Independent Signaling of Hepatoma Derived Growth Factor and Tumor Necrosis Factor-Alpha in Human Gastric Cancer Organoids Infected by Helicobacter pylori
Abstract
1. Introduction
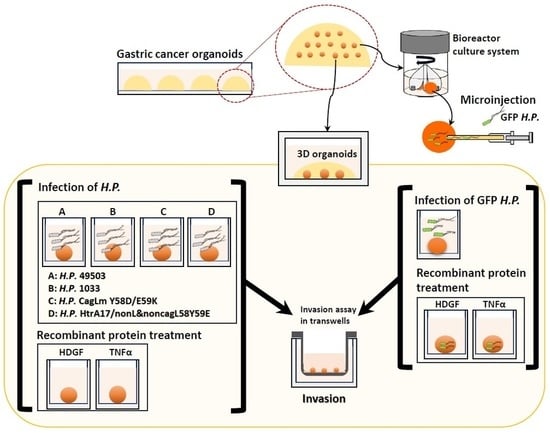
2. Results
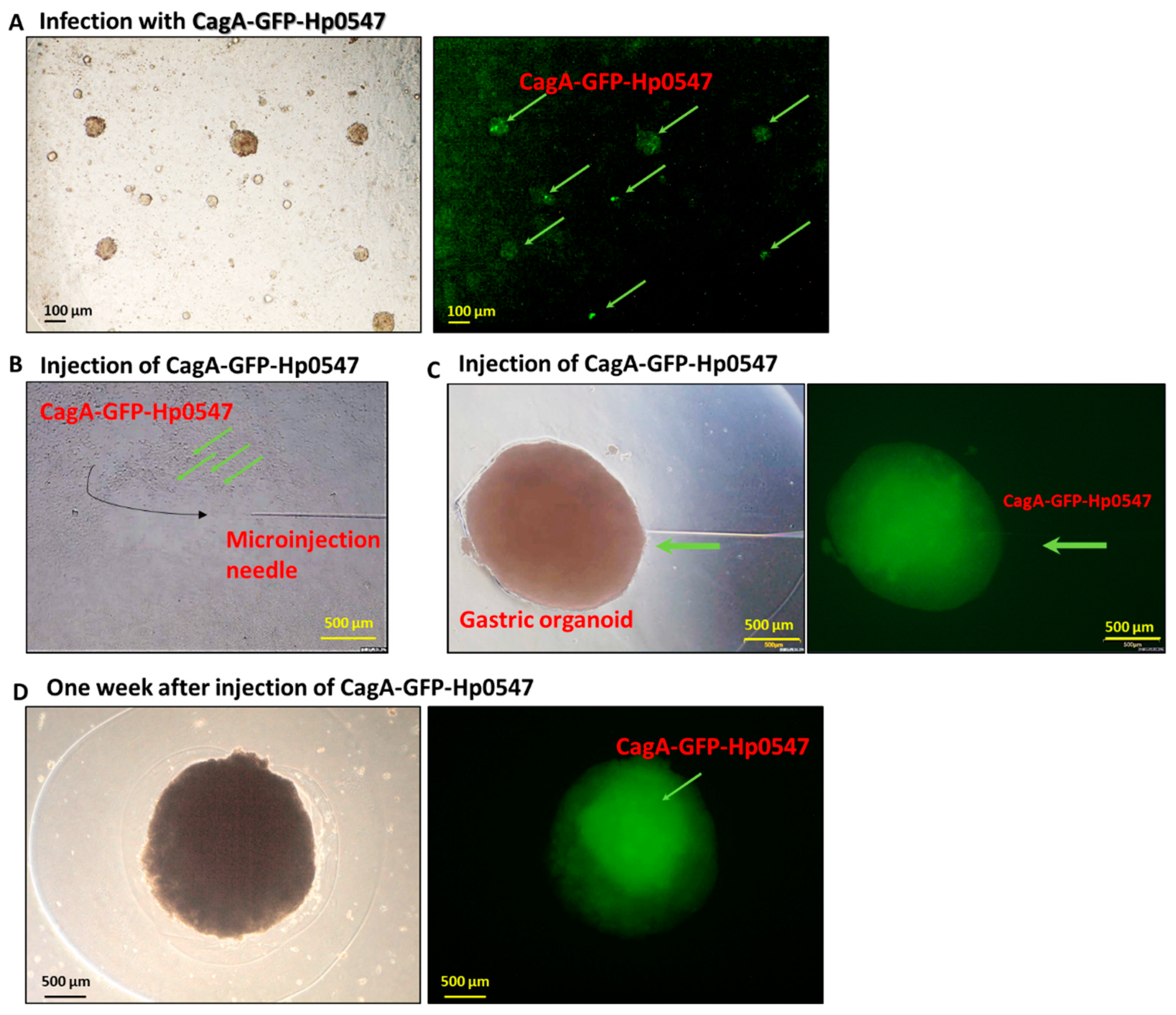
2.1. Injection of GFP-H. pylori into Gastric Cancer Organoid
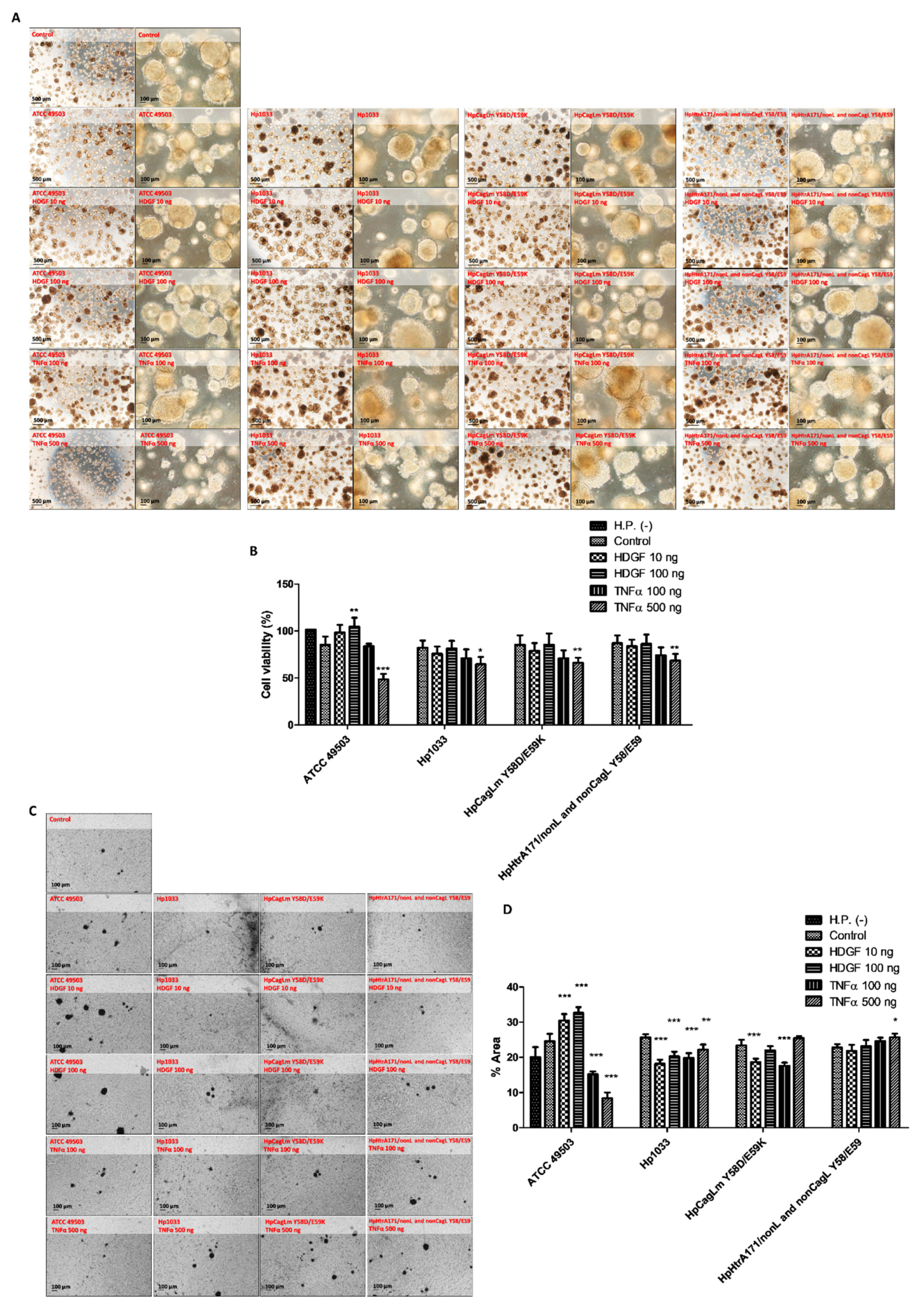
2.2. H. pylori Species with Three Different Toxicities Were Compared with the In Vitro Expansion of Gastric Cancer Organoids
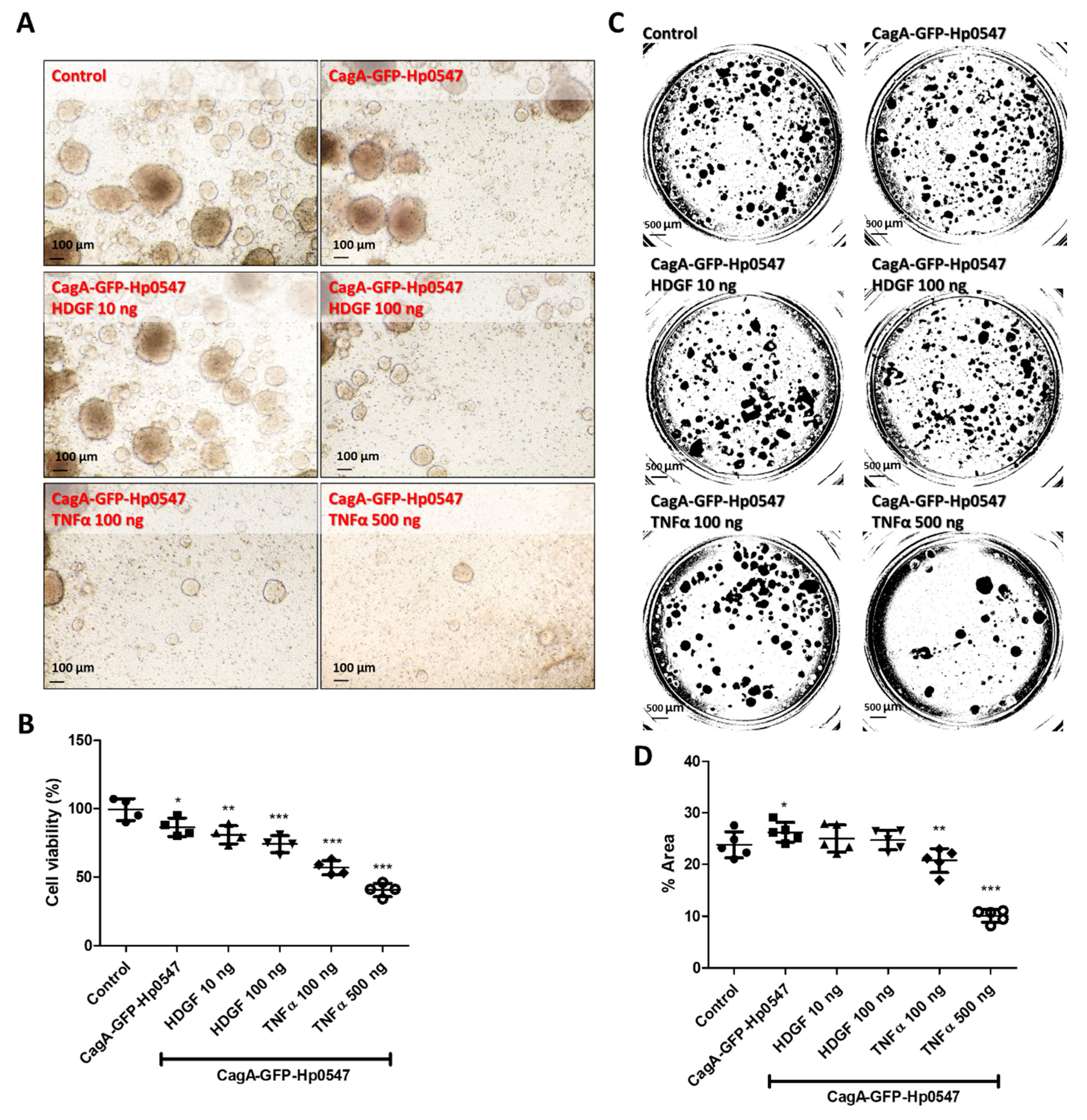
2.3. Expansion of Human Gastric Cancer Organoids Infected by H. pylori and Effects of Recombinant HDGF and TNFα on Their Viabilities and Invasion Activities
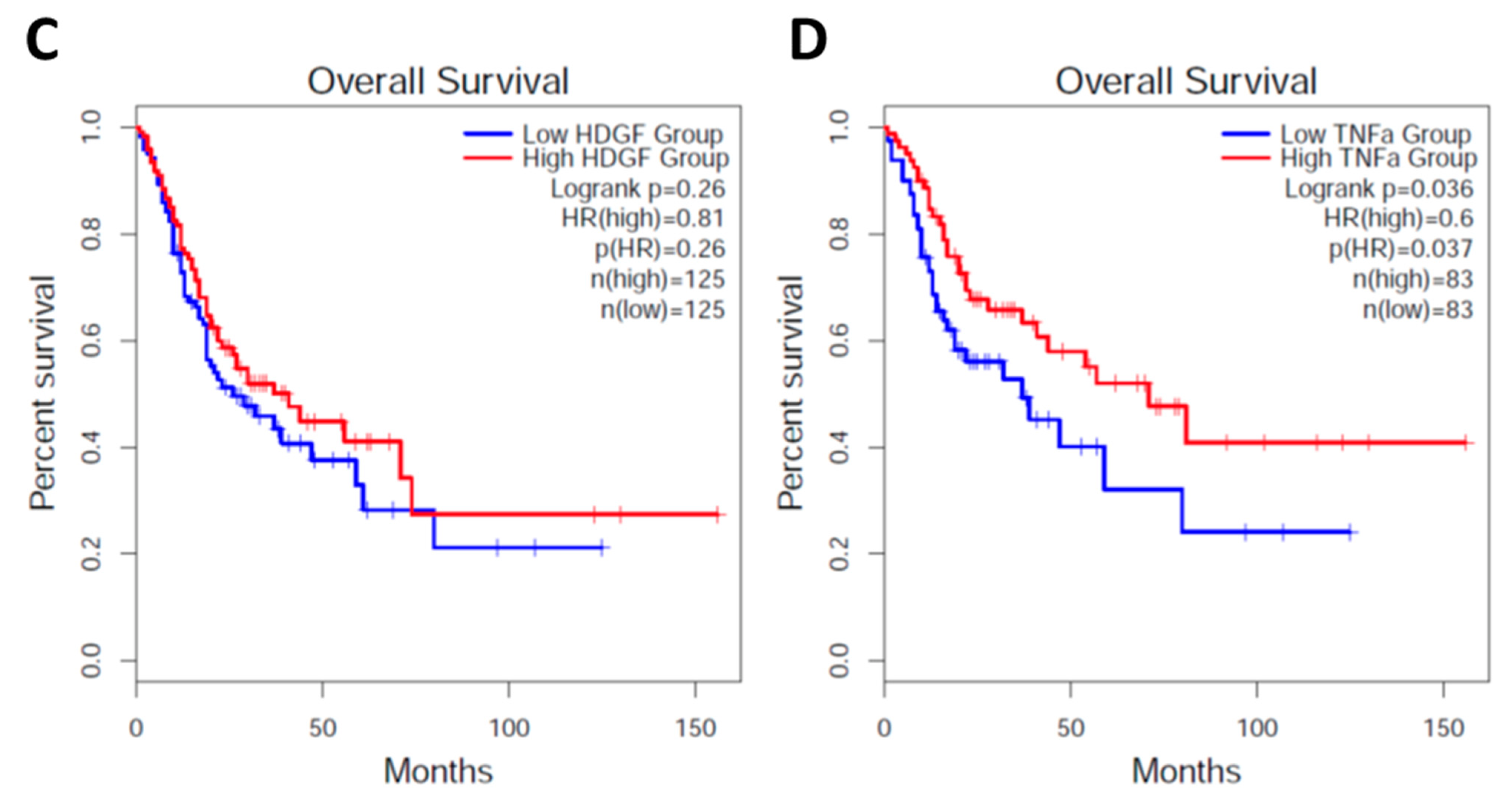
2.4. Survey of High and Low HDGF and TNFα in H. pylori-Infected Patients
3. Discussion
4. Materials and Methods
4.1. Helicobacter pylori Strains, Culture, and Microinjection
4.2. Organoid Preparation and Preparation of Recombinant HDGF and TNFα Proteins
4.3. Bioreactor Cultivation
4.4. Measurement of Viable Cells by 3-D Cell Viability Assay
4.5. Invasion Assay
4.6. Cancer Genome Atlas/GEPIA2 Portal Analysis
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; Ferlay, J.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Ni, M.; Zhu, H.; Cao, J.; Zhou, L.; Shen, S.; Peng, C.; Lv, Y.; Xu, G.; Wang, L.; et al. Differential prognostic implications of gastric adenocarcinoma based on Lauren’s classification: A Surveillance, Epidemiology, and End Results (SEER)-based cohort study. Ann. Transl. Med. 2021, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T., Jr. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Burnette, B.R.; Cross, J.V.; Mitra, S.; Ernst, P.B.; Bhakat, K.K.; Crowe, S.E. Acetylation of apurinic/apyrimidinic endonuclease-1 regulates Helicobacter pylori-mediated gastric epithelial cell apoptosis. Gastroenterology 2009, 136, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Broda, T.R.; McCracken, K.W.; Wells, J.M. Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat. Protoc. 2019, 14, 28–50. [Google Scholar] [CrossRef]
- Kalabis, J.; Wong, G.S.; Vega, M.E.; Natsuizaka, M.; Robertson, E.S.; Herlyn, M.; Nakagawa, H.; Rustgi, A.K. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat. Protoc. 2012, 7, 235–246. [Google Scholar] [CrossRef]
- McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.-H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Chu, T.H.; Huang, S.T.; Yang, S.F.; Li, C.J.; Lin, G.W.; Weng, B.C.; Yang, S.M.; Huang, S.C.; Wu, J.C.; Chang, Y.C.; et al. Hepatoma-derived growth factor participates in Helicobacter Pylori-induced neutrophils recruitment, gastritis and gastric carcinogenesis. Oncogene 2019, 38, 6461–6477. [Google Scholar] [CrossRef]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, 6409. [Google Scholar] [CrossRef]
- Gomez-Mejiba, S.E.; Zhai, Z.; Gimenez, M.S.; Ashby, M.T.; Chilakapati, J.; Kitchin, K.; Mason, R.P.; Ramirez, D.C. Myeloperoxidase-induced genomic DNA-centered radicals. J. Biol. Chem. 2010, 285, 20062–20071. [Google Scholar] [CrossRef]
- Ku, C.-C.; Wuputra, K.; Pan, J.-B.; Li, C.-P.; Liu, C.-J.; Liu, Y.-C.; Saito, S.; Chan, T.-F.; Lin, C.-S.; Wu, D.-C.; et al. Generation of Human Stomach Cancer iPSC-Derived Organoids Induced by Helicobacter pylori Infection and Their Application to Gastric Cancer Research. Cells 2022, 11, 184. [Google Scholar] [CrossRef]
- Wuputra, K.; Ku, C.C.; Kato, K.; Wu, D.C.; Saito, S.; Yokoyama, K.K. Translational models of 3-D organoids and cancer stem cells in gastric cancer research. Stem Cell Res. Ther. 2021, 12, 492. [Google Scholar] [CrossRef]
- Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Liu, C.-J.; Liu, Y.-C.; Saito, S.; Kato, K.; Lin, Y.-C.; Kuo, K.-K.; Chan, T.-F.; et al. Stem Cell Biomarkers and Tumorigenesis in Gastric Cancer. J. Pers. Med. 2022, 12, 929. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Kuo, H.K.; Chang, H.-L.; Yang, H.B.; Lu, C.C.; Cheng, H.C.; Wu, M.-S.; Sheu, B.S.H. H. Pylori isolates with amino acid sequence polymorphisms as presence of both HtrA-L171 & CagL-Y58.E59 increase the risk of gastric cancer. J. Biomed. Sci. 2019, 26, 4–8. [Google Scholar]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015, 148, 126–136.e126. [Google Scholar] [CrossRef]
- Lu, P.J.; Hsu, P.I.; Chen, C.H.; Hsiao, M.; Chang, W.C.; Tseng, H.H.; Lin, K.H.; Chuah, S.K.; Chen, H.C. Gastric juice acidity in upper gastrointestinal diseases. World J. Gastroenterol. 2010, 16, 5496–5501. [Google Scholar] [CrossRef]
- Peek, R.M., Jr.; Blaser, M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2002, 2, 28–37. [Google Scholar] [PubMed]
- Yeh, Y.C.; Cheng, H.C.; Yang, H.B.; Chang, W.L.; Sheu, B.S.H. H. pylori CagL-Y58/E59 prime higher integrin α5β1 in adverse pH condition to enhance hypochlorhydria vicious cycle for gastric carcinogenesis. PLoS ONE 2013, 8, e72735. [Google Scholar] [CrossRef]
- Shimoyama, T.; Crabtree, J.E. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut 1998, 43 (Suppl. 1), S2–S5. [Google Scholar] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Sigal, M.; Rothenberg, M.E.; Logan, C.Y.; Lee, J.Y.; Honaker, R.W.; Cooper, R.L.; Passarelli, B.; Camorlinga, M.; Bouley, D.M.; Alvarez, G.; et al. Helicobacter pylori Activates and Expands Lgr5+ Stem Cells through Direct Colonization of the Gastric Glands. Gastroenterology 2015, 148, 1392–1404.e21. [Google Scholar] [CrossRef]
- Oh, J.D.; Karam, S.M.; Gordon, J.I. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl. Acad. Sci. USA. 2005, 102, 5186–5191. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef]
- Fumagalli, A.; Drost, J.; Suijkerbuijk, S.J.; van Boxtel, R.; de Ligt, J.; Offerhaus, G.J.; Begthel, H.; Beerling, E.; Tan, E.H.; Sansom, O.J.; et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl. Acad. Sci. USA 2017, 114, E2357–E2364. [Google Scholar] [CrossRef]
- Schlaermann, P.; Toelle, B.; Berger, H.; Schmidt, S.C.; Glanemann, M.; Ordemann, J.; Bartfeld, S.; Mollenkopf, H.J.; Meyer, T.F. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 2016, 65, 202–213. [Google Scholar] [CrossRef]
- Lin, W.-C.; Tsai, H.-F.; Kuo, S.-H.; Wu, M.-S.; Lin, C.-W.; Hsu, P.-I.; Cheng, A.-L.; Hsu, P.-N. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010, 70, 5740–5748. [Google Scholar] [CrossRef]
- Barrozo, R.M.; Cooke, C.L.; Hansen, L.M.; Lam, A.M.; Gaddy, J.A.; Johnson, E.M.; Cariaga, T.A.; Suarez, G.; Peek, R.M., Jr.; Cover, T.L.; et al. Functional plasticity in the type IV secretion system of Helicobacter pylori. PLoS Pathog. 2013, 9, e1003189. [Google Scholar] [CrossRef]
- Przepiorski, A.; Sander, V.; Tran, T.; Hollywood, J.A.; Sorrenson, B.; Shih, J.-H.; Wolvetang, E.J.; McMahon, A.P.; Holm, T.M.; Davidson, A.J. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 470–484. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- De Angelis, M.L.; Francescangeli, F.; Nicolazzo, C.; Signore, M.; Giuliani, A.; Colace, L.; Boe, A.; Magri, V.; Baiocchi, M.; Ciardi, A.; et al. An organoid model of colorectal circulating tumor cells with stem cell features, hybrid EMT state and distinctive therapy response profile. J. Exp. Clin. Cancer Res. 2022, 41, 86. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Liu, C.-J.; Kato, K.; Lin, Y.-C.; Liu, Y.-C.; Lin, C.-S.; Hsiao, M.; Tai, M.-H.; et al. Independent Signaling of Hepatoma Derived Growth Factor and Tumor Necrosis Factor-Alpha in Human Gastric Cancer Organoids Infected by Helicobacter pylori. Int. J. Mol. Sci. 2023, 24, 6567. https://doi.org/10.3390/ijms24076567
Wuputra K, Ku C-C, Pan J-B, Liu C-J, Kato K, Lin Y-C, Liu Y-C, Lin C-S, Hsiao M, Tai M-H, et al. Independent Signaling of Hepatoma Derived Growth Factor and Tumor Necrosis Factor-Alpha in Human Gastric Cancer Organoids Infected by Helicobacter pylori. International Journal of Molecular Sciences. 2023; 24(7):6567. https://doi.org/10.3390/ijms24076567
Chicago/Turabian StyleWuputra, Kenly, Chia-Chen Ku, Jia-Bin Pan, Chung-Jung Liu, Kohsuke Kato, Ying-Chu Lin, Yi-Chang Liu, Chang-Shen Lin, Michael Hsiao, Ming-Hong Tai, and et al. 2023. "Independent Signaling of Hepatoma Derived Growth Factor and Tumor Necrosis Factor-Alpha in Human Gastric Cancer Organoids Infected by Helicobacter pylori" International Journal of Molecular Sciences 24, no. 7: 6567. https://doi.org/10.3390/ijms24076567
APA StyleWuputra, K., Ku, C.-C., Pan, J.-B., Liu, C.-J., Kato, K., Lin, Y.-C., Liu, Y.-C., Lin, C.-S., Hsiao, M., Tai, M.-H., Chong, I.-W., Hu, H.-M., Kuo, C.-H., Wu, D.-C., & Yokoyama, K. K. (2023). Independent Signaling of Hepatoma Derived Growth Factor and Tumor Necrosis Factor-Alpha in Human Gastric Cancer Organoids Infected by Helicobacter pylori. International Journal of Molecular Sciences, 24(7), 6567. https://doi.org/10.3390/ijms24076567