COVID-19 and Diarylamidines: The Parasitic Connection
Abstract
:1. Introduction
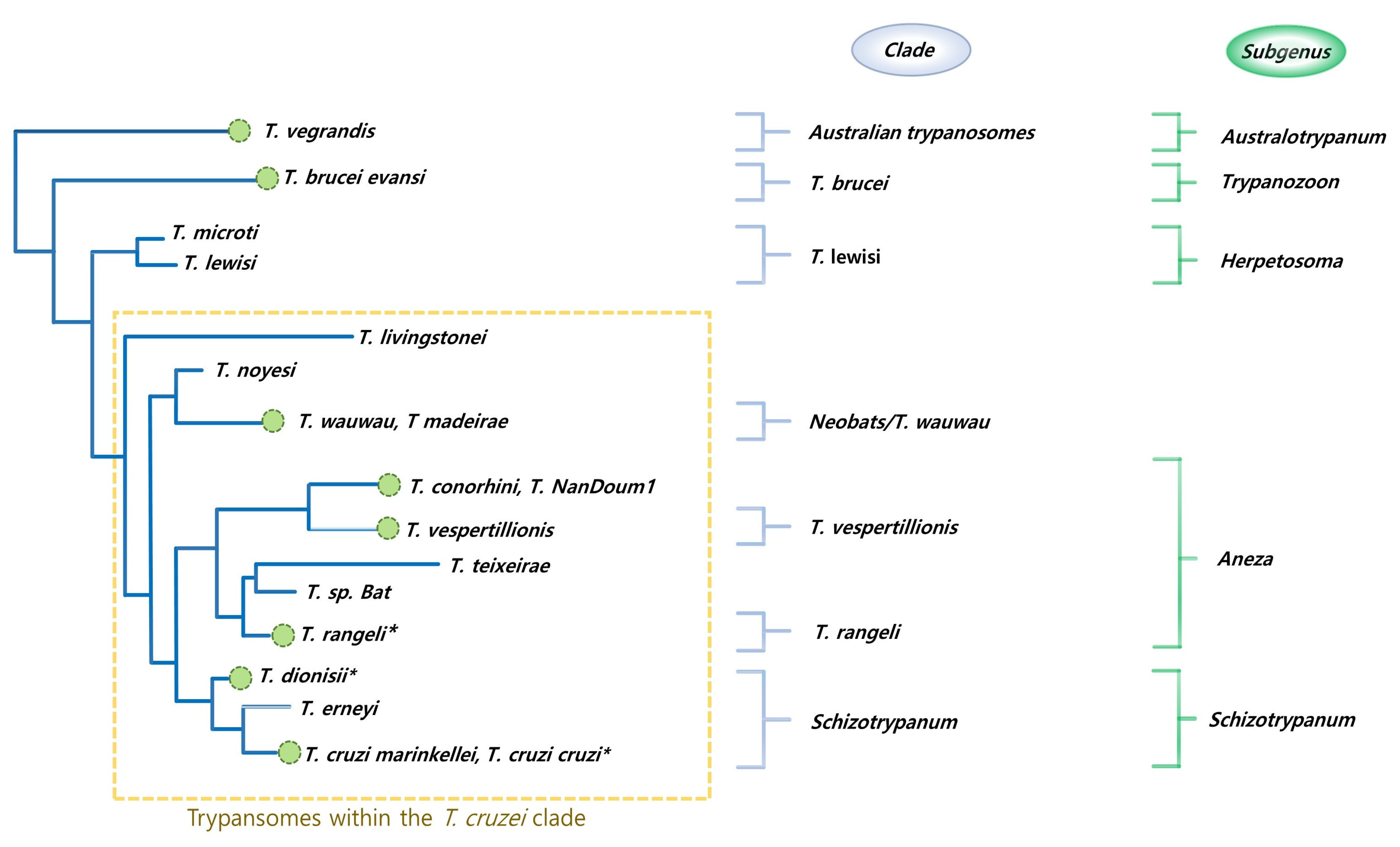
2. Bats, Pangolins, Parasites, and Diarylamidines: Making the Connection
2.1. Revisiting the Renin-Angiotensin System (RAS)
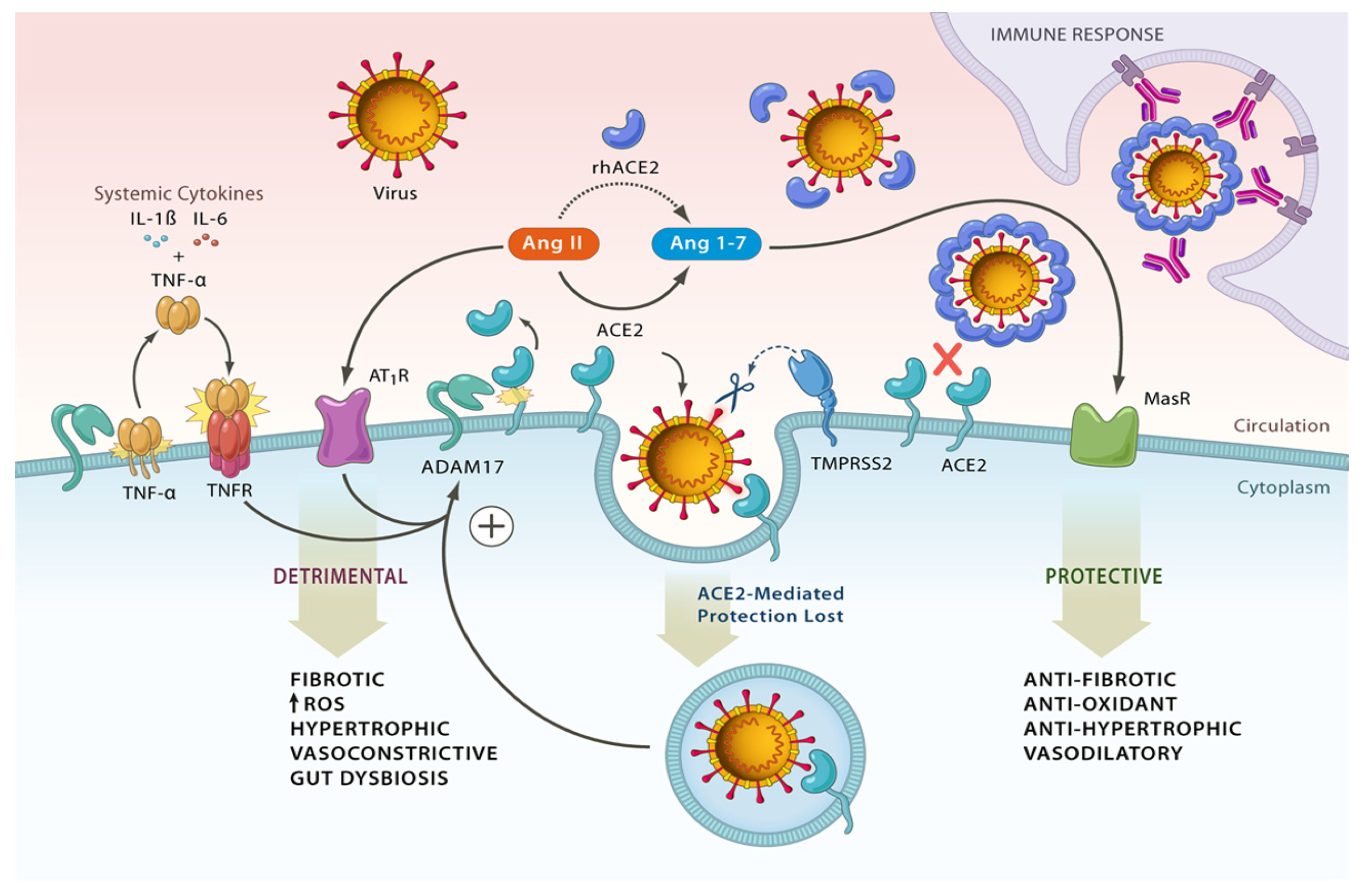
2.2. SARS-CoV-2, ACE2, TMPRSS2, and Shredases
2.3. ACE2 Potentiation and SARS-CoV-2
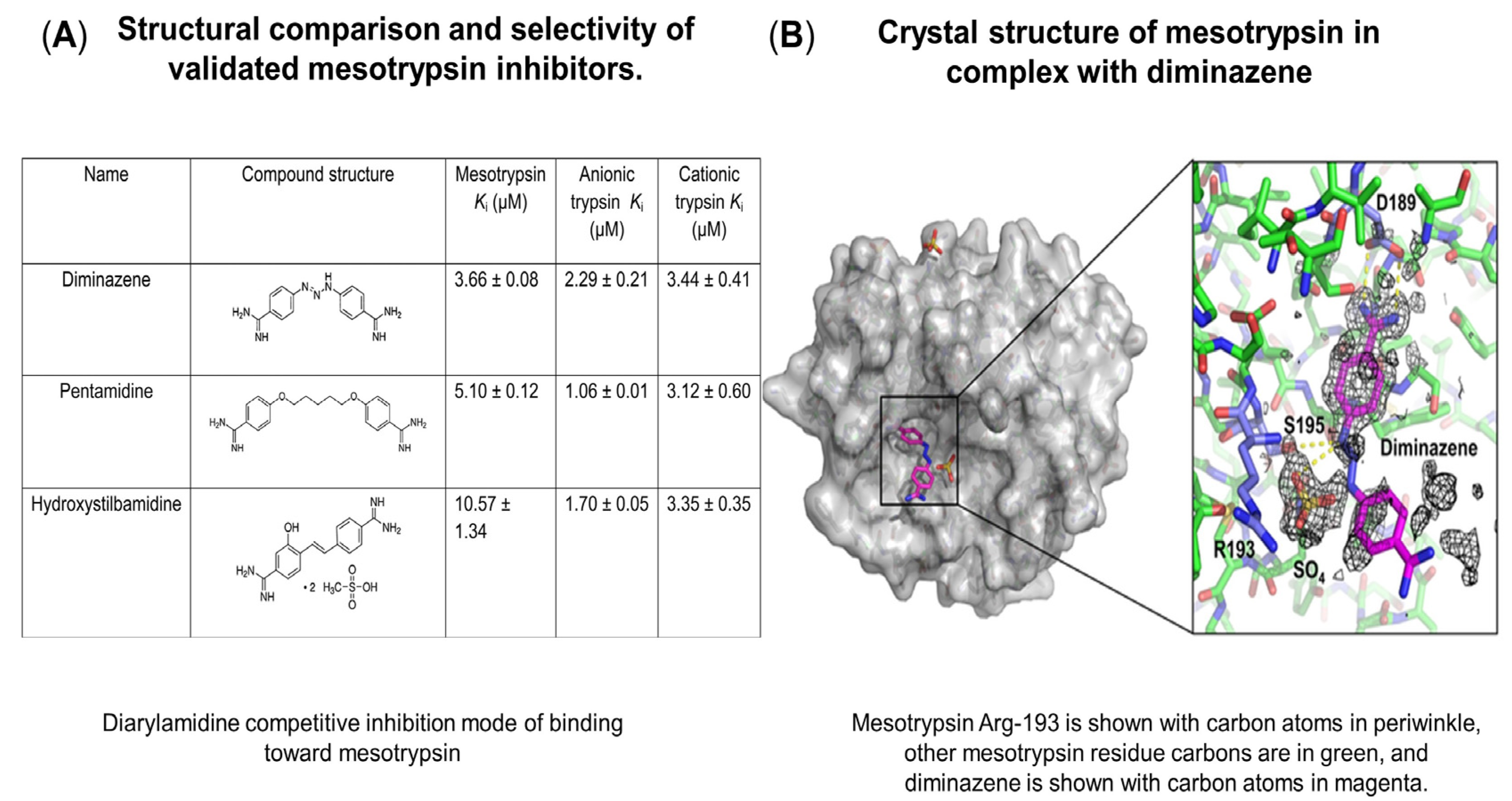
3. Diarylamidines, Serine Proteases
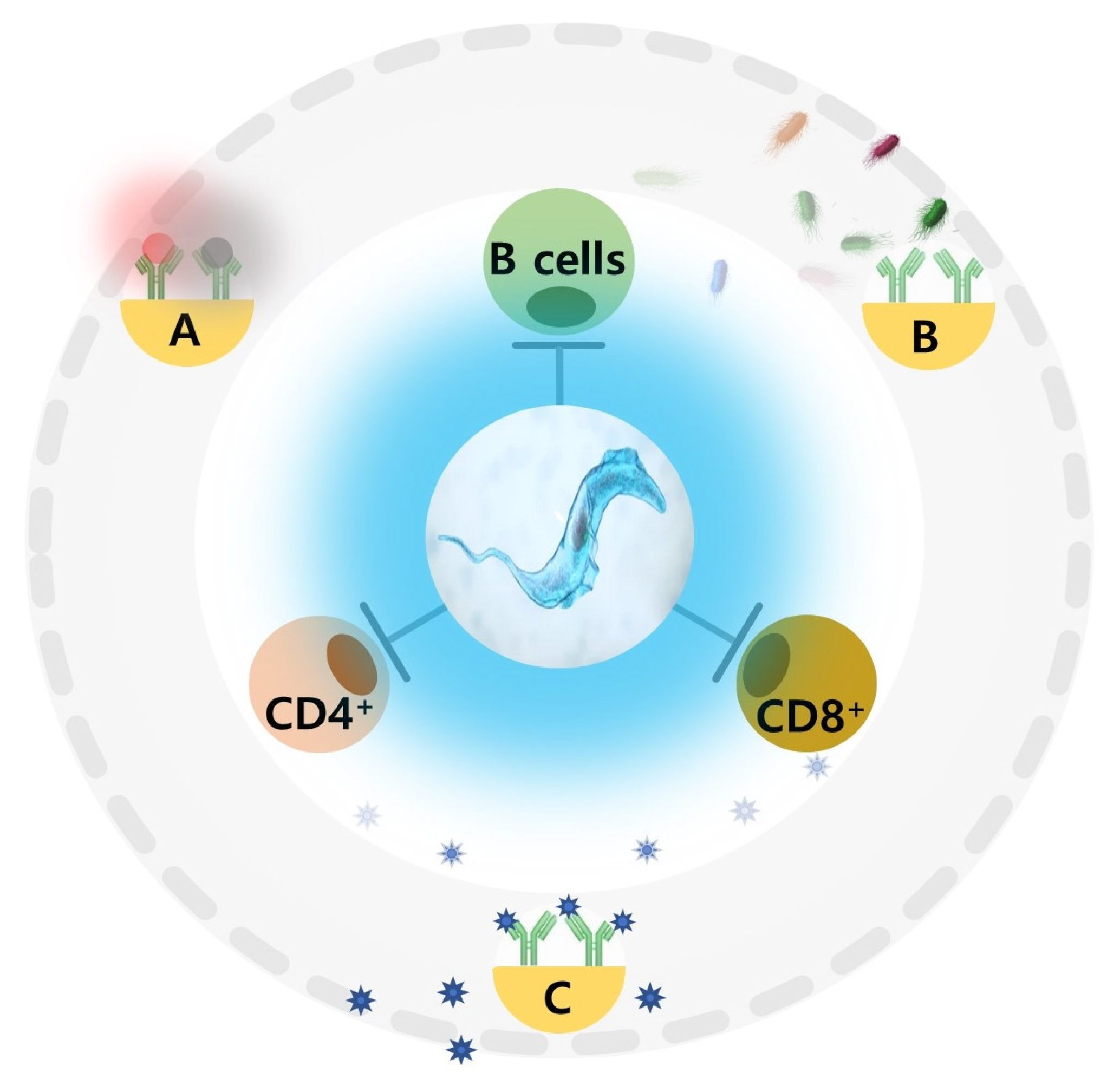
3.1. Immunosuppression, Vaccinations, and Epigenetic Potential
3.2. Long COVID and DIZE
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Vázquez, L.D.; Arenas, M. Molecular Evolution of SARS-CoV-2 during the COVID-19 Pandemic. Genes 2023, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, X.; Wei, C.; Li, S.; Zhao, J.; Zheng, Y.; Liu, X.; Zeng, X.; Yuan, W.; Peng, S. Molecular Evolutionary Characteristics of SARS-CoV-2 Emerging in the United States. J. Med. Virol. 2022, 94, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Peng, L.; Tsang, T.K.; Cowling, B.J. Epidemiology of SARS-CoV-2 Omicron BA.5 Infections, Macau, June-July 2022. Emerg. Infect. Dis. 2023, 29, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Bhiman, J.N.; Richardson, S.I.; Lambson, B.E.; Kgagudi, P.; Mzindle, N.; Kaldine, H.; Crowther, C.; Gray, G.; Bekker, L.-G.; Koen, A.; et al. Novavax NVX-COV2373 Triggers Neutralization of Omicron Sub-Lineages. Sci. Rep. 2023, 13, 1222. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Quiroga, R.; McConnell, S.; Johnson, M.C.; Casadevall, A. Convergent Evolution in SARS-CoV-2 Spike Creates a Variant Soup from Which New COVID-19 Waves Emerge. Int. J. Mol. Sci. 2023, 24, 2264. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Lin, R.-L.; Maskey, A.P.; Pandey, S.; Lin, A.-H.; Lee, L.-Y. A Distinct Difference Between Air and Mucosal Temperatures in Human Respiratory Tract. Front. Med. 2021, 8, 650637. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ng, K.-C.; Ho, J.C.W.; Yeung, H.-W.; Ching, R.H.H.; Gu, H.; Chung, J.C.K.; Chow, V.L.Y.; Sit, K.-Y.; Hsin, M.K.Y.; et al. Replication of SARS-CoV-2 Omicron BA.2 Variant in Ex Vivo Cultures of the Human Upper and Lower Respiratory Tract. eBioMedicine 2022, 83, 104232. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, J.; Guo, J.; Hao, C.; Zheng, M.; Zhang, R.; Huang, Q.; Yao, X.; Li, R.; Jin, Y. Reinfection in Patients with COVID-19: A Systematic Review. Glob. Health Res. Policy 2022, 7, 12. [Google Scholar] [CrossRef]
- Tiwari, R.; Dhama, K.; Sharun, K.; Iqbal Yatoo, M.; Malik, Y.S.; Singh, R.; Michalak, I.; Sah, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. COVID-19: Animals, Veterinary and Zoonotic Links. Vet. Q. 2020, 40, 169–182. [Google Scholar] [CrossRef]
- Goldberg, A.R.; Langwig, K.E.; Marano, J.; Sharp, A.K.; Brown, K.L.; Ceci, A.; Kailing, M.J.; Briggs, R.; Roby, C.; Brown, A.M.; et al. Wildlife Exposure to SARS-CoV-2 across a Human Use Gradient: Wildlife Exposure to SARS-CoV-2. bioRxiv, 2022; preprint. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The Furin Cleavage Site in the SARS-CoV-2 Spike Protein Is Required for Transmission in Ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Schussman, M.K.; McLellan, S.L. Effect of Time and Temperature on SARS-CoV-2 in Municipal Wastewater Conveyance Systems. Water 2022, 14, 1373. [Google Scholar] [CrossRef]
- Gao, S.; Guo, H.; Luo, G. Omicron Variant (B.1.1.529) of SARS-CoV-2, a Global Urgent Public Health Alert! J. Med. Virol. 2022, 94, 1255–1256. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Where Did Omicron Come from? Three Key Theories. Nature 2022, 602, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Chavarria-Miró, G.; Anfruns-Estrada, E.; Guix, S.; Paraira, M.; Galofré, B.; Sánchez, G.; Pintó, R.M.; Bosch, A. Sentinel Surveillance of SARS-CoV-2 in Wastewater Anticipates the Occurrence of COVID-19 Cases. medRxiv 2020. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Chen, D. Human Coronaviruses: Origin, Host and Receptor. J. Clin. Virol. 2022, 155, 105246. [Google Scholar] [CrossRef]
- Van Vo, G.; Bagyinszky, E.; Park, Y.S.; Hulme, J.; An, S.S.A. SARS-CoV-2 (COVID-19): Beginning to Understand a New Virus. Adv. Exp. Med. Biol. 2021, 1321, 3–19. [Google Scholar] [CrossRef]
- Luis, A.D.; Hayman, D.T.S.; O’Shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.C.; Mills, J.N.; Timonin, M.E.; Willis, C.K.R.; Cunningham, A.A.; et al. A Comparison of Bats and Rodents as Reservoirs of Zoonotic Viruses: Are Bats Special? Proc. R. Soc. B Biol. Sci. 2013, 280, 20122753. [Google Scholar] [CrossRef] [Green Version]
- Szentiványi, T.; Christe, P.; Glaizot, O. Bat Flies and Their Microparasites: Current Knowledge and Distribution. Front. Vet. Sci. 2019, 6, 115. [Google Scholar] [CrossRef]
- Njiokou, F.; Simo, G.; Nkinin, S.W.; Laveissière, C.; Herder, S. Infection Rate of Trypanosoma Brucei s.l., T. Vivax, T. Congolense “Forest Type”, and T. Simiae in Small Wild Vertebrates in South Cameroon. Acta Trop. 2004, 92, 139–146. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Y.; Chen, X.; Shi, M.; Fu, S.; Yang, W.; Liu, W.J.; Gao, G.F.; Liang, G. Virome of Bat-Infesting Arthropods: Highly Divergent Viruses in Different Vectors. J. Virol. 2022, 96, 4. [Google Scholar] [CrossRef] [PubMed]
- Austen, J.M.; Barbosa, A.D. Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective. Pathogens 2021, 10, 1148. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, X.; Zhang, N.; Li, J.; Gong, P.; He, B.; Zhang, X. First Report of the Prevalence and Genotype of Trypanosoma spp. in Bats in Yunnan Province, Southwestern China. Acta Trop. 2019, 198, 105105. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Teixeira, M.M.G.; Stevens, J.R. The Evolution of Trypanosoma Cruzi: The “bat Seeding” Hypothesis. Trends Parasitol. 2012, 28, 136–141. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Hernández, C.; Montilla, M.; Zambrano, P.; Flórez, A.C.; Parra, E.; Cucunubá, Z.M. First Report of Human Trypanosoma Cruzi Infection Attributed to TcBat Genotype. Zoonoses Public Health 2014, 61, 477–479. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Interim Guidelines for the Treatment of Gambiense Human African Trypanosomiasis; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-155056-7. [Google Scholar]
- Yodsheewan, R.; Sukmak, M.; Sangkharak, B.; Kaolim, N.; Ploypan, R.; Phongphaew, W. First Report on Detection of Babesia spp. in Confiscated Sunda Pangolins (Manis javanica) in Thailand. Veter World 2021, 14, 2380–2385. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Latinne, A.; Thuy, H.B.; Long, N.V.; Ngoc, P.T.B.; Anh, N.T.L.; Thai, N.V.; Phuong, T.Q.; Thai, H.V.; Hai, L.K.; et al. Evidence of SARS-CoV-2 Related Coronaviruses Circulating in Sunda Pangolins (Manis javanica) Confiscated From the Illegal Wildlife Trade in Viet Nam. Front. Public Health 2022, 10, 826116. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Q.; Xue, J.; Chen, S.; Huang, D.; Yu, Y.; Cai, Y.; Lu, Y.; Song, P.; Zhang, R.; et al. Spreading of Human Babesiosis in China: Current Epidemiological Status and Future Challenges. China CDC Wkly. 2020, 2, 634–637. [Google Scholar] [CrossRef]
- Puri, A.; Bajpai, S.; Meredith, S.; Aravind, L.; Krause, P.J.; Kumar, S. Babesia Microti: Pathogen Genomics, Genetic Variability, Immunodominant Antigens, and Pathogenesis. Front. Microbiol. 2021, 12, 697669. [Google Scholar] [CrossRef]
- Jacobs, J.W.; Siddon, A.J. Concurrent COVID-19 and Babesiosis in an Older, Splenectomized Patient. Blood 2021, 138, 2154. [Google Scholar] [CrossRef]
- Alberca, R.W.; Yendo, T.M.; Leuzzi Ramos, Y.Á.; Fernandes, I.G.; Oliveira, L.d.M.; Teixeira, F.M.E.; Beserra, D.R.; de Oliveira, E.A.; Gozzi-Silva, S.C.; Andrade, M.M.d.S.; et al. Case Report: COVID-19 and Chagas Disease in Two Coinfected Patients. Am. J. Trop. Med. Hyg. 2020, 103, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Bhanot, P. Babesia Microti—Borrelia Burgdorferi Coinfection. Pathogens 2019, 8, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akoolo, L.; Rocha, S.C.; Parveen, N. Protozoan Co-Infections and Parasite Influence on the Efficacy of Vaccines against Bacterial and Viral Pathogens. Front. Microbiol. 2022, 13, 1020029. [Google Scholar] [CrossRef]
- Anyanwu, M.U. The Association between Malaria Prevalence and COVID-19 Mortality. BMC Infect. Dis. 2021, 21, 975. [Google Scholar] [CrossRef]
- Osei, S.A.; Biney, R.P.; Anning, A.S.; Nortey, L.N.; Ghartey-Kwansah, G. Low Incidence of COVID-19 Case Severity and Mortality in Africa; Could Malaria Co-Infection Provide the Missing Link? BMC Infect. Dis. 2022, 22, 78. [Google Scholar] [CrossRef]
- Kuriakose, S.M.; Onyilagha, C.; Singh, R.; Jia, P.; Uzonna, J.E. Diminazene Aceturate (Berenil) Downregulates Trypanosoma Congolense-Induced Proinflammatory Cytokine Production by Altering Phosphorylation of MAPK and STAT Proteins. Immunol. Res. 2018, 67, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Koning, P.D.H. The Drugs of Sleeping Sickness: Their Mechanisms of Action and Resistance, and a Brief History. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- van Genderen, P.J.J.; Nouwen, J.L.; De Mendonça Melo, M.; Rijnders, B.J.A.; van Hellemond, J.J. Single-Dose Pentamidine Substantially Reduces Viability of Trypanosomes in Human East African Trypanosomiasis. J. Travel Med. 2021, 28, taab080. [Google Scholar] [CrossRef]
- Baneth, G. Antiprotozoal Treatment of Canine Babesiosis. Vet. Parasitol. 2018, 254, 58–63. [Google Scholar] [CrossRef]
- Millan, C.R.; Acosta-Reyes, F.J.; Lagartera, L.; Ebiloma, G.U.; Lemgruber, L.; Nué Martínez, J.J.; Saperas, N.; Dardonville, C.; de Koning, H.P.; Campos, J.L. Functional and Structural Analysis of AT-Specific Minor Groove Binders That Disrupt DNA–Protein Interactions and Cause Disintegration of the Trypanosoma Brucei Kinetoplast. Nucleic Acids Res. 2017, 45, 8378–8391. [Google Scholar] [CrossRef] [Green Version]
- Kuriakose, S.; Muleme, H.M.; Onyilagha, C.; Singh, R.; Jia, P.; Uzonna, J.E. Diminazene Aceturate (Berenil) Modulates the Host Cellular and Inflammatory Responses to Trypanosoma Congolense Infection. PLoS ONE 2012, 7, e48696. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Park, G.-Y.; Kim, S.-H.; Hulme, J.; An, S.S.A. Diminazene Aceturate: An Antibacterial Agent for Shiga-Toxin-Producing Escherichia Coli O157:H7. Drug Des. Dev. Ther. 2016, 10, 3363–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios, T.B.; Silva, O.N.; de Souza, C.M.; Fensterseifer, I.C.M.; Mehta, A.; Franco, O.L. Repurposing Streptomycin and Chloramphenicol against Bacterial Pathogens by Combination with Diminazene Aceturate. Lett. Appl. Microbiol. 2023, 76, ovac009. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.F.; Ezeh, I.O.; Okpala, M.I.; Idika, I.K.; Mbe, N.; Nwobi, L.G.; Ezeokonkwo, R.C. Azithromycin and Diminazene Aceturate Combination Therapy in Experimental Multidrug-Resistant Trypanosoma Brucei Brucei Infection in Albino Rats. Vet. Parasitol. 2020, 282, 109138. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.; Joshua, M.; John, C.; Alain, J.; Michael, M. In Vitro Activity and in Vivo Efficacy of a Combination Therapy of Diminazene and Chloroquine against Murine Visceral Leishmaniasis. J. Biomed. Res. 2015, 29, 214–223. [Google Scholar] [CrossRef]
- Stokes, J.M.; Macnair, C.R.; Ilyas, B.; French, S.; Côté, J.P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine Sensitizes Gram-Negative Pathogens to Antibiotics and Overcomes Acquired Colistin Resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef] [Green Version]
- Macnair, C.R.; Farha, M.A.; Serrano-Wu, M.H.; Lee, K.K.; Hubbard, B.; Côté, J.P.; Carfrae, L.A.; Tu, M.M.; Gaulin, J.L.; Hunt, D.K.; et al. Preclinical Development of Pentamidine Analogs Identifies a Potent and Nontoxic Antibiotic Adjuvant. ACS Infect. Dis. 2022, 8, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Zhang, B.; Jin, Y.; Zhang, L. Pentamidine Ninety Years on: The Development and Applications of Pentamidine and Its Analogs. Curr. Med. Chem. 2022, 29, 4602–4609. [Google Scholar] [CrossRef]
- Kuriakose, S.; Muleme, H.; Onyilagha, C.; Okeke, E.; Uzonna, J.E. Diminazene Aceturate (Berenil) Modulates LPS Induced pro-Inflammatory Cytokine Production by Inhibiting Phosphorylation of MAPKs and STAT Proteins. Innate Immun. 2013, 20, 760–773. [Google Scholar] [CrossRef]
- Ge, P.; Yao, X.; Li, J.; Jiang, R.; Dai, J.; Zhang, L. Diminazene Aceturate Alleviated Lipopolysaccharide/D-Galactosamine-Induced Fulminant Hepatitis in Mice. Biomed. Pharmacother. 2018, 98, 142–148. [Google Scholar] [CrossRef]
- Lund, N.C.; Kayode, Y.; McReynolds, M.R.; Clemmer, D.C.; Hudson, H.; Clerc, I.; Hong, H.-K.; Brenchley, J.M.; Bass, J.; D’Aquila, R.T.; et al. MTOR Regulation of Metabolism Limits LPS-Induced Monocyte Inflammatory and Procoagulant Responses. Commun. Biol. 2022, 5, 878. [Google Scholar] [CrossRef]
- Chaiwut, R.; Kasinrerk, W. Very Low Concentration of Lipopolysaccharide Can Induce the Production of Various Cytokines and Chemokines in Human Primary Monocytes. BMC Res. Notes 2022, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Fajtová, P.; Štefanić, S.; Hradilek, M.; Dvořák, J.; Vondrášek, J.; Jílková, A.; Ulrychová, L.; McKerrow, J.H.; Caffrey, C.R.; Mareš, M.; et al. Prolyl Oligopeptidase from the Blood Fluke Schistosoma Mansoni: From Functional Analysis to Anti-Schistosomal Inhibitors. PLoS Negl. Trop. Dis. 2015, 9, e0003827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, R.; Xue, X.; Zhang, Q.-Q.; Wang, S.-Y.; Gong, P.-Y.; Yan, E.; Jiang, T.; Zhang, Y.-D. ACE2 Activator Diminazene Aceturate Ameliorates Alzheimer’s Disease-like Neuropathology and Rescues Cognitive Impairment in SAMP8 Mice. Aging 2020, 12, 14819–14829. [Google Scholar] [CrossRef]
- Velkoska, E.; Patel, S.K.; Griggs, K.; Burrell, L.M. Diminazene Aceturate Improves Cardiac Fibrosis and Diastolic Dysfunction in Rats with Kidney Disease. PLoS ONE 2016, 11, e0161760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Shenoy, V.; Zhang, J.; Katovich, M.; Raizada, M. Small Molecule ACE2 Activator, Diminazene Aceturate (DIZE) Attenuates MI-Induced Cardiac Pathophysiology. FASEB J. 2013, 27, lb682. [Google Scholar] [CrossRef]
- Stachowicz, A.; Wiśniewska, A.; Kuś, K.; Białas, M.; Łomnicka, M.; Totoń-Żurańska, J.; Kiepura, A.; Stachyra, K.; Suski, M.; Bujak-Giżycka, B.; et al. Diminazene Aceturate Stabilizes Atherosclerotic Plaque and Attenuates Hepatic Steatosis in Apoe-Knockout Mice by Influencing Macrophages Polarization and Taurine Biosynthesis. Int. J. Mol. Sci. 2021, 22, 5861. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.; Gjymishka, A.; Jarajapu, Y.P.; Qi, Y.; Afzal, A.; Rigatto, K.; Ferreira, A.J.; Fraga-Silva, R.A.; Kearns, P.; Douglas, J.Y.; et al. Diminazene Attenuates Pulmonary Hypertension and Improves Angiogenic Progenitor Cell Functions in Experimental Models. Am. J. Respir. Crit. Care Med. 2013, 187, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Rajapaksha, I.G.; Mak, K.Y.; Huang, P.; Burrell, L.M.; Angus, P.W.; Herath, C.B. The Small Molecule Drug Diminazene Aceturate Inhibits Liver Injury and Biliary Fibrosis in Mice. Sci. Rep. 2018, 8, 10175. [Google Scholar] [CrossRef] [Green Version]
- Goru, S.K.; Kadakol, A.; Malek, V.; Pandey, A.; Sharma, N.; Gaikwad, A.B. Diminazene Aceturate Prevents Nephropathy by Increasing Glomerular ACE2 and AT2 Receptor Expression in a Rat Model of Type1 Diabetes. Br. J. Pharmacol. 2017, 174, 3118–3130. [Google Scholar] [CrossRef] [Green Version]
- Gasperetti, T.; Prasad Sharma, G.; Frei, A.C.; Pierce, L.; Veley, D.; Szalewski, N.; Narayanan, J.; Fish, B.L.; Himburg, H.A. Mitigation of Multi-Organ Radiation Injury with ACE2 Agonist Diminazene Aceturate. Radiat. Res. 2022, 198, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Joviano-Santos, J.V.; Santos-Miranda, A.; Joca, H.C.; Cruz, J.S.; Ferreira, A.J. Diminazene Aceturate (DIZE) Has Cellular and in Vivo Antiarrhythmic Effects. Clin. Exp. Pharmacol. Physiol. 2020, 47, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.M.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; Kelaidonis, K.; Ligielli, I.; Moschovou, K.; Georgiou, N.; Plotas, P.; Chasapis, C.T.; et al. Diminazene Aceturate Reduces Angiotensin II Constriction and Interacts with the Spike Protein of Severe Acute Respiratory Syndrome Coronavirus 2. Biomedicines 2022, 10, 1731. [Google Scholar] [CrossRef]
- Royea, J.; Martinot, P.; Hamel, E. Memory and Cerebrovascular Deficits Recovered Following Angiotensin IV Intervention in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2020, 134, 104644. [Google Scholar] [CrossRef] [PubMed]
- Dhande, I.; Ma, W.; Hussain, T. Angiotensin AT2 Receptor Stimulation Is Anti-Inflammatory in Lipopolysaccharide-Activated THP-1 Macrophages via Increased Interleukin-10 Production. Hypertens. Res. 2014, 38, 21–29. [Google Scholar] [CrossRef]
- Tao, X.; Fan, J.; Kao, G.; Zhang, X.; Su, L.; Yin, Y.; Zrenner, B. Angiotensin-(1-7) Attenuates Angiotensin II-Induced Signalling Associated with Activation of a Tyrosine Phosphatase in Sprague-Dawley Rats Cardiac Fibroblasts. Biol. Cell 2014, 106, 182–192. [Google Scholar] [CrossRef]
- Shah, A.; Gul, R.; Yuan, K.; Gao, S.; Oh, Y.-B.; Kim, U.-H.; Kim, S.H. Angiotensin-(1–7) Stimulates High Atrial Pacing-Induced ANP Secretion via Mas/PI3-Kinase/Akt Axis and Na+/H+ Exchanger. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1365–H1374. [Google Scholar] [CrossRef]
- Mikusic, N.L.R.; Pineda, A.M.; Gironacci, M.M. Angiotensin-(1-7) and Mas Receptor in the Brain. Explor. Med. 2021, 2, 268–293. [Google Scholar] [CrossRef]
- Zhang, L.; Zetter, M.A.; Guerra, E.C.; Hernández, V.S.; Mahata, S.K.; Eiden, L.E. ACE2 in the Second Act of COVID-19 Syndrome: Peptide Dysregulation and Possible Correction with Oestrogen. J. Neuroendocrinol. 2021, 33, e12935. [Google Scholar] [CrossRef]
- Michard, F.; Vieillard-Baron, A. Critically Ill Patients with COVID-19: Are They Hemodynamically Unstable and Do We Know Why? Intensiv. Care Med. 2020, 47, 254–255. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-Regulatory Renin-Angiotensin System in Cardiovascular Disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, L.; Wu, J.; Yu, Y.; Liu, S.; Li, T.; Li, Q.; Ding, R.; Wang, H.; Nie, J.; et al. A Second Functional Furin Site in the SARS-CoV-2 Spike Protein. Emerg. Microbes Infect. 2022, 11, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Azouz, N.P.; Klingler, A.; Callahan, V.; Akhrymuk, I.; Elez, K.; Raich, L.; Henry, B.; Benoit, J.; Benoit, S.; Noé, F.; et al. Alpha 1 Antitrypsin Is an Inhibitor of the SARS-CoV-2–Priming Protease TMPRSS2. Pathog. Immun. 2021, 6, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Mercadante, M.; Nilsson-Payant, B.; Johnson, J.L.; Jaimes, J.A.; Muecksch, F.; Weisblum, Y.; Bram, Y.; Chandar, V.; Whittaker, G.R.; et al. Coagulation Factors Directly Cleave SARS-CoV-2 Spike and Enhance Viral Entry. eLife 2022, 11, e77444. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Nägele, F.; Graber, M.; Hirsch, J.; Pölzl, L.; Sahanic, S.; Fiegl, M.; Hau, D.; Engler, C.; Lechner, S.; Stalder, A.K.; et al. Correlation between Structural Heart Disease and Cardiac SARS-CoV-2 Manifestations. Commun. Med. 2022, 2, 142. [Google Scholar] [CrossRef]
- Bastolla, U. Mathematical Model of SARS-CoV-2 Propagation Versus ACE2 Fits COVID-19 Lethality Across Age and Sex and Predicts That of SARS. Front. Mol. Biosci. 2021, 8, 706122. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Cheng, D.; Wang, Q.; Pei, Z.; Zhu, N.; Fang, W.; Yu, Q. Potential Role of a Disintegrin and Metalloproteinase-17 (ADAM17) in Age-Associated Ventricular Remodeling of Rats. RSC Adv. 2019, 9, 14321–14330. [Google Scholar] [CrossRef] [Green Version]
- Schuler, B.A.; Habermann, A.C.; Plosa, E.J.; Taylor, C.J.; Jetter, C.; Negretti, N.M.; Kapp, M.E.; Benjamin, J.T.; Gulleman, P.; Nichols, D.S.; et al. Age-Determined Expression of Priming Protease TMPRSS2 and Localization of SARS-CoV-2 in Lung Epithelium. J. Clin. Investig. 2021, 131, e140766. [Google Scholar] [CrossRef]
- Keller, C.; Böttcher-Friebertshäuser, E.; Lohoff, M. TMPRSS2, a Novel Host-Directed Drug Target against SARS-CoV-2. Signal Transduct. Target. Ther. 2022, 7, 1–3. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.; Lidsky, P.V.; Wu, C.-T.; Bonser, L.R.; Peng, S.; Garcia-Knight, M.A.; Tassetto, M.; Chung, C.-I.; Li, X.; et al. Fluorogenic Reporter Enables Identification of Compounds That Inhibit SARS-CoV-2. Nat. Microbiol. 2023, 8, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, J.; Patel, S.K.; Kearney, L.G.; Matalanis, G.; Farouque, O.; Srivastava, P.M.; Burrell, L.M. Plasma ACE2 Activity Predicts Mortality in Aortic Stenosis and Is Associated With Severe Myocardial Fibrosis. JACC Cardiovasc. Imaging 2020, 13, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ketabchi, F.; Jamzad, S. Therapeutic Approaches in COVID-19 Patients: The Role of the Renin-Angiotensin System. Can. Respir. J. 2022, 2022, 8698825. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, F.; Névo, N.; Piovesana, E.; Lahaye, X.; Buchrieser, J.; Schwartz, O.; Manel, N.; Tkach, M.; Théry, C.; Martin-Jaular, L. Extracellular Vesicles Containing ACE2 Efficiently Prevent Infection by SARS-CoV-2 Spike Protein-containing Virus. J. Extracell. Vesicles 2020, 10, e12050. [Google Scholar] [CrossRef]
- Zhou, X.; Miao, Y.; Wang, Y.; He, S.; Guo, L.; Mao, J.; Chen, M.; Yang, Y.; Zhang, X.; Gan, Y. Tumour-Derived Extracellular Vesicle Membrane Hybrid Lipid Nanovesicles Enhance SiRNA Delivery by Tumour-Homing and Intracellular Freeway Transportation. J. Extracell. Vesicles 2022, 11, e12198. [Google Scholar] [CrossRef]
- da Silva Oliveira, G.L.; de Freitas, R.M. Diminazene Aceturate—An Antiparasitic Drug of Antiquity: Advances in Pharmacology & Therapeutics. Pharmacol. Res. 2015, 102, 138–157. [Google Scholar] [CrossRef]
- Thatcher, S.E.; Zhang, X.; Howatt, D.A.; Yiannikouris, F.; Gurley, S.B.; Ennis, T.; Curci, J.A.; Daugherty, A.; Cassis, L.A. Angiotensin-Converting Enzyme 2 Decreases Formation and Severity of Angiotensin II-Induced Abdominal Aortic Aneurysms. Arter. Thromb. Vasc. Biol. 2014, 34, 2617–2623. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.; Song, H.; Ji, Y.; Huo, D.; Han, F.; Li, F.; Jiang, N. Identification of Therapeutic Drugs against COVID-19 through Computational Investigation on Drug Repurposing and Structural Modification. J. Biomed. Res. 2020, 34, 458–469. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X Receptor (F.X.R.): Structures and Ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- Fiorillo, B.; Marchianò, S.; Moraca, F.; Sepe, V.; Carino, A.; Rapacciuolo, P.; Biagioli, M.; Limongelli, V.; Zampella, A.; Catalanotti, B.; et al. Discovery of Bile Acid Derivatives as Potent ACE2 Activators by Virtual Screening and Essential Dynamics. J. Chem. Inf. Model. 2022, 62, 196–209. [Google Scholar] [CrossRef]
- Steri, R.; Achenbach, J.; Steinhilber, D.; Schubert-Zsilavecz, M.; Proschak, E. Investigation of Imatinib and Other Approved Drugs as Starting Points for Antidiabetic Drug Discovery with FXR Modulating Activity. Biochem. Pharmacol. 2012, 83, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Brevini, T.; Maes, M.; Webb, G.J.; John, B.V.; Fuchs, C.D.; Buescher, G.; Wang, L.; Griffiths, C.; Brown, M.L.; Scott, W.E.; et al. FXR Inhibition May Protect from SARS-CoV-2 Infection by Reducing ACE2. Nature 2023, 615, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Onyeyili, P.A.; Anika, S.M. Diminazene Aceturate Residues in the Tissues of Healthy, Trypanosoma Congolense and Trypanosoma Brucei Brucei Infected Dogs. Br. Vet. J. 1991, 147, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Lenzig, P.; Oslender-Bujotzek, A.; Kusch, J.; Dias Lucas, S.; Gründer, S.; Wiemuth, D. The Bile Acid-Sensitive Ion Channel (BASIC) Is Activated by Alterations of Its Membrane Environment. PLoS ONE 2014, 9, e111549. [Google Scholar] [CrossRef] [Green Version]
- Wiegreffe, S.; Löhrer, D.; Wirtz, M.; Wiemuth, D. The Bile Acid-Sensitive Ion Channel (BASIC) Mediates Bile Acid-Dependent Currents in Bile Duct Epithelial Cells. Pflugers. Arch. 2021, 473, 1841–1850. [Google Scholar] [CrossRef]
- Wiemuth, D.; Gründer, S. The Pharmacological Profile of Brain Liver Intestine Na+ Channel: Inhibition by Diarylamidines and Activation by Fenamates. Mol. Pharmacol. 2011, 80, 911–919. [Google Scholar] [CrossRef]
- Su, R.; Zeng, J.; O’Shaughnessy, B. Host Cell Membrane Capture by the SARS-CoV-2 Spike Protein Fusion Intermediate. bioRxiv 2021. [CrossRef]
- Zhou, J.; Le, V.; Kalia, D.; Nakayama, S.; Mikek, C.; Lewis, E.A.; Sintim, H.O. Diminazene or Berenil, a Classic Duplex Minor Groove Binder, Binds to G-Quadruplexes with Low Nanomolar Dissociation Constants and the Amidine Groups Are Also Critical for G-Quadruplex Binding. Mol. Biosyst. 2014, 10, 2724–2734. [Google Scholar] [CrossRef] [Green Version]
- Kayode, O.; Huang, Z.; Soares, A.S.; Caulfield, T.R.; Dong, Z.; Bode, A.M.; Radisky, E.S. Small Molecule Inhibitors of Mesotrypsin from a Structure-Based Docking Screen. PLoS ONE 2017, 12, e0176694. [Google Scholar] [CrossRef] [Green Version]
- Krauson, A.J.; Rooney, J.G.; Carattino, M.D. Molecular Basis of Inhibition of Acid Sensing Ion Channel 1A by Diminazene. PLoS ONE 2018, 13, e0196894. [Google Scholar] [CrossRef]
- Arias, R.L.; Sung, M.-L.A.; Vasylyev, D.; Zhang, M.-Y.; Albinson, K.; Kubek, K.; Kagan, N.; Beyer, C.; Lin, Q.; Dwyer, J.M.; et al. Amiloride Is Neuroprotective in an MPTP Model of Parkinson’s Disease. Neurobiol. Dis. 2008, 31, 334–341. [Google Scholar] [CrossRef]
- Wong, H.K.; Bauer, P.O.; Kurosawa, M.; Goswami, A.; Washizu, C.; Machida, Y.; Tosaki, A.; Yamada, M.; Knöpfel, T.; Nakamura, T.; et al. Blocking Acid-Sensing Ion Channel 1 Alleviates Huntington’s Disease Pathology via an Ubiquitin-Proteasome System-Dependent Mechanism. Hum. Mol. Genet. 2008, 17, 3223–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Z.-G.; Zhu, X.-M.; Chu, X.-P.; Minami, M.; Hey, J.; Wei, W.-L.; MacDonald, J.F.; Wemmie, J.A.; Price, M.P.; Welsh, M.J.; et al. Neuroprotection in Ischemia: Blocking Calcium-Permeable Acid-Sensing Ion Channels. Cell 2004, 118, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friese, M.A.; Craner, M.J.; Etzensperger, R.; Vergo, S.; Wemmie, J.A.; Welsh, M.J.; Vincent, A.; Fugger, L. Acid-Sensing Ion Channel-1 Contributes to Axonal Degeneration in Autoimmune Inflammation of the Central Nervous System. Nat. Med. 2007, 13, 1483–1489. [Google Scholar] [CrossRef]
- Mango, D.; Nisticò, R. Neurodegenerative Disease: What Potential Therapeutic Role of Acid-Sensing Ion Channels? Front. Cell. Neurosci. 2021, 15, 730641. [Google Scholar] [CrossRef] [PubMed]
- Perilo, C.S.; Pereira, M.T.; Santoro, M.M.; Nagem, R.A.P. Structural Binding Evidence of the Trypanocidal Drugs Berenil® and Pentacarinate® Active Principles to a Serine Protease Model. Int. J. Biol. Macromol. 2010, 46, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Zhigulin, A.S.; Tikhonov, D.B.; Barygin, O.I. Mechanisms of Acid-Sensing Ion Channels Inhibition by Nafamostat, Sepimostat and Diminazene. Eur. J. Pharmacol. 2023, 938, 175394. [Google Scholar] [CrossRef]
- Evans, S.A.; Olson, S.T.; Shore, J.D. P-Aminobenzamidine as a Fluorescent Probe for the Active Site of Serine Proteases. J. Biol. Chem. 1982, 257, 3014–3017. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Inacio, M.C.; Liu, M.X.; Gunatilaka, A.A.L. Discovery of Diminazene as a Dual Inhibitor of SARS-CoV-2 Human Host Proteases TMPRSS2 and Furin Using Cell-Based Assays. Curr. Res. Chem. Biol. 2022, 2, 100023. [Google Scholar] [CrossRef]
- Hernández-Mitre, M.P.; Tong, S.Y.C.; Denholm, J.T.; Dore, G.J.; Bowen, A.C.; Lewin, S.R.; Venkatesh, B.; Hills, T.E.; McQuilten, Z.; Paterson, D.L.; et al. Nafamostat Mesylate for Treatment of COVID-19 in Hospitalised Patients: A Structured, Narrative Review. Clin. Pharmacokinet. 2022, 61, 1331–1343. [Google Scholar] [CrossRef]
- Santos, E.S.; Silva, P.C.; Sousa, P.S.A.; Aquino, C.C.; Pacheco, G.; Teixeira, L.F.L.S.; Araujo, A.R.; Sousa, F.B.M.; Barros, R.O.; Ramos, R.M.; et al. Antiviral Potential of Diminazene Aceturate against SARS-CoV-2 Proteases Using Computational and in Vitro Approaches. Chem. Biol. Interact. 2022, 367, 110161. [Google Scholar] [CrossRef]
- Morty, R.E.; Troeberg, L.; Pike, R.N.; Jones, R.; Nickel, P.; Lonsdale-Eccles, J.D.; Coetzer, T.H. A Trypanosome Oligopeptidase as a Target for the Trypanocidal Agents Pentamidine, Diminazene and Suramin. FEBS Lett. 1998, 433, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, D.E.; Timofeev, V.I.; Britikov, V.V.; Britikova, E.V.; Kleymenov, S.Y.; Vlaskina, A.V.; Kuranova, I.P.; Mikhailova, A.G.; Rakitina, T.V. First Crystal Structure of Bacterial Oligopeptidase B in an Intermediate State: The Roles of the Hinge Region Modification and Spermine. Biology 2021, 10, 1021. [Google Scholar] [CrossRef]
- Mikhailova, A.G.; Rakitina, T.V.; Timofeev, V.I.; Karlinsky, D.M.; Korzhenevskiy, D.A.; Agapova, Y.K.; Vlaskina, A.V.; Ovchinnikova, M.V.; Gorlenko, V.A.; Rumsh, L.D. Activity Modulation of the Oligopeptidase B from Serratia Proteamaculans by Site-Directed Mutagenesis of Amino Acid Residues Surrounding Catalytic Triad Histidine. Biochimie 2017, 139, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.L.; Garlick, J.M.; Wu, Y.; Soellner, M.B.; Brooks, C.L.; Mapp, A.K. TMPRSS2 Inhibitor Discovery Facilitated through an in Silico and Biochemical Screening Platform. bioRxiv, 2021; preprint. [Google Scholar] [CrossRef]
- Morty, R.E.; Pellé, R.; Vadász, I.; Uzcanga, G.L.; Seeger, W.; Bubis, J. Oligopeptidase B from Trypanosoma Evansi: A Parasite Peptidase That Inactivates Atrial Natriuretic Factor In The Bloodstream Of Infected Hosts*. J. Biol. Chem. 2005, 280, 10925–10937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motta, F.N.; Azevedo, C.d.S.; Neves, B.P.; de Araújo, C.N.; Grellier, P.; de Santana, J.M.; Bastos, I.M.D. Oligopeptidase B, a Missing Enzyme in Mammals and a Potential Drug Target for Trypanosomatid Diseases. Biochimie 2019, 167, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Grellier, P.; Vendeville, S.; Joyeau, R.; Bastos, I.M.; Drobecq, H.; Frappier, F.; Teixeira, A.R.; Schrével, J.; Davioud-Charvet, E.; Sergheraert, C.; et al. Trypanosoma Cruzi Prolyl Oligopeptidase Tc80 Is Involved in Nonphagocytic Mammalian Cell Invasion by Trypomastigotes. J. Biol. Chem. 2001, 276, 47078–47086. [Google Scholar] [CrossRef] [Green Version]
- de Brito, M.G.; Mengarda, A.C.; Oliveira, G.L.; Cirino, M.E.; Silva, T.C.; de Oliveira, R.N.; Allegretti, S.M.; de Moraes, J. Therapeutic Effect of Diminazene Aceturate on Parasitic Blood Fluke Schistosoma Mansoni Infection. Antimicrob. Agents Chemother. 2020, 64, 11. [Google Scholar] [CrossRef]
- Hajissa, K.; Muhajir, A.E.M.A.; Eshag, H.A.; Alfadel, A.; Nahied, E.; Dahab, R.; Ali, S.M.; Mohammed, M.; Gaafar, M.; Mohamed, Z. Prevalence of Schistosomiasis and Associated Risk Factors among School Children in Um-Asher Area, Khartoum, Sudan. BMC Res. Notes 2018, 11, 779. [Google Scholar] [CrossRef]
- Wang, Q.; Da’dara, A.A.; Skelly, P.J. The Blood Fluke Schistosoma Mansoni Cleaves the Coagulation Protein High Molecular Weight Kininogen (HK) but Does Not Generate the Vasodilator Bradykinin. Parasites Vectors 2018, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, T.; Mideo, N.; Alizon, S. Coevolution of Virulence and Immunosuppression in Multiple Infections. J. Evol. Biol. 2018, 31, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Chevaisrakul, P.; Lumjiaktase, P.; Kietdumrongwong, P.; Chuatrisorn, I.; Chatsangjaroen, P.; Phanuphak, N. Hybrid and Herd Immunity 6 Months after SARS-CoV-2 Exposure among Individuals from a Community Treatment Program. Sci. Rep. 2023, 13, 763. [Google Scholar] [CrossRef]
- Costa-Madeira, J.C.; Trindade, G.B.; Almeida, P.H.P.; Silva, J.S.; Carregaro, V. T Lymphocyte Exhaustion During Human and Experimental Visceral Leishmaniasis. Front. Immunol. 2022, 13, 835711. [Google Scholar] [CrossRef]
- Gigley, J.P.; Bhadra, R.; Moretto, M.M.; Khan, I.A. T Cell Exhaustion in Protozoan Disease. Trends Parasitol. 2012, 28, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abulsoud, A.I.; El-Husseiny, H.M.; El-Husseiny, A.A.; El-Mahdy, H.A.; Ismail, A.; Elkhawaga, S.Y.; Khidr, E.G.; Fathi, D.; Mady, E.A.; Najda, A.; et al. Mutations in SARS-CoV-2: Insights on Structure, Variants, Vaccines, and Biomedical Interventions. Biomed. Pharmacother. 2023, 157, 113977. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Devnath, P.; Emran, T.B.; Tallei, T.E.; Mitra, S.; Dhama, K. Oral and Intranasal Vaccines against SARS-CoV-2: Current Progress, Prospects, Advantages, and Challenges. Immun. Inflamm. Dis. 2022, 10, e604. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir Remain Active against SARS-CoV-2 Omicron and Other Variants of Concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, A.; Tagliazucchi, L.; Lima, C.; Venuti, F.; Malpezzi, G.; Magoulas, G.E.; Santarem, N.; Calogeropoulou, T.; Cordeiro-da-Silva, A.; Costi, M.P. Current Treatments to Control African Trypanosomiasis and One Health Perspective. Microorganisms 2022, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Radwanska, M.; Guirnalda, P.; Trez, C.D.; Ryffel, B.; Black, S.; Magez, S. Trypanosomiasis-Induced B Cell Apoptosis Results in Loss of Protective Anti-Parasite Antibody Responses and Abolishment of Vaccine-Induced Memory Responses. PLoS Pathog. 2008, 4, e1000078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Trez, C.; Katsandegwaza, B.; Caljon, G.; Magez, S. Experimental African Trypanosome Infection by Needle Passage or Natural Tsetse Fly Challenge Thwarts the Development of Collagen-Induced Arthritis in DBA/1 Prone Mice via an Impairment of Antigen Specific B Cell Autoantibody Titers. PLoS ONE 2015, 10, e0130431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Trez, C.; Khan, S.; Magez, S.T. Brucei Infections Abrogate Diverse Plasma Cell-Mediated Effector B Cell Responses, Independently of Their Specificity, Affinity and Host Genetic Background. PLoS Negl. Trop. Dis. 2020, 14, e0008358. [Google Scholar] [CrossRef] [PubMed]
- Männe, C.; Takaya, A.; Yamasaki, Y.; Mursell, M.; Hojyo, S.; Wu, T.-Y.; Sarkander, J.; McGrath, M.A.; Cornelis, R.; Hahne, S.; et al. Salmonella SiiE Prevents an Efficient Humoral Immune Memory by Interfering with IgG+ Plasma Cell Persistence in the Bone Marrow. Proc. Natl. Acad. Sci. USA 2019, 116, 7425–7430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, V.N.; Sawatsky, B.; Han, A.X.; Laksono, B.M.; Walz, L.; Parker, E.; Pieper, K.; Anderson, C.A.; de Vries, R.D.; Lanzavecchia, A.; et al. Incomplete Genetic Reconstitution of B Cell Pools Contributes to Prolonged Immunosuppression after Measles. Sci. Immunol. 2019, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Li, J.; Wang, L.; Wu, Z.; Sun, X. Extracellular Vesicle-Mediated Communication Within Host-Parasite Interactions. Front. Immunol. 2019, 9, 3066. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, M.; Mirsamadi, E.S.; Mirjalali, H.; Zali, M.R. Isolation and Functions of Extracellular Vesicles Derived from Parasites: The Promise of a New Era in Immunotherapy, Vaccination, and Diagnosis. Int. J. Nanomed. 2020, 15, 2957–2969. [Google Scholar] [CrossRef]
- Yaseen, I.; Kaur, P.; Nandicoori, V.K.; Khosla, S. Mycobacteria Modulate Host Epigenetic Machinery by Rv1988 Methylation of a Non-Tail Arginine of Histone H3. Nat. Commun. 2015, 6, 8922. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, A.R.; Nishiguchi, T.; Mace, E.M.; Rajapakshe, K.; Mtetwa, G.; Kay, A.; Maphalala, G.; Secor, W.E.; Mejia, R.; Orange, J.S.; et al. Schistosomiasis Induces Persistent DNA Methylation and Tuberculosis-Specific Immune Changes. J. Immunol. 2018, 201, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Hamdane, N.; Jühling, F.; Crouchet, E.; Saghire, H.E.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.R.; et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, B.; Oestreich, K.J.; Ha, S.-J.; Duraiswamy, J.; Akondy, R.S.; West, E.E.; Wei, Z.; Lu, P.; Austin, J.W.; Riley, J.L.; et al. Chronic Virus Infection Enforces Demethylation of the Locus That Encodes PD-1 in Antigen-Specific CD8(+) T Cells. Immunity 2011, 35, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Cross, D.; Drury, R.; Hill, J.; Pollard, A.J. Epigenetics in Sepsis: Understanding Its Role in Endothelial Dysfunction, Immunosuppression, and Potential Therapeutics. Front. Immunol. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Hulme, J. Staphylococcus Infection: Relapsing Atopic Dermatitis and Microbial Restoration. Antibiotics 2023, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, A. Taurine Regulation of Neuroendocrine Function. Adv. Exp. Med. Biol. 2019, 1155, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, S.; West, N.; Singhal, N.K.; Clements, R.; Basu, S.; Tripathi, A.; Dutta, R.; Freeman, E.J.; McDonough, J. The BHMT-Betaine Methylation Pathway Epigenetically Modulates Oligodendrocyte Maturation. PLoS ONE 2021, 16, e0250486. [Google Scholar] [CrossRef] [PubMed]
- Mládková, J.; Hladílková, J.; Diamond, C.E.; Tryon, K.; Yamada, K.; Garrow, T.A.; Jungwirth, P.; Koutmos, M.; Jiráček, J. Specific Potassium Ion Interactions Facilitate Homocysteine Binding to Betaine-Homocysteine S-Methyltransferase. Proteins Struct. Funct. Bioinform. 2014, 82, 2552–2564. [Google Scholar] [CrossRef]
- Neidhart, M.; Karouzakis, E.; Jüngel, A.; Gay, R.E.; Gay, S. Inhibition of Spermidine/Spermine N1-Acetyltransferase Activity: A New Therapeutic Concept in Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 1723–1733. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kato, A.; Ota, Y.; Ohba, R.; Komatsu, K. Bisbenzamidine Derivative, Pentamidine Represses DNA Damage Response through Inhibition of Histone H2A Acetylation. Mol. Cancer 2010, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Alabed, S.J.; Zihlif, M.; Taha, M. Discovery of New Potent Lysine Specific Histone Demythelase-1 Inhibitors (LSD-1) Using Structure Based and Ligand Based Molecular Modelling and Machine Learning. RSC Adv. 2022, 12, 35873–35895. [Google Scholar] [CrossRef]
- Galkin, F.; Parish, A.; Bischof, E.; Zhang, J.; Mamoshina, P.; Zhavoronkov, A. Increased Pace of Aging in COVID-Related Mortality. Life 2021, 11, 730. [Google Scholar] [CrossRef]
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A.; et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int. J. Mol. Sci. 2021, 22, 6151. [Google Scholar] [CrossRef]
- Ying, K.; Zhai, R.; Pyrkov, T.V.; Shindyapina, A.V.; Mariotti, M.; Fedichev, P.O.; Shen, X.; Gladyshev, V.N. Genetic and Phenotypic Analysis of the Causal Relationship between Aging and COVID-19. Commun. Med. 2021, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Kokoris, S. COVID-19 Sequelae: Can Long-Term Effects Be Predicted? Lancet Infect. Dis. 2022, 22, 1651–1652. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. The Potential Role of Ischaemia-Reperfusion Injury in Chronic, Relapsing Diseases Such as Rheumatoid Arthritis, Long COVID, and ME/CFS: Evidence, Mechanisms, and Therapeutic Implications. Biochem. J. 2022, 479, 1653–1708. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A Central Role for Amyloid Fibrin Microclots in Long COVID/PASC: Origins and Therapeutic Implications. Biochem. J. 2022, 479, 537–559. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Moremi, K.; Oladejo, S.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Combined Triple Treatment of Fibrin Amyloid Microclots and Platelet Pathology in Individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC.) Can Resolve Their Persistent Symptoms. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Sait, A.; Angeli, C.; Doig, A.J.; Day, P.J.R. Viral Involvement in Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 1049–1060. [Google Scholar] [CrossRef]
- Bu, X.-L.; Liu, Y.-H.; Wang, Q.-H.; Jiao, S.-S.; Zeng, F.; Yao, X.-Q.; Gao, D.; Chen, J.-C.; Wang, Y.-J. Serum Amyloid-Beta Levels Are Increased in Patients with Obstructive Sleep Apnea Syndrome. Sci. Rep. 2015, 5, 13917. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kanwar, B.; Khattak, A.; Balentine, J.; Nguyen, N.H.; Kast, R.E.; Lee, C.J.; Bourbeau, J.; Altschuler, E.L.; Sergi, C.M.; et al. COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs. Int. J. Mol. Sci. 2022, 23, 13260. [Google Scholar] [CrossRef]
- Cho, S.C.; Rhim, J.H.; Choi, H.R.; Son, Y.H.; Lee, S.J.; Song, K.Y.; Park, S.C. Protective Effect of 4,4′-Diaminodiphenylsulfone against Paraquat-Induced Mouse Lung Injury. Exp. Mol. Med. 2011, 43, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, C.J.; Park, J.; Lee, S.J.; Choi, S. The Neuroinflammasome in Alzheimer’s Disease and Cerebral Stroke. Dement. Geriatr. Cogn. Discord. Extra 2021, 11, 159–167. [Google Scholar] [CrossRef]
- Macedo, L.M.; Souza, P.D.S.; Maria, M.L.D.A.D.; Borges, C.L.; Soares, C.M.D.A.; Pedrino, G.R.; Colugnati, D.B.; dos Santos, R.A.S.; Mendes, E.P.; Ferreira, A.J.; et al. Cardioprotective Effects of Diminazene Aceturate in Pressure-Overloaded Rat Hearts. Life Sci. 2016, 155, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan Impairment in Low-Risk Individuals with Post-COVID-19 Syndrome: A Prospective, Community-Based Study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.K.M.; Nogueira, K.M.; Araújo, T.S.L.; Sousa, N.A.; Sousa, F.B.M.; Oliveira, A.P.; Sales, T.; Silva, K.; Rocha, T.M.; Leal, L.K.A.M.; et al. Anti-Diarrheal Therapeutic Potential of Diminazene Aceturate Stimulation of the ACE II/Ang-(1-7)/Mas Receptor Axis in Mice: A Trial Study. Biochem. Pharmacol. 2021, 186, 114500. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Hulme, J.; Tran, H.D.; Vo, T.K.; Vo, G.V. The Potential Impact of COVID-19 on Male Reproductive Health. J. Endocrinol. Investig. 2022, 45, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, X.; Li, J.; Wang, Z.; Cheng, S.; Chen, L.; Qiang, Y.; Li, H.; Ni, J. Preparation and Pharmacokinetics of an Injectable Thermosensitive Hydrogel of Diminazene Aceturate. J. Drug Deliv. Sci. Technol. 2013, 23, 531–535. [Google Scholar] [CrossRef]
- Andreana, I.; Bincoletto, V.; Milla, P.; Dosio, F.; Stella, B.; Arpicco, S. Nanotechnological Approaches for Pentamidine Delivery. Drug Deliv. Transl. Res. 2022, 12, 1911–1927. [Google Scholar] [CrossRef]
- Prata, L.O.; Rodrigues, C.R.; Martins, J.M.; Vasconcelos, P.C.; Oliveira, F.M.S.; Ferreira, A.J.; Rodrigues-Machado, M.d.G.; Caliari, M.V. Original Research: ACE2 Activator Associated with Physical Exercise Potentiates the Reduction of Pulmonary Fibrosis. Exp. Biol. Med. 2017, 242, 8–21. [Google Scholar] [CrossRef] [Green Version]
| Diarylamidines & Combinations | Pathogen/Disease | Hosts/Experimental | Findings | References |
|---|---|---|---|---|
| DIZE & AZI | T. brucei | Albino Rats | ↓ Parasitaemia ↓ Leukocytosis | [45] |
| DIZE | T. congolense | BALB/c mice C57BL/6 mice | ↓ LPS-induced production ↓ IL-6, IL-12, TNF and IFN-γ | [42] |
| DIZE | S. mansoniex vivo | mice | ↓ Aminotransferase levels ↓ Immature and adult parasites | [54] |
| DIZE | T. congolense | Pre-treated macrophages | ↓ Phosphorylation of mitogen-activated protein kinase (p38), STAT1 and STAT3 | [50] |
| CLD, DIZE, ACE, IMD | C. babesiosis | Dogs | ↓ Malaise, fatigue, chills, fever | [40] |
| DIZE, CHL, STP | Antibiotic-resistant K. pneumoniae, S. aureus and E. coli | Minimal inhibitory concentrations (MICs) assay | ↑ Gram-negative and positive bacterial sensitivity to antibiotics ↓ in MIC | [44] |
| PENT, colistin | T. brucei rhodesiense | 66-year-old female | ↓ Parasitaemia by 75% (trypomastigotes) | [39] |
| PENT | A. baumannii | Murine Infection Model | ↑ sensitivity of colistin-resistant A. baumannii to gram-positive antibiotics | [47] |
| PENT analogue (P35) | A. baumannii & K. pneumoniae. | Murine model | ↑ sensitivity of colistin-resistant A. baumannii to gram-positive antibiotics | [48] |
| Diarylamidine | Disease/Pathology | Hosts/Experimental | Findings | References |
|---|---|---|---|---|
| DIZE | AD | SAMP8 mice | ↓ Neuropathology | [55] |
| DIZE | Liver injury & BF | MDR gene-2 knockout mice | ↓ NOX enzyme assembly and ROS generation ↑ myofibroblasts & tissue repair | [60] |
| DIZE | ATP & HPS | ApoE-Knockout mice | Modulating macrophage response & taurine biosynthesis | [58] |
| DIZE | CF & DAD | W rats | ↑ Protective effect on the heart under the pathological condition of kidney injury | [56] |
| DIZE | PHY | SD male rats | ↑ Vasoprotective axis of the LRAS, ↑ pulmonary vasoreactivity, ↑ enhanced cardiac function, ↓ inflammatory cytokines | [57] |
| DIZE | NPP | W diabetic male rats | ↑ Glomerular ACE2 & AT2 receptor expression ↓ fibrosis and apoptosis | [61] |
| DIZE | MORI | WAG/RijCmcr rats | ↑ Survivability in rat models of H-ARS and DEARE | [62] |
| DIZE | CAR | W rats | ↑ Acute antiarrhythmic-mic potential in vivo modulation of cardiomyocytes contraction and excitability properties | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulme, J. COVID-19 and Diarylamidines: The Parasitic Connection. Int. J. Mol. Sci. 2023, 24, 6583. https://doi.org/10.3390/ijms24076583
Hulme J. COVID-19 and Diarylamidines: The Parasitic Connection. International Journal of Molecular Sciences. 2023; 24(7):6583. https://doi.org/10.3390/ijms24076583
Chicago/Turabian StyleHulme, John. 2023. "COVID-19 and Diarylamidines: The Parasitic Connection" International Journal of Molecular Sciences 24, no. 7: 6583. https://doi.org/10.3390/ijms24076583
APA StyleHulme, J. (2023). COVID-19 and Diarylamidines: The Parasitic Connection. International Journal of Molecular Sciences, 24(7), 6583. https://doi.org/10.3390/ijms24076583






