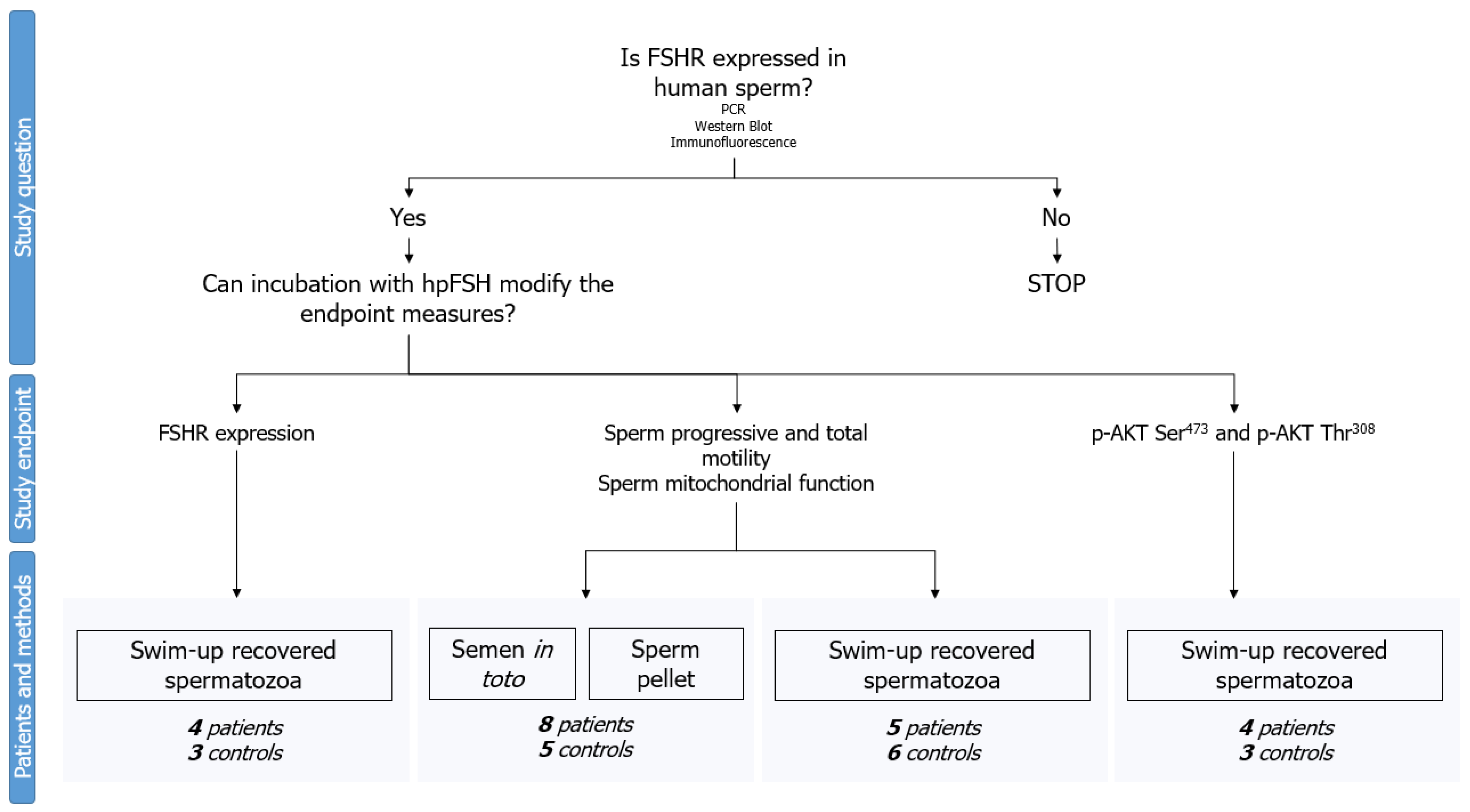
:1. Introduction
2. Results
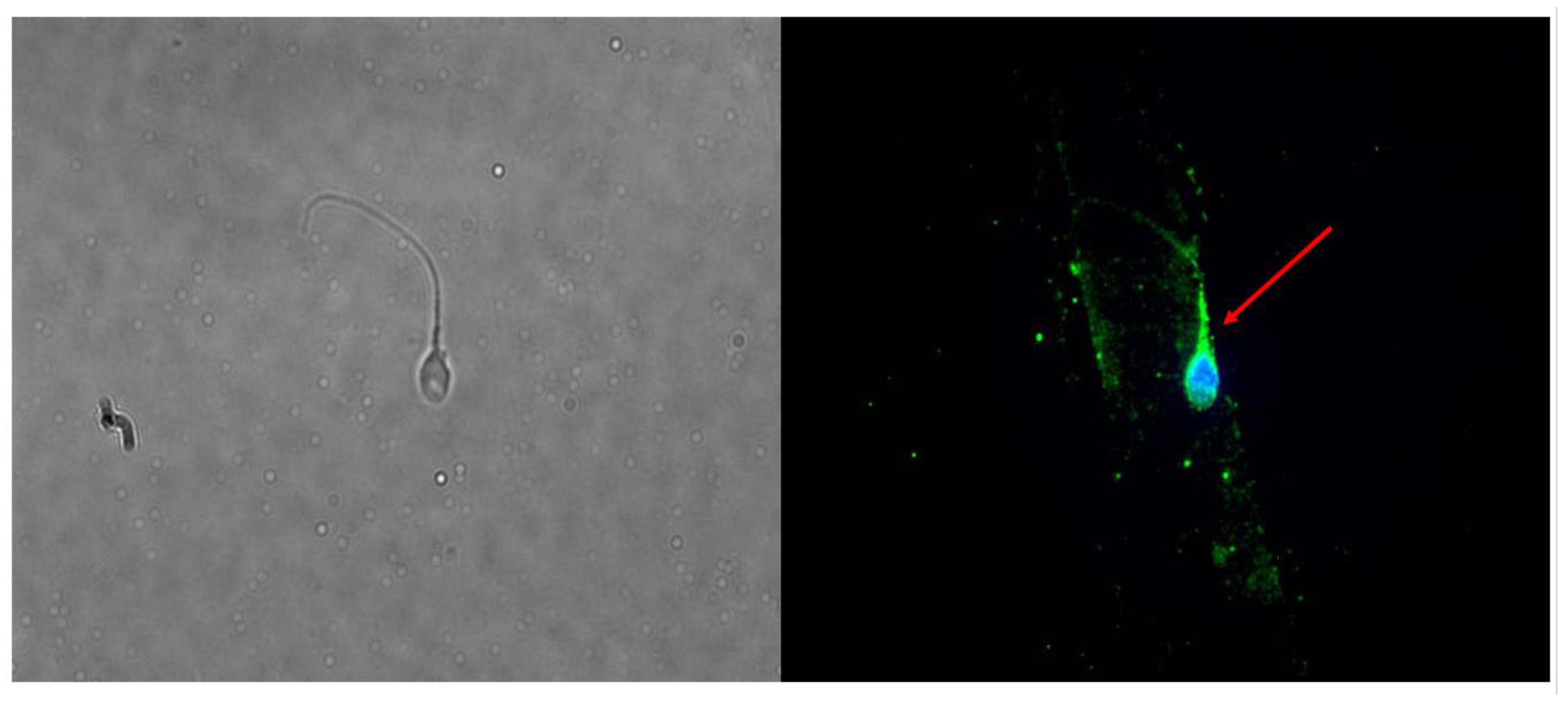
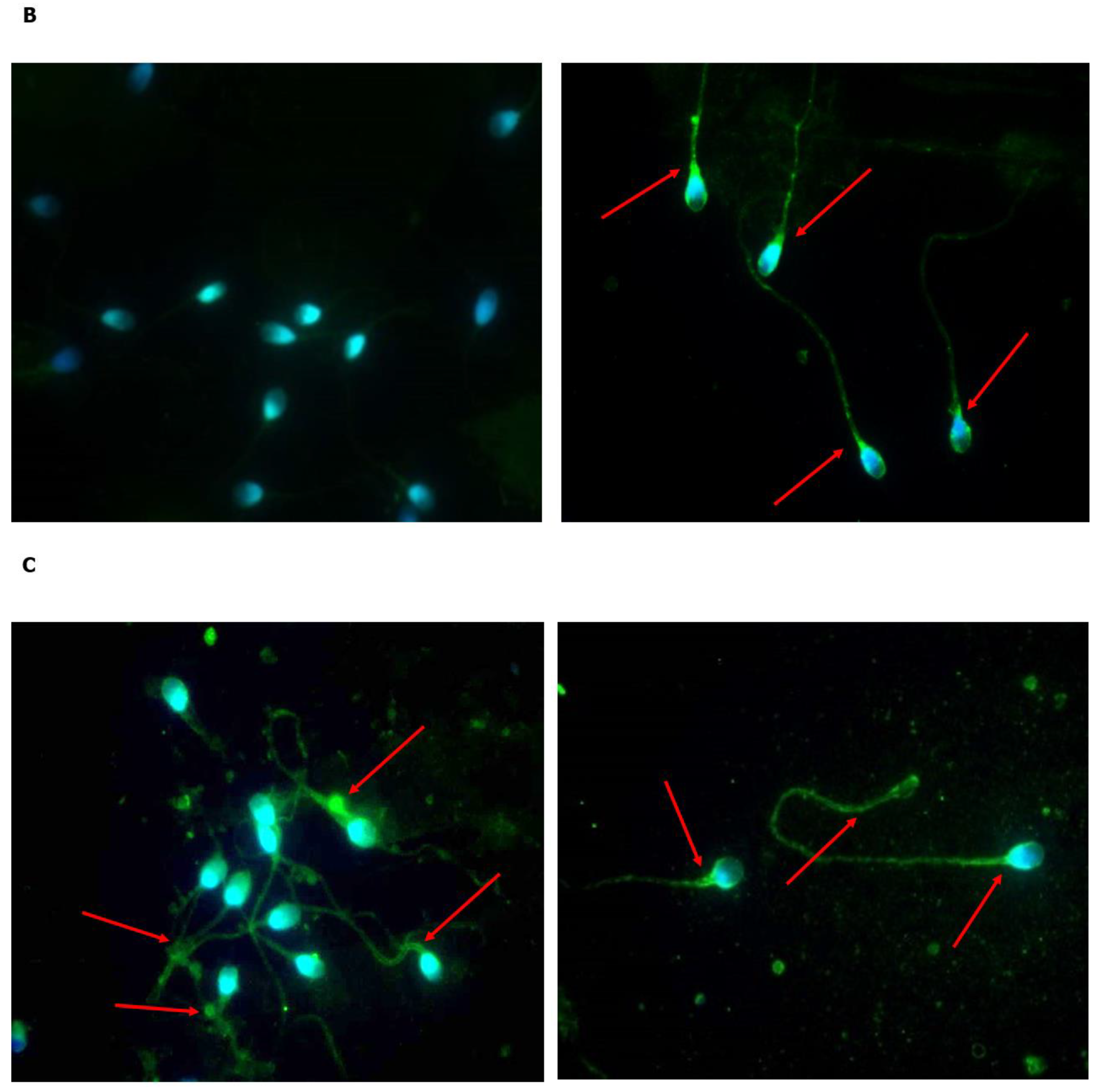
2.1. Is FSHR Expressed in Human Spermatozoa?
2.2. Does Incubation with FSH Influence the Expression and Localization of FSHR?
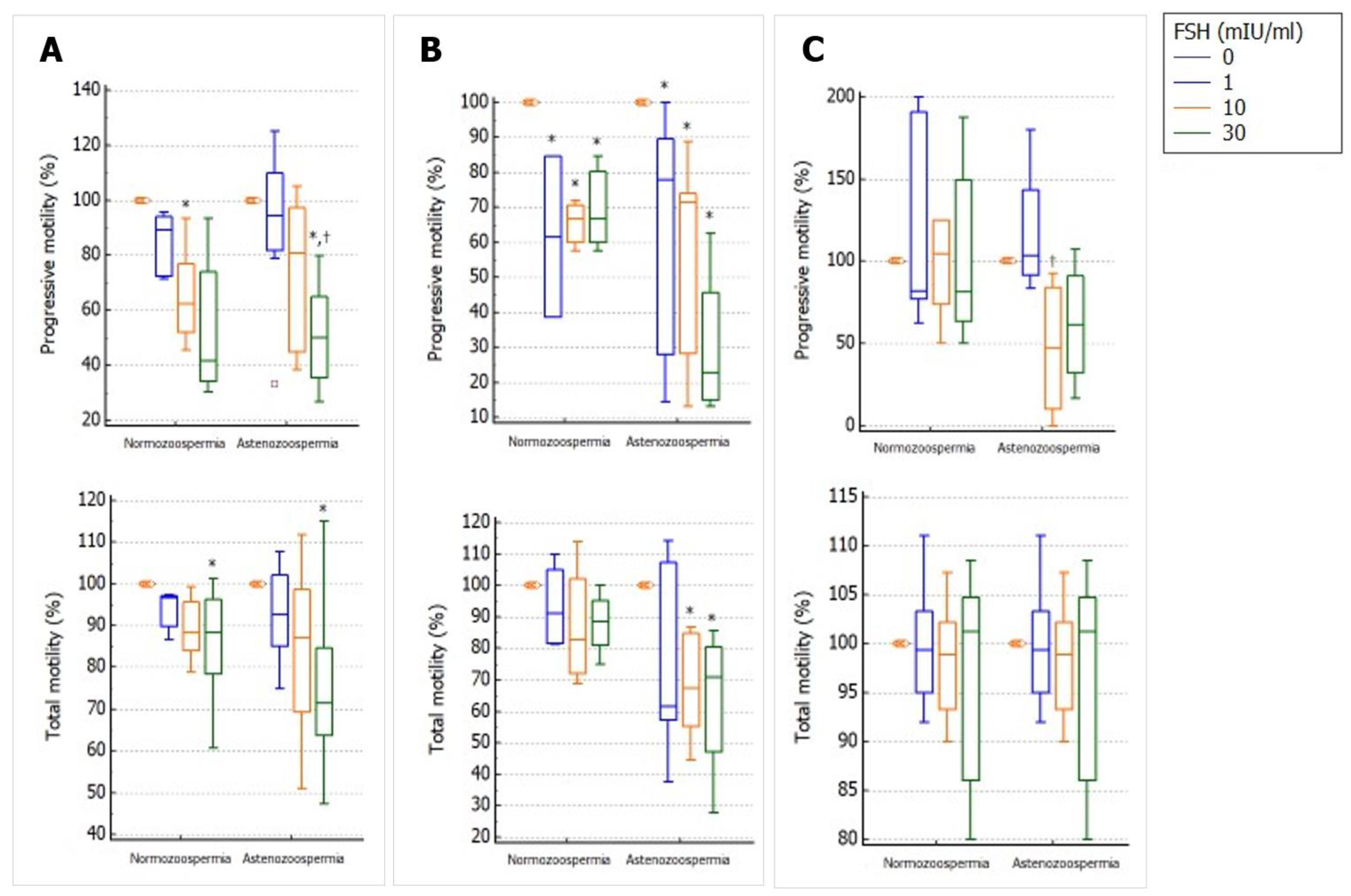
2.3. Does Incubation with FSH Influence Progressive and Total Sperm Motility?
2.4. Does Incubation with FSH Influence Sperm Mitochondrial Function?
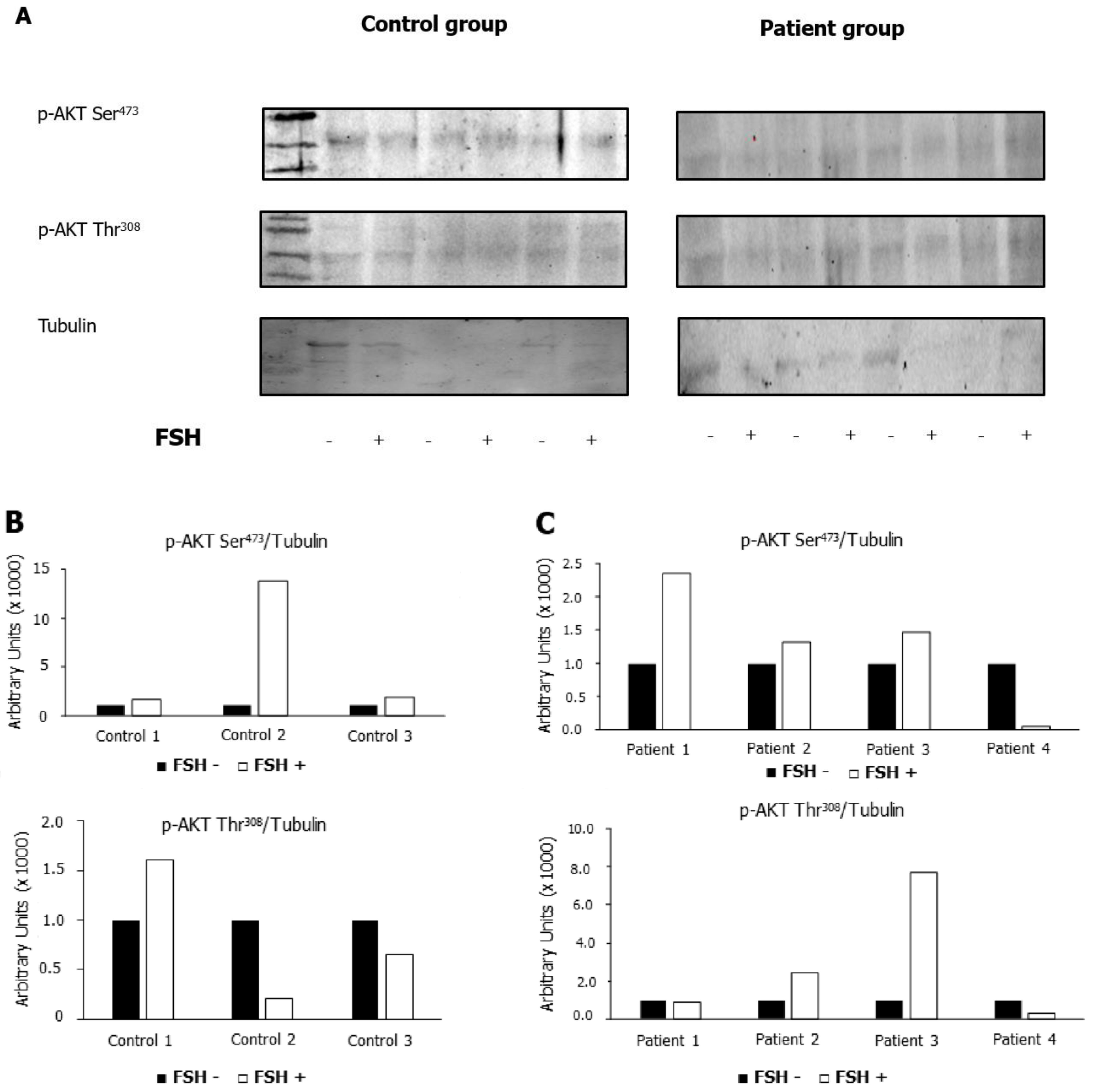
2.5. Does Incubation with FSH Influence the Phosphorylation of AKT473 and AKT308?
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Patients Selection
4.3. Semen Collection and Processing
4.4. Treatment
4.5. Motility Assessment
4.6. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
4.7. Western Blot Analysis
4.8. Immunofluorescence Analysis
4.9. Evaluation of Sperm Mitochondrial Membrane Potential
4.10. Power Analysis
4.11. Statistical Analysis
4.12. Ethical Approval
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanehzad, M.; Abbaszadeh, R.; Holakuyee, M.; Modarressi, M.H.; Nourashrafeddin, S.M. FSH regulates RA signaling to commit spermatogonia into differentiation pathway and meiosis. Reprod. Biol. Endocrinol. 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.C. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol. Cell. Endocrinol. 2008, 288, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, D.S.; Olivas, E.; Di Candeloro, P.; Wright, W.W. Stage-specific changes in GDNF expression by rat Sertoli cells: A possible regulator of the replication and differentiation of stem spermatogonia. Biol. Reprod. 2011, 85, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Hipkin, R.W.; Ascoli, M. A polyclonal antibody to a synthetic peptide derived from the rat follicle-stimulating hormone receptor reveals the recombinant receptor as a 74-kilodalton protein. Endocrinology 1993, 133, 2098–2104. [Google Scholar] [CrossRef]
- Davis, D.; Liu, X.; Segaloff, D.L. Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in FSH receptor function. Mol. Endocrinol. 1995, 9, 159–170. [Google Scholar]
- Tan, D.; Zhao, Y.; Ma, D.; Tong, F. Role of FSH and FSH receptor on HUVECs migration. Gene Ther. 2021, 28, 155–161. [Google Scholar] [CrossRef]
- Stilley, J.A.; Guan, R.; Santillan, D.A.; Mitchell, B.F.; Lamping, K.G.; Segaloff, D.L. Differential Regulation of Human and Mouse Myometrial Contractile Activity by FSH as a Function of FSH Receptor Density. Biol. Reprod. 2016, 95, 36. [Google Scholar] [CrossRef]
- Sun, D.; Bai, M.; Jiang, Y.; Hu, M.; Wu, S.; Zheng, W.; Zhang, Z. Roles of follicle stimulating hormone and its receptor in human metabolic diseases and cancer. Am. J. Transl. Res. 2020, 12, 3116–3132. [Google Scholar]
- Alamo, A.; De Luca, C.; Mongioì, L.M.; Barbagallo, F.; Cannarella, R.; La Vignera, S.; Calogero, A.E.; Condorelli, R.A. Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility. Biomedicines 2020, 8, 196. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Panza, S.; Giordano, F.; De Rose, D.; Panno, M.L.; De Amicis, F.; Santoro, M.; Malivindi, R.; Rago, V.; Aquila, S. FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders. Life 2020, 10, 336. [Google Scholar] [CrossRef]
- Cannarella, R.; Arato, I.; Condorelli, R.A.; Luca, G.; Barbagallo, F.; Alamo, A.; Bellucci, C.; Lilli, C.; La Vignera, S.; Calafiore, R.; et al. The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells. J. Clin. Med. 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluss, P.M.; Ewing, J.F.; Schneyer, A.L. Phospholipase C-mediated release of low molecular weight follicle-stimulating hormone receptor-binding inhibitor from testis membranes. Biol. Reprod. 1990, 43, 1026–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluss, P.M.; Schneyer, A.L.; Cockett, A.T.; Cromie, W.J. Identification of a potential FSH modulatory protein in human testis and seminal plasma. J. Androl. 1989, 10, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Tourkova, I.; Wang, Y.; Sharrow, A.C.; Landau, M.S.; Yaroslavskiy, B.B.; Sun, L.; Zaidi, M.; Blair, H.C. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 2010, 394, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef]
- Casarini, L.; Crépieux, P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef] [Green Version]
- Nelson, L.; Young, M.J.; Gardner, M.E. Sperm motility and calcium transport: A neurochemically controlled process. Life Sci. 1980, 26, 1739–1749. [Google Scholar] [CrossRef]
- Cannarella, R.; La Vignera, S.; Condorelli, R.A.; Mongioì, L.M.; Calogero, A.E. FSH dosage effect on conventional sperm parameters: A meta-analysis of randomized controlled studies. Asian J. Androl. 2020, 22, 309–316. [Google Scholar] [CrossRef]
- Schoenfeld, C.; Amelar, R.D.; Dubin, L.; Numeroff, M. Follicle-stimulating hormone, luteinizing hormone, and testosterone levels found in human seminal plasma. Fertil. Steril. 1978, 29, 69–71. [Google Scholar] [CrossRef]
- Shirai, M.; Matsuda, S.; Mitsukawa, S.; Nakamura, M.; Yonezawa, K. FSH, LH and testosterone levels in human seminal plasma. Tohoku J. Exp. Med. 1975, 116, 201–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, J.M.; Weinbauer, G.F.; Nieschlag, E. Differential effects of FSH and testosterone on the maintenance of spermatogenesis in the adult hypophysectomized rat. J. Endocrinol. 1989, 121, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Musso, N.; Condorelli, R.A.; Musmeci, M.; Stefani, S.; La Vignera, S.; Calogero, A.E. Combined Effects of the FSHR 2039 A/G and FSHR -29 G/A Polymorphisms on Male Reproductive Parameters. World J. Men’s Health 2020, 28. [Google Scholar] [CrossRef]
- De Rocco Ponce, M.; Foresta, C.; Rago, R.; Dal Lago, A.; Balercia, G.; Calogero, A.E.; La Vignera, S.; Cosci, I.; Di Nisio, A.; Garolla, A. Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study. J. Clin. Med. 2020, 9, 1478. [Google Scholar] [CrossRef] [PubMed]
- Libman, J.; Gabriel, M.S.; Sairam, M.R.; Zini, A. Catalase can protect spermatozoa of FSH receptor knock-out mice against oxidant-induced DNA damage in vitro. Int. J. Androl. 2010, 33, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Smith, C.E.; Gregory, M.; Cyr, D.G.; Sairam, M.R.; Hermo, L. Effects of FSH receptor deletion on epididymal tubules and sperm morphology, numbers, and motility. Mol. Reprod. Dev. 2005, 72, 135–144. [Google Scholar] [CrossRef]
- Henkel, R.R.; Schill, W.B. Sperm preparation for ART. Reprod. Biol. Endocrinol. 2003, 1, 108. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Luca, G.; Arato, I.; Mancuso, F.; Calvitti, M.; Falabella, G.; Murdolo, G.; Basta, G.; Cameron, D.F.; Hansen, B.C.; Fallarino, F.; et al. Xenograft of microencapsulated Sertoli cells restores glucose homeostasis in db/db mice with spontaneous diabetes mellitus. Xenotransplantation 2016, 23, 429–439. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Dal Bosco, A.; Collodel, G.; Pistilli, A.; Stabile, A.M.; Macchioni, L.; Mancuso, F.; Luca, G.; Rende, M. In Vitro effect of nerve growth factor on the main traits of rabbit sperm. Reprod. Biol. Endocrinol. 2019, 17, 93. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, F.; Arato, I.; Di Michele, A.; Antognelli, C.; Angelini, L.; Bellucci, C.; Lilli, C.; Boncompagni, S.; Fusella, A.; Bartolini, D.; et al. Effects of Titanium Dioxide Nanoparticles on Porcine Prepubertal Sertoli Cells: An “In Vitro” Study. Front. Endocrinol. 2022, 12, 751915. [Google Scholar] [CrossRef] [PubMed]


| In-Toto Semen | Semen Pellet | Spermatozoa Separated by Swim-Up | ||||
|---|---|---|---|---|---|---|
| FSH Concentration (mIU/mL) | Asthenozoospemia | Normozoospermia | Asthenozoospemia | Normozoospermia | Asthenozoospemia | Normozoospermia |
| High mitochondrial membrane potential (%) | ||||||
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | 103.7 ± 2.4 | 94.2 ± 3.3 * | 104.6 ± 3.9 | 92.9 ± 9.7 | 100.2 ± 0.9 | 100.5 ± 0.3 |
| 10 | 105.8 ± 6.8 | 92.9 ± 2.3 * | 94.1 ± 5.0 | 102.4 ± 1.1 | 99.4 ± 0.3 | 97.9 ± 1.5 |
| 30 | 92.6 ± 3.6 | 92.0 ± 3.8 * | 87.8 ± 5.0 | 83.4 ± 19.3 | 99.4 ± 0.1 | 101.2 ± 1.6 |
| Low mitochondrial membrane potential (%) | ||||||
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | 89.3 ± 7.5 | 114.7 ± 19.1 | 88.8 ± 11.5 | 118.2 ± 26.7 | 100.7 ± 18.6 | 90.8 ± 7.1 |
| 10 | 99.6 ± 23.1 | 179.9 ± 60.8 | 110.4 ± 18.7 | 73.2 ± 10.0 | 120.6 ± 0.8 | 124.5 ± 30.6 |
| 30 | 119.7 ± 8.2 | 188.3 ± 53.8 | 181.0 ± 38.3 | 141.7 ± 71.6 | 123.9 ± 10.1 | 91.9 ± 26.7 |
| Parameters | All Cohort | Samples Used for In-Toto Semen and Pellet | Samples Used for Swim-Up | |||
|---|---|---|---|---|---|---|
| Patients (n = 13) | Controls (n = 11) | Patients (n = 8) | Controls (n = 5) | Patients (n = 5) | Controls (n = 6) | |
| Sperm concentration (million/mL) | 41.5 ± 38.7 | 59.0 ± 29.4 | 39.8 ± 32.4 | 68.0 ± 25.1 | 44.2 ± 51.3 | 50.0 ± 33.4 |
| Total sperm count (million/ejaculate) | 108.8 ± 95.0 | 169.8 ± 109.8 | 118.4 ± 113.2 | 224.5 ± 104.9 | 93.3 ± 64.3 | 115.0 ± 92.9 |
| Progressive sperm motility (%) | 21.9 ± 7.8 * | 33.9 ± 5.6 | 26.4 ± 6.2 | 33.0 ± 6.7 | 14.8 ± 3.3 * | 34.8 ± 4.8 |
| Total sperm motility (%) | 59.0 ± 12.7 * | 69.2 ± 5.9 | 65.3 ± 11.7 | 73.0 ± 5.7 | 49.0 ± 6.5 * | 65.4 ± 3.2 |
| Spermatozoa with normal morphology (%) | 7.2 ± 2.4 * | 10.8 ± 3.7 | 8.3 ± 2.3 | 11.4 ± 5.2 | 5.4 ± 1.1 * | 10.2 ± 1.8 |
| Leukocyte concentration (million/mL) | 0.6 ± 0.5 | 0.8 ± 0.5 | 0.4 ± 0.3 | 0.7 ± 0.4 | 0.8 ± 0.6 | 0.9 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarella, R.; Mancuso, F.; Barone, N.; Arato, I.; Lilli, C.; Bellucci, C.; Musmeci, M.; Luca, G.; La Vignera, S.; Condorelli, R.A.; et al. Effects of Follicle-Stimulating Hormone on Human Sperm Motility In Vitro. Int. J. Mol. Sci. 2023, 24, 6536. https://doi.org/10.3390/ijms24076536
Cannarella R, Mancuso F, Barone N, Arato I, Lilli C, Bellucci C, Musmeci M, Luca G, La Vignera S, Condorelli RA, et al. Effects of Follicle-Stimulating Hormone on Human Sperm Motility In Vitro. International Journal of Molecular Sciences. 2023; 24(7):6536. https://doi.org/10.3390/ijms24076536
Chicago/Turabian StyleCannarella, Rossella, Francesca Mancuso, Nunziata Barone, Iva Arato, Cinzia Lilli, Catia Bellucci, Marco Musmeci, Giovanni Luca, Sandro La Vignera, Rosita A. Condorelli, and et al. 2023. "Effects of Follicle-Stimulating Hormone on Human Sperm Motility In Vitro" International Journal of Molecular Sciences 24, no. 7: 6536. https://doi.org/10.3390/ijms24076536
APA StyleCannarella, R., Mancuso, F., Barone, N., Arato, I., Lilli, C., Bellucci, C., Musmeci, M., Luca, G., La Vignera, S., Condorelli, R. A., & Calogero, A. E. (2023). Effects of Follicle-Stimulating Hormone on Human Sperm Motility In Vitro. International Journal of Molecular Sciences, 24(7), 6536. https://doi.org/10.3390/ijms24076536









