Preleukemic Fusion Genes Induced via Ionizing Radiation
Abstract
1. Ionizing Radiation and DNA Damage
2. Mechanisms of Fusion Genes Formation and Its Prevalence
3. IR Induced Leukemia
4. PFGs Induced by IR
4.1. Induction of BCR-ABL1 Gene Fusions
4.2. Induction of RUNX1-RUNX1T1 Gene Fusion
4.3. Induction of KMT2A (MLL)—Translocations
4.4. Induction of PML-RARA—Translocations
5. Probability of BCR-ABL1, RUNX1-RUNX1T1, and PML-RARA Induction
- A = 4 × (200 × 103 × 3 × 103)/(6.4 × 109)2 = 5.8 × 10−11
- B = 5.8 × 10−11 × 0.057 = 3.3 × 10−12
- C = 3.3 × 10−12/(20 × 106 × 1400 × 10−2) = 1.18 × 10−4
- A = 4 × (25 × 103 × 15 × 103)/(6.4 × 109)2 = 3.6 × 10−11
- B = 3.6 × 10−11 × 0.057 = 2.05 × 10−12
- C = 2.05 × 10−12/(20 × 106 × 1400 × 10−2) = 7.3 × 10−5
- A = 4 × (2.8 × 103 × 15 × 103)/(6.4 × 109)2 = 4.1 × 10−12
- B = 4.1 × 10−12 × 0.057 = 2.3 × 10−13
- C = 2.3 × 10−13/(20 × 106 × 1400 × 10−2) = 8.2 × 10−6
6. Discussion
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchinson, F. Chemical changes induced in DNA by ionizing radiation. Prog. Nucleic Acid Res. Mol. Biol. 1985, 32, 115–154. [Google Scholar] [PubMed]
- Abolfath, R.M.; Carlson, D.J.; Chen, Z.J.; Nath, R. A molecular dynamics simulation of DNA damage induction by ionizing radiation. Phys. Med. Biol. 2013, 58, 7143–7157. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. Int. J. Radiat. Biol. 1994, 65, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Nikjoo, H.; Uehara, S.; Wilson, W.E.; Hoshi, M.; Goodhead, D.T. Track structure in radiation biology: Theory and applications. Int. J. Radiat. Biol. 1998, 73, 355–364. [Google Scholar] [CrossRef]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Cannan, W.J.; Pederson, D.S. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J. Cell. Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef]
- Thadathil, N.; Hori, R.; Xiao, J.; Khan, M.M. DNA double-strand breaks: A potential therapeutic target for neurodegenerative diseases. Chromosome Res. 2019, 27, 345–364. [Google Scholar] [CrossRef]
- Rothkamm, K.; Kruger, I.; Thompson, L.H.; Lobrich, M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 2003, 23, 5706–5715. [Google Scholar] [CrossRef]
- Boffetta, P.; van der Hel, O.; Norppa, H.; Fabianova, E.; Fucic, A.; Gundy, S.; Lazutka, J.; Cebulska-Wasilewska, A.; Puskailerova, D.; Znaor, A.; et al. Chromosomal aberrations and cancer risk: Results of a cohort study from Central Europe. Am. J. Epidemiol. 2007, 165, 36–43. [Google Scholar] [CrossRef]
- Yun, S.M.; Yoon, K.; Lee, S.; Kim, E.; Kong, S.H.; Choe, J.; Kang, J.M.; Han, T.S.; Kim, P.; Choi, Y.; et al. PPP1R1B-STARD3 chimeric fusion transcript in human gastric cancer promotes tumorigenesis through activation of PI3K/AKT signaling. Oncogene 2014, 33, 5341–5347. [Google Scholar] [CrossRef]
- Chen, M.; Foster, J.P., 2nd; Lock, I.C.; Leisenring, N.H.; Daniel, A.R.; Floyd, W.; Xu, E.; Davis, I.J.; Kirsch, D.G. Radiation-Induced Phosphorylation of a Prion-Like Domain Regulates Transformation by FUS-CHOP. Cancer Res. 2021, 81, 4939–4948. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, L.K.; Poplawski, A.; Grandt, C.L.; Schwarz, H.; Hankeln, T.; Rapp, S.; Zahnreich, S.; Galetzka, D.; Schmitt, I.; Grad, C.; et al. Comparison of time and dose dependent gene expression and affected pathways in primary human fibroblasts after exposure to ionizing radiation. Mol. Med. 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.; Barreau, K.; Kling, T.; Tisell, M.; Caren, H. Accumulation of DNA methylation alterations in paediatric glioma stem cells following fractionated dose irradiation. Clin. Epigenet. 2020, 12, 26. [Google Scholar] [CrossRef]
- Miousse, I.R.; Ewing, L.E.; Kutanzi, K.R.; Griffin, R.J.; Koturbash, I. DNA Methylation in Radiation-Induced Carcinogenesis: Experimental Evidence and Clinical Perspectives. Crit. Rev. Oncog. 2018, 23, 1–11. [Google Scholar] [CrossRef]
- Di Nisio, E.; Lupo, G.; Licursi, V.; Negri, R. The Role of Histone Lysine Methylation in the Response of Mammalian Cells to Ionizing Radiation. Front. Genet. 2021, 12, 639602. [Google Scholar] [CrossRef]
- Kumar, R.; Horikoshi, N.; Singh, M.; Gupta, A.; Misra, H.S.; Albuquerque, K.; Hunt, C.R.; Pandita, T.K. Chromatin modifications and the DNA damage response to ionizing radiation. Front. Oncol. 2013, 2, 214. [Google Scholar] [CrossRef]
- Julienne, H.; Zoufir, A.; Audit, B.; Arneodo, A. Human Genome Replication Proceeds through Four Chromatin States. PLoS Comput. Biol. 2013, 9, e1003233. [Google Scholar] [CrossRef] [PubMed]
- Weckselblatt, B.; Rudd, M.K. Human Structural Variation: Mechanisms of Chromosome Rearrangements. Trends Genet. 2015, 31, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Aplan, P.D. Causes of oncogenic chromosomal translocation. Trends Genet. 2006, 22, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I. Molecular targets and mechanisms in formation of chromosomal aberrations: Contributions of Soviet scientists. Cytogenet. Genome Res. 2004, 104, 56–64. [Google Scholar] [CrossRef]
- Thandla, S.P.; Ploski, J.E.; Raza-Egilmez, S.Z.; Chhalliyil, P.P.; Block, A.W.; de Jong, P.J.; Aplan, P.D. ETV6-AML1 translocation breakpoints cluster near a purine/pyrimidine repeat region in the ETV6 gene. Blood 1999, 93, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Aplan, P.D.; Raimondi, S.C.; Kirsch, I.R. Disruption of the SCL gene by a t(1;3) translocation in a patient with T cell acute lymphoblastic leukemia. J. Exp. Med. 1992, 176, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Hayes, K.; Cortes, J.; Albitar, M.; Glassman, A.; Talpaz, M.; Kantarjian, H.M. Translocation t(17;18)(q10;q10): A new nonrandom chromosomal translocation of clonal evolution in chronic myeloid leukemia. Cancer 2001, 91, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, M.; Lindsey, R.H., Jr.; Felix, C.A.; Grimwade, D.; Osheroff, N. Topoisomerase II and leukemia. Ann. N. Y. Acad. Sci. 2014, 1310, 98–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Rowley, J.D. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair 2006, 5, 1282–1297. [Google Scholar] [CrossRef]
- Gue, M.; Sun, J.S.; Boudier, T. Simultaneous localization of MLL, AF4 and ENL genes in interphase nuclei by 3D-FISH: MLL translocation revisited. BMC Cancer 2006, 6, 20. [Google Scholar] [CrossRef]
- Neves, H.; Ramos, C.; da Silva, M.G.; Parreira, A.; Parreira, L. The nuclear topography of ABL, BCR, PML, and RARalpha genes: Evidence for gene proximity in specific phases of the cell cycle and stages of hematopoietic differentiation. Blood 1999, 93, 1197–1207. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Stringer, J.R.; Blough, R.; Medvedovic, M.; Fagin, J.A.; Nikiforov, Y.E. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science 2000, 290, 138–141. [Google Scholar] [CrossRef]
- Gandhi, M.; Medvedovic, M.; Stringer, J.R.; Nikiforov, Y.E. Interphase chromosome folding determines spatial proximity of genes participating in carcinogenic RET/PTC rearrangements. Oncogene 2006, 25, 2360–2366. [Google Scholar] [CrossRef]
- Hamatani, K.; Eguchi, H.; Ito, R.; Mukai, M.; Takahashi, K.; Taga, M.; Imai, K.; Cologne, J.; Soda, M.; Arihiro, K.; et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008, 68, 7176–7182. [Google Scholar] [CrossRef]
- Caudill, C.M.; Zhu, Z.W.; Ciampi, R.; Stringer, J.R.; Nikiforov, Y.E. Dose-dependent generation of RET/PTC in human thyroid cells after in vitro exposure to gamma-radiation: A model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J. Clin. Endocr. Metab. 2005, 90, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Song, Z.; Babiceanu, M.; Song, Y.; Facemire, L.; Singh, R.; Adli, M.; Li, H. Discovery of CTCF-sensitive Cis-spliced fusion RNAs between adjacent genes in human prostate cells. PLoS Genet. 2015, 11, e1005001. [Google Scholar]
- Li, H.; Wang, J.; Ma, X.; Sklar, J. Gene fusions and RNA trans-splicing in normal and neoplastic human cells. Cell Cycle 2009, 8, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Akiva, P.; Toporik, A.; Edelheit, S.; Peretz, Y.; Diber, A.; Shemesh, R.; Novik, A.; Sorek, R. Transcription-mediated gene fusion in the human genome. Genome Res. 2006, 16, 30–36. [Google Scholar] [CrossRef]
- Jividen, K.; Li, H. Chimeric RNAs generated by intergenic splicing in normal and cancer cells. Genes. Chromosomes Cancer 2014, 53, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Qin, F.; Movassagh, M.; Park, H.; Golden, W.; Xie, Z.; Zhang, P.; Sklar, J.; Li, H. A chimeric RNA characteristic of rhabdomyosarcoma in normal myogenesis process. Cancer Discov. 2013, 3, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, M.; Yuan, H.; Park, H.G.; Frierson, H.F.; Li, H. Chimeric transcript generated by cis-splicing of adjacent genes regulates prostate cancer cell proliferation. Cancer Discov. 2012, 2, 598–607. [Google Scholar] [CrossRef]
- Campbell, P.J.; Stephens, P.J.; Pleasance, E.D.; O’Meara, S.; Li, H.; Santarius, T.; Stebbings, L.A.; Leroy, C.; Edkins, S.; Hardy, C.; et al. Identification of somatically acquired rearrangements in cancer using genome-wide massively parallel paired-end sequencing. Nat. Genet. 2008, 40, 722–729. [Google Scholar] [CrossRef]
- Mata-Rocha, M.; Rangel-Lopez, A.; Jimenez-Hernandez, E.; Morales-Castillo, B.A.; Gonzalez-Torres, C.; Gaytan-Cervantes, J.; Alvarez-Olmos, E.; Nunez-Enriquez, J.C.; Fajardo-Gutierrez, A.; Martin-Trejo, J.A.; et al. Identification and Characterization of Novel Fusion Genes with Potential Clinical Applications in Mexican Children with Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2019, 20, 2394. [Google Scholar] [CrossRef]
- Engvall, M.; Cahill, N.; Jonsson, B.I.; Hoglund, M.; Hallbook, H.; Cavelier, L. Detection of leukemia gene fusions by targeted RNA-sequencing in routine diagnostics. BMC Med. Genom. 2020, 13, 106. [Google Scholar] [CrossRef]
- Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. 2022. Mitelman, F., Johansson, B., Mertens, F., (Eds.). Available online: https://mitelmandatabase.isb-cgc.org (accessed on 20 August 2022).
- Ping, L.; Chen, J.J.; Liao, C.S.; Liu, G.H.; Ming, Z. Silencing of circ_0009910 inhibits acute myeloid leukemia cell growth through increasing miR-20a-5p. Blood Cell Mol. Dis. 2019, 75, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, C.Q.; Jiao, J.; Li, P.; Cui, B.X.; Ji, C.Y.; Ma, D.X. Characterization of hsa_circ_0004277 as a New Biomarker for Acute Myeloid Leukemia via Circular RNA Profile and Bioinformatics Analysis. Int. J. Mol. Sci. 2017, 18, 597. [Google Scholar] [CrossRef] [PubMed]
- Guarnerio, J.; Bezzi, M.; Jeong, J.C.; Paffenholz, S.V.; Berry, K.; Naldini, M.M.; Lo-Coco, F.; Tay, Y.; Beck, A.H.; Pandolfi, P.P. Oncogenic Role of Fusion-circRNAs Derived from Cancer-Associated Chromosomal Translocations. Cell 2016, 166, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Babin, L.; Piganeau, M.; Renouf, B.; Lamribet, K.; Thirant, C.; Deriano, L.; Mercher, T.; Giovannangeli, C.; Brunet, E.C. Chromosomal Translocation Formation Is Sufficient to Produce Fusion Circular RNAs Specific to Patient Tumor Cells. iScience 2018, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; Robles, A.I.; Harris, C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer 2010, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Rudich, A.; Garzon, R.; Dorrance, A. Non-Coding RNAs Are Implicit in Chronic Myeloid Leukemia Therapy Resistance. Int. J. Mol. Sci. 2022, 23, 12271. [Google Scholar] [CrossRef]
- Anderson, K.; Lutz, C.; van Delft, F.W.; Bateman, C.M.; Guo, Y.; Colman, S.M.; Kempski, H.; Moorman, A.V.; Titley, I.; Swansbury, J.; et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 2011, 469, 356–361. [Google Scholar] [CrossRef]
- Castor, A.; Nilsson, L.; Astrand-Grundstrom, I.; Buitenhuis, M.; Ramirez, C.; Anderson, K.; Strombeck, B.; Garwicz, S.; Bekassy, A.N.; Schmiegelow, K.; et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat. Med. 2005, 11, 630–637. [Google Scholar] [CrossRef]
- Long, N.A.; Golla, U.; Sharma, A.; Claxton, D.F. Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications. Stem Cell Rev. Rep. 2022, 18, 1211–1226. [Google Scholar] [CrossRef]
- Greaves, M. A natural history for pediatric acute leukemia. Blood 1993, 82, 1043–1051. [Google Scholar] [CrossRef]
- Greaves, M.F.; Maia, A.T.; Wiemels, J.L.; Ford, A.M. Leukemia in twins: Lessons in natural history. Blood 2003, 102, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J. Chromosomal Translocations in Childhood Leukemia: Natural History, Mechanisms, and Epidemiology. JNCI Monogr. 2008, 2008, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, E.L.; Spector, L.G.; Mendes-de-Almeida, D.P.; Nelson, H.H. The Prenatal Origin of Childhood Leukemia: Potential Applications for Epidemiology and Newborn Screening. Front. Pediatr. 2021, 9, 639479. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G. Genetics and prognosis of ALL in children vs adults. Hematol.-Am. Soc. Hematol. 2018, 2018, 137–145. [Google Scholar] [CrossRef]
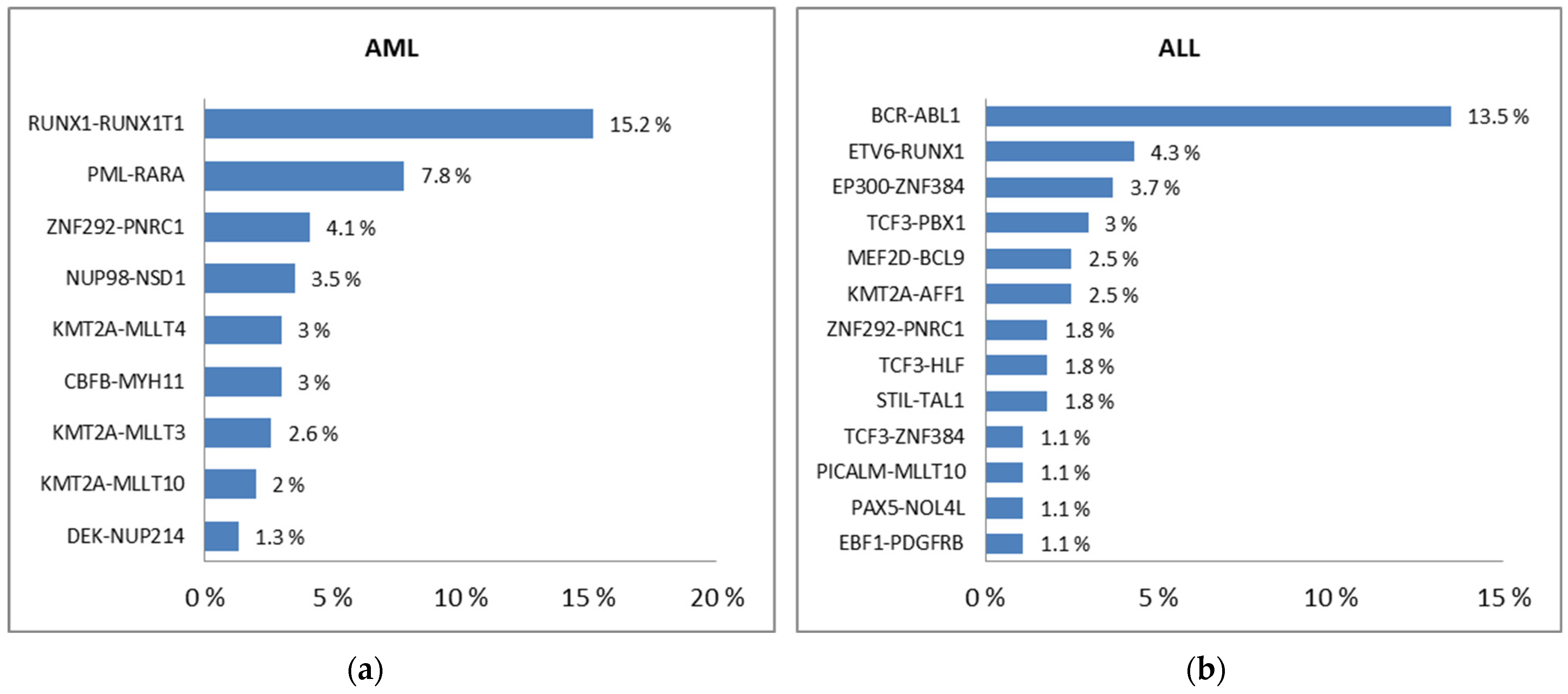
- Chen, X.; Wang, F.; Zhang, Y.; Ma, X.; Cao, P.; Yuan, L.; Wang, L.; Chen, J.; Zhou, X.; Wu, Q.; et al. Fusion gene map of acute leukemia revealed by transcriptome sequencing of a consecutive cohort of 1000 cases in a single center. Blood Cancer J. 2021, 11, 112. [Google Scholar] [CrossRef]
- Hsu, W.L.; Preston, D.L.; Soda, M.; Sugiyama, H.; Funamoto, S.; Kodama, K.; Kimura, A.; Kamada, N.; Dohy, H.; Tomonaga, M.; et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat. Res. 2013, 179, 361–382. [Google Scholar] [CrossRef]
- Kodama, K.; Mabuchi, K.; Shigematsu, I. A long-term cohort study of the atomic-bomb survivors. J. Epidemiol. 1996, 6 (Suppl. 3), S95–S105. [Google Scholar] [CrossRef]
- Preston, D.L.; Ron, E.; Tokuoka, S.; Funamoto, S.; Nishi, N.; Soda, M.; Mabuchi, K.; Kodama, K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 2007, 168, 1–64. [Google Scholar] [CrossRef]
- Grant, E.J.; Brenner, A.; Sugiyama, H.; Sakata, R.; Sadakane, A.; Utada, M.; Cahoon, E.K.; Milder, C.M.; Soda, M.; Cullings, H.M.; et al. Solid Cancer Incidence among the Life Span Study of Atomic Bomb Survivors: 1958–2009. Radiat. Res. 2017, 187, 513–537. [Google Scholar] [CrossRef]
- Preston, D.L.; Pierce, D.A.; Shimizu, Y.; Cullings, H.M.; Fujita, S.; Funamoto, S.; Kodama, K. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat. Res. 2004, 162, 377–389. [Google Scholar] [CrossRef]
- Ron, E.; Preston, D.L.; Mabuchi, K.; Thompson, D.E.; Soda, M. Cancer incidence in atomic bomb survivors. Part IV: Comparison of cancer incidence and mortality. Radiat. Res. 1994, 137 (Suppl. 2), S98–S112. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Pawel, D.; Misumi, M.; Hamada, N.; Cullings, H.M.; Wakeford, R.; Ozasa, K. Lifetime Mortality Risk from Cancer and Circulatory Disease Predicted from the Japanese Atomic Bomb Survivor Life Span Study Data Taking Account of Dose Measurement Error. Radiat. Res. 2020, 194, 566. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Jordan, R.; Sun, J.; Ma, H.; Hsieb, A.W. Dose-dependent changes in the spectrum of mutations induced by ionizing radiation. Radiat. Res. 2000, 153, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Grosovsky, A.J.; Little, J.B. Evidence for linear response for the induction of mutations in human cells by x-ray exposures below 10 rads. Proc. Natl. Acad. Sci. USA 1985, 82, 2092–2095. [Google Scholar] [CrossRef]
- Ludovici, G.M.; Cascone, M.G.; Huber, T.; Chierici, A.; Gaudio, P.; de Souza, S.O.; D’Errico, F.; Malizia, A. Cytogenetic bio-dosimetry techniques in the detection of dicentric chromosomes induced by ionizing radiation: A review. Eur. Phys. J. Plus 2021, 136, 1–21. [Google Scholar] [CrossRef]
- Tawn, E.J.; Whitehouse, C.A.; Tarone, R.E. FISH chromosome aberration analysis on retired radiation workers from the sellafield nuclear facility. Radiat. Res. 2004, 162, 249–256. [Google Scholar] [CrossRef]
- Tucker, J.D.; Sorensen, K.J.; Chu, C.S.; Nelson, D.O.; Ramsey, M.J.; Urlando, C.; Heddle, J.A. The accumulation of chromosome aberrations and Dlb-1 mutations in mice with highly fractionated exposure to gamma radiation. Mutat. Res.-Fund. Mol. Mech. Mutagen. 1998, 400, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.N.; Hill, F.; Burk, C.; Fester, T.; Straume, T. Dose-Response Curve for Chromosome Translocations Measured in Human-Lymphocytes Exposed to Co-60 Gamma-Rays. Health Phys. 1995, 68, 761–765. [Google Scholar] [CrossRef]
- Romm, H.; Stephan, G. Dose dependency of FISH-detected translocations in stable and unstable cells after Cs-137 gamma irradiation of human lymphocytes in vitro. Cytogenet. Genome Res. 2004, 104, 162–167. [Google Scholar] [CrossRef]
- Wright, E.G. Ionizing radiation and leukaemia: More questions than answers. Hematol. Oncol. 2005, 23, 119–126. [Google Scholar] [CrossRef]
- National Research Council. Health Effects of Exposure to Low Levels of Ionizing Radiation: BEIR V; The National Academies Press: Washington, DC, USA, 1990. [Google Scholar] [CrossRef]
- Preston, D.L.; Kusumi, S.; Tomonaga, M.; Izumi, S.; Ron, E.; Kuramoto, A.; Kamada, N.; Dohy, H.; Matsuo, T.; Matsui, T.; et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat. Res. 1994, 137 (Suppl. 2), S68–S97. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.A.; Linton, O.W. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, medical exposure—Are we doing less with more, and is there a role for health physicists? Health Phys. 2009, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Kwan, M.L.; Marlow, E.C.; Theis, M.K.; Bolch, W.; Cheng, S.Y.; Bowles, E.J.A.; Duncan, J.R.; Greenlee, R.T.; Kushi, L.H.; et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. JAMA-J. Am. Med. Assoc. 2019, 322, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Berrington de Gonzalez, A.; Salotti, J.A.; McHugh, K.; Little, M.P.; Harbron, R.W.; Lee, C.; Ntowe, E.; Braganza, M.Z.; Parker, L.; Rajaraman, P.; et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: Assessment of the impact of underlying conditions. Br. J. Cancer 2016, 114, 388–394. [Google Scholar] [CrossRef]
- Little, M.P.; Wakeford, R.; Borrego, D.; French, B.; Zablotska, L.B.; Adams, M.J.; Allodji, R.; de Vathaire, F.; Lee, C.; Brenner, A.V.; et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: A pooled analysis of nine historical cohort studies. Lancet Haematol. 2018, 5, e346–e358. [Google Scholar]
- Hauptmann, M.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; Lubin, J.H.; Preston, D.L.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. J. Natl. Cancer Inst. Monogr. 2020, 2020, 188–200. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Sir Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Kendall, G.M.; Little, M.P.; Wakeford, R.; Bunch, K.J.; Miles, J.C.H.; Vincent, T.J.; Meara, J.R.; Murphy, M.F.G. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia 2013, 27, 3–9. [Google Scholar] [CrossRef]
- Mazzei-Abba, A.; Folly, C.L.; Coste, A.; Wakeford, R.; Little, M.P.; Raaschou-Nielsen, O.; Kendall, G.; Hemon, D.; Nikkila, A.; Spix, C.; et al. Epidemiological studies of natural sources of radiation and childhood cancer: Current challenges and future perspectives. J. Radiol. Prot. 2020, 40, R1–R23. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent Neoplasms in 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. JNCI-J. Natl. Cancer I 2010, 102, 1083–1095. [Google Scholar] [CrossRef]
- Leone, G.; Pagano, L.; Ben-Yehuda, D.; Voso, M.T. Therapy-related leukemia and myelodysplasia: Susceptibility and incidence. Haematologica 2007, 92, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; St Clair, C.M.; Deutsch, I.; Burke, W.M.; Gorrochurn, P.; Sun, X.; Herzog, T.J. Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer 2010, 116, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Ahmed, S. Characteristics of BCR-ABL gene variants in patients of chronic myeloid leukemia. Open Med. 2021, 16, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Seyama, T.; Mizuno, T.; Hayashi, T.; Iwamoto, K.S.; Dohi, K.; Nakamura, N.; Akiyama, M. Induction of BCR-ABL fusion genes by in vitro X-irradiation. Jpn. J. Cancer Res. 1993, 84, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Granter, N. Leukemia patient-derived lymphoblastoid cell lines exhibit increased induction of leukemia-associated transcripts following high-dose irradiation. Exp. Hematol. 1999, 27, 1397–1401. [Google Scholar] [CrossRef]
- Spencer, A.; Yan, X.H.; Chase, A.; Goldman, J.M.; Melo, J.V. BCR-ABL-positive lymphoblastoid cells display limited proliferative capacity under in vitro culture conditions. Br. J. Haematol. 1996, 94, 654–658. [Google Scholar] [CrossRef]
- Martin, P.J.; Najfeld, V.; Hansen, J.A.; Penfold, G.K.; Jacobson, R.J.; Fialkow, P.J. Involvement of the B-lymphoid system in chronic myelogenous leukaemia. Nature 1980, 287, 49–50. [Google Scholar] [CrossRef]
- Mizuno, T.; Kyoizumi, S.; Suzuki, T.; Iwamoto, K.S.; Seyama, T. Continued expression of a tissue specific activated oncogene in the early steps of radiation-induced human thyroid carcinogenesis. Oncogene 1997, 15, 1455–1460. [Google Scholar] [CrossRef]
- Mizuno, T.; Iwamoto, K.S.; Kyoizumi, S.; Nagamura, H.; Shinohara, T.; Koyama, K.; Seyama, T.; Hamatani, K. Preferential induction of RET/PTC1 rearrangement by X-ray irradiation. Oncogene 2000, 19, 438–443. [Google Scholar] [CrossRef]
- Tanaka, K.; Takechi, M.; Hong, J.; Shigeta, C.; Oguma, N.; Kamada, N.; Takimoto, Y.; Kuramoto, A.; Dohy, H.; Kyo, T. 9-22 Translocation and Bcr Rearrangements in Chronic Myelocytic-Leukemia Patients among Atomic-Bomb Survivors. J. Radiat. Res. 1989, 30, 352–358. [Google Scholar] [CrossRef]
- Kosik, P.; Durdik, M.; Jakl, L.; Skorvaga, M.; Markova, E.; Vesela, G.; Vokalova, L.; Kolarikova, L.; Horvathova, E.; Kozics, K.; et al. DNA damage response and preleukemic fusion genes induced by ionizing radiation in umbilical cord blood hematopoietic stem cells. Sci. Rep. 2020, 10, 13722. [Google Scholar] [CrossRef] [PubMed]
- Kosik, P.; Durdik, M.; Skorvaga, M.; Klimova, D.; Kochanova, D.; Cerna, Z.; Kubes, M.; Holop, M.; Belyaev, I. Induction of AML Preleukemic Fusion Genes in HSPCs and DNA Damage Response in Preleukemic Fusion Gene Positive Samples. Antioxidants 2021, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, K.; Duque-Afonso, J.; Lubbert, M. AML1/ETO and its function as a regulator of gene transcription via epigenetic mechanisms. Oncogene 2021, 40, 5665–5676. [Google Scholar] [CrossRef]
- Deininger, M.W.N.; Bose, S.; Gara-Tybor, J.; Yan, X.H.; Goldman, J.M.; Melo, J.V. Selective induction of leukemia-associated fusion genes by high-dose ionizing radiation. Cancer Res. 1998, 58, 421–425. [Google Scholar] [PubMed]
- Britten, O.; Ragusa, D.; Tosi, S.; Kamel, Y.M. MLL-Rearranged Acute Leukemia with t(4;11)(q21;q23)-Current Treatment Options. Is There a Role for CAR-T Cell Therapy? Cells 2019, 8, 1341. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.; Schnittger, S.; Klaus, M.; Kern, W.; Hiddemann, W.; Haferlach, T. AML with 11q23/MLL abnormalities as defined by the WHO classification: Incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood 2003, 102, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Burmeister, T.; Groger, D.; Tsaur, G.; Fechina, L.; Renneville, A.; Sutton, R.; Venn, N.C.; Emerenciano, M.; Pombo-de-Oliveira, M.S.; et al. The MLL recombinome of acute leukemias in 2017. Leukemia 2018, 32, 273–284. [Google Scholar] [CrossRef]
- Meyer, C.; Lopes, B.A.; Caye-Eude, A.; Cave, H.; Arfeuille, C.; Cuccuini, W.; Sutton, R.; Venn, N.C.; Oh, S.H.; Tsaur, G.; et al. Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL-USP2 fusions. Leukemia 2019, 33, 2306–2310. [Google Scholar] [CrossRef]
- Stanulla, M.; Wang, J.; Chervinsky, D.S.; Aplan, P.D. Topoisomerase II inhibitors induce DNA double-strand breaks at a specific site within the AML1 locus. Leukemia 1997, 11, 490–496. [Google Scholar] [CrossRef]
- Betti, C.J.; Villalobos, M.J.; Diaz, M.O.; Vaughan, A.T.M. Apoptotic triggers initiate translocations within the MLL gene involving the nonhomologous end joining repair system. Cancer Res. 2001, 61, 4550–4555. [Google Scholar]
- Le, H.; Singh, S.; Shih, S.J.; Du, N.; Schnyder, S.; Loredo, G.A.; Bien, C.; Michaelis, L.; Toor, A.; Diaz, M.O.; et al. Rearrangements of the MLL gene are influenced by DNA secondary structure, potentially mediated by topoisomerase II binding. Genes Chromosomes Cancer 2009, 48, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, S.V.; Bink, K.; Trott, K.R.; Bebeshko, V.G.; Bazyka, D.A.; Dmytrenko, I.V.; Abramenko, I.V.; Bilous, N.I.; Zitzelsberger, H.; Misurin, A.V.; et al. MLL gene alterations in radiation-associated acute myeloid leukemia. Exp. Oncol. 2005, 27, 71–75. [Google Scholar] [PubMed]
- de The, H.; Chomienne, C.; Lanotte, M.; Degos, L.; Dejean, A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 1990, 347, 558–561. [Google Scholar] [CrossRef]
- Grisolano, J.L.; Wesselschmidt, R.L.; Pelicci, P.G.; Ley, T.J. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RAR alpha under control of cathepsin G regulatory sequences. Blood 1997, 89, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Quina, A.S.; Gameiro, P.; Sa da Costa, M.; Telhada, M.; Parreira, L. PML-RARA fusion transcripts in irradiated and normal hematopoietic cells. Genes Chromosomes Cancer 2000, 29, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M. Is the Primary Event in Radiation-Induced Chronic Myelogenous Leukemia the Induction of the T(9-22) Translocation. Leuk. Res. 1992, 16, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Kwon, D.; Doi, K.; Simon, S.L.; Preston, D.L.; Doody, M.M.; Lee, T.; Miller, J.S.; Kampa, D.M.; Bhatti, P.; et al. Association of chromosome translocation rate with low dose occupational radiation exposures in U.S. radiologic technologists. Radiat. Res. 2014, 182, 1–17. [Google Scholar] [CrossRef]
- Silva, A.; Anderson, A.R.; Gatenby, R. A multiscale model of the bone marrow and hematopoiesis. Math. Biosci. Eng. 2011, 8, 643–658. [Google Scholar]
- Prokopishyn, N.L.; Logan, B.R.; Kiefer, D.M.; Sees, J.A.; Chitphakdithai, P.; Ahmed, I.A.; Anderlini, P.N.; Beitinjaneh, A.M.; Bredeson, C.; Cerny, J.; et al. The Concentration of Total Nucleated Cells in Harvested Bone Marrow for Transplantation Has Decreased over Time. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2019, 25, 1325–1330. [Google Scholar] [CrossRef]
- Macedo, A.; Orfao, A.; Ciudad, J.; Gonzalez, M.; Vidriales, B.; Lopez-Berges, M.C.; Martinez, A.; Landolfi, C.; Canizo, C.; San Miguel, J.F. Phenotypic analysis of CD34 subpopulations in normal human bone marrow and its application for the detection of minimal residual disease. Leukemia 1995, 9, 1896–1901. [Google Scholar]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.; Hogge, D.E.; Sutherland, H.J. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(-)/HLA-DR-. Blood 1998, 92, 4325–4335. [Google Scholar] [CrossRef]
- Kong, Y.; Yoshida, S.; Saito, Y.; Doi, T.; Nagatoshi, Y.; Fukata, M.; Saito, N.; Yang, S.; Iwamoto, C.; Okamura, J.; et al. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia 2008, 22, 1207–1213. [Google Scholar] [CrossRef]
- Ishimaru, T.; Otake, M.; Ichimaru, M. Dose-Response Relationship of Neutrons and Gamma- Rays to Leukemia Incidence among Atomic-Bomb Survivors in Hiroshima and Nagasaki by Type of Leukemia, 1950-1971. Radiat. Res. 1979, 77, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Nesta, A.V.; Tafur, D.; Beck, C.R. Hotspots of Human Mutation. Trends Genet. 2021, 37, 717–729. [Google Scholar] [CrossRef]
- Wintrobe, M.M. Clinical Hematology, 8th ed.; Lea Febiger: Phila, PA, USA, 1981. [Google Scholar]
- Mori, H.; Colman, S.M.; Xiao, Z.J.; Ford, A.M.; Healy, L.E.; Donaldson, C.; Hows, J.M.; Navarrete, C.; Greaves, M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc. Natl. Acad. Sci. USA 2002, 99, 8242–8247. [Google Scholar] [CrossRef]
- Skorvaga, M.; Nikitina, E.; Kubes, M.; Kosik, P.; Gajdosechova, B.; Leitnerova, M.; Copakova, L.; Belyaev, I. Incidence of common preleukemic gene fusions in umbilical cord blood in Slovak population. PLoS ONE 2014, 9, e91116. [Google Scholar] [CrossRef]
- Brown, P. TEL-AML1 in cord blood: 1% or 0.01%? Blood 2011, 117, 2–4. [Google Scholar] [CrossRef]
- Ishihara, T.; Kumatori, T. Chromosome aberrations in human leukocytes irradiated in vivo and in vitro. Nihon Ketsueki Gakkai Zasshi 1965, 28, 291–307. [Google Scholar]
- Kumatori, T.; Ishihara, T.; Ueda, T.; Miyoshi, K. Medical survey of Japanese exposed to fall-out radiation in 1954: A report after 10 years. Chiba Natl. Inst. Radiol. Sci. Chiba 1965, 1–14. [Google Scholar]
- Kohno, S.I.; Ishihara, T. Radiation-induced aneusomic clones in bone marrow of rats. Mutat. Res. 1976, 35, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Kohno, S.; Minamihisamatsu, M. Radiation exposure and chromosome abnormalities. Human cytogenetic studies at the National Institute of Radiological Sciences, Japan, 1963–1988. Cancer Genet. Cytogenet. 1990, 45, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kang, J.K.; Lee, Y.H.; Yoon, H.J.; Yang, S.S.; Kim, S.H.; Jang, S.; Park, S.; Heo, D.H.; Jang, W.I.; et al. Chromosome aberration dynamics in breast cancer patients treated with radiotherapy: Implications for radiation biodosimetry. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 872, 503419. [Google Scholar] [CrossRef]
- Kozubek, S.; Lukasova, E.; Mareckova, A.; Skalnikova, M.; Kozubek, M.; Bartova, E.; Kroha, V.; Krahulcova, E.; Slotova, J. The topological organization of chromosomes 9 and 22 in cell nuclei has a determinative role in the induction of t(9,22) translocations and in the pathogenesis of t(9,22) leukemias. Chromosoma 1999, 108, 426–435. [Google Scholar] [CrossRef]
- Schafer, D.; Olsen, M.; Lahnemann, D.; Stanulla, M.; Slany, R.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood 2018, 131, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.; Hjalgrim, H.; Melbye, M.; Madsen, H.O.; Schmiegelow, K. RT-PCR screening for ETV6-RUNX1-positive clones in cord blood from newborns in the Danish National Birth Cohort. J. Pediatr. Hematol./Oncol. 2012, 34, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N. A hypothesis: Radiation-related leukemia is mainly attributable to the small number of people who carry pre-existing clonally expanded preleukemic cells. Radiat. Res. 2005, 163, 258–265. [Google Scholar] [CrossRef]
- Nikitina, V.; Nugis, V.; Astrelina, T.; Zheglo, D.; Kobzeva, I.; Kozlova, M.; Galstyan, I.; Lomonosova, E.; Zhanataev, A.; Karaseva, T.; et al. Pattern of chromosomal aberrations persisting over 30 years in a Chernobyl Nuclear Power Plant accident survivor: Study using mFISH. J. Radiat. Res. 2022, 63, 202–212. [Google Scholar] [CrossRef]
- Nakano, M.; Kodama, Y.; Ohtaki, K.; Itoh, M.; Awa, A.A.; Cologne, J.; Kusunoki, Y.; Nakamura, N. Estimating the number of hematopoietic or lymphoid stem cells giving rise to clonal chromosome aberrations in blood T lymphocytes. Radiat. Res. 2004, 161, 273–281. [Google Scholar] [CrossRef]
- George, K.; Durante, M.; Willingham, V.; Cucinotta, F.A. Chromosome aberrations of clonal origin are present in astronauts’ blood lymphocytes. Cytogenet. Genome Res. 2004, 104, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Namura, K.; Uchida, R.; Fuchida, S.; Okano, A.; Okamoto, M.; Shimazaki, C. The unbalanced chromosomal translocation der(15)t(1;15)(q21;p13) in multiple myeloma. Int. J. Hematol. 2005, 81, 437–438. [Google Scholar] [CrossRef] [PubMed]
| 0 Gy | 10 Gy | 50 Gy | 100 Gy | |
|---|---|---|---|---|
| BCR-ABL1 in all cell lines | 0% | 5% | 14.2% | 41.9% |
| 8505C | 0% | 0% | 50% | 20% |
| Daudi | 0% | 0% | 0% | 30% |
| G-401 | 0% | 10% | 10% | 27.3% |
| HT1080 | 0% | 10% | 20% | 60% |
| RUNX1-RUNX1T1 | PML-RARA | KMT2A-MLLT3 | |
|---|---|---|---|
| Po1-C | 0/6 | ND | 0/6 |
| Po1-I | 0/6 | 0/3 | 0/6 |
| Po2-C | 0/9 | 0/6 | 0/9 |
| Po2-I | 0/9 | 0/6 | 0/9 |
| Po3-C | 1/9 | 0/9 | 0/9 |
| Po3-I | 0/9 | 0/9 | 0/9 |
| Po5-C | 0/12 | 0/9 | 0/12 |
| Po5-I | 0/12 | 0/9 | 0/12 |
| Po6-C | 0/9 | 0/12 | 0/12 |
| Po6-I | 0/9 | 0/12 | 0/12 |
| Po7-C | 0/12 | 0/12 | 0/12 |
| Po7-I | 0/12 | 0/12 | 0/12 |
| Po8-C | 0/9 | 0/12 | 0/9 |
| Po8-I | 3/12 | 0/12 | 0/12 |
| Study | Type of Radiation/Dose | Cells (Cell Lines) | PFGs (Preleukemic Fusion Genes) | Results |
|---|---|---|---|---|
| Ito, Seyama et al., 1993 [86] | X-rays 100 Gy | HL-60 | BCR-ABL1 | Induction |
| Mizuno, Kyoizumi et al., 1997 [90] | X-rays 50 Gy | Human thyroid tissues in mice | BCR-ABL1 H4-RET (thyroid papillary carcinoma) | Induction for BCR-ABL1 (at 2 days only), induction for H4-RET |
| Deininger, Bose et al., 1998 [96] | γ-radiation 50, 100 Gy | HL-60 KG1 | BCR-ABL1 RUNX1-RUNX1T1 DEK-NUP214 | No induction for BCR-ABL and DEK-NUP214, induction for RUNX1-RUNX1T1 (only in KG1) |
| Spencer and Granter 1999 [87] | γ-radiation 50, 100 Gy | AML–LCL CML–LCL Non–LCL | BCR-ABL1 | Induction (only in AML and CML derived LCL) |
| Mizuno, Iwamoto et al., 2000 [91] | X-rays 10, 50, 100 Gy | 8505C Daudi G-401 HT1080 | BCR-ABL1 | Induction (linear dose response) |
| Quina, Gameiro et al., 2000 [107] | γ-radiation 10 Gy | IM9 | PML-RARA | No induction |
| Kosik, Durdik et al., 2020 [93] | γ-radiation 0.1, 0.5, 2, 5, 10 and 30 Gy | UCB–MNCs | BCR-ABL1 ETV6-RUNX1 RUNX1-RUNX1T1 KMT2A-AFF1 | No induction for all PFGs except for BCR-ABL1 at doses ≤ 0.5 Gy |
| Kosik, Durdik et al., 2021 [94] | γ-radiation 0.5 Gy | UCB–HSPCs | RUNX1-RUNX1T1 KMT2A-MLLT3 PML-RARA | No induction for all PFGs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosik, P.; Skorvaga, M.; Belyaev, I. Preleukemic Fusion Genes Induced via Ionizing Radiation. Int. J. Mol. Sci. 2023, 24, 6580. https://doi.org/10.3390/ijms24076580
Kosik P, Skorvaga M, Belyaev I. Preleukemic Fusion Genes Induced via Ionizing Radiation. International Journal of Molecular Sciences. 2023; 24(7):6580. https://doi.org/10.3390/ijms24076580
Chicago/Turabian StyleKosik, Pavol, Milan Skorvaga, and Igor Belyaev. 2023. "Preleukemic Fusion Genes Induced via Ionizing Radiation" International Journal of Molecular Sciences 24, no. 7: 6580. https://doi.org/10.3390/ijms24076580
APA StyleKosik, P., Skorvaga, M., & Belyaev, I. (2023). Preleukemic Fusion Genes Induced via Ionizing Radiation. International Journal of Molecular Sciences, 24(7), 6580. https://doi.org/10.3390/ijms24076580







