The Expression of Insulin in the Central Nervous System: What Have We Learned So Far?
Abstract
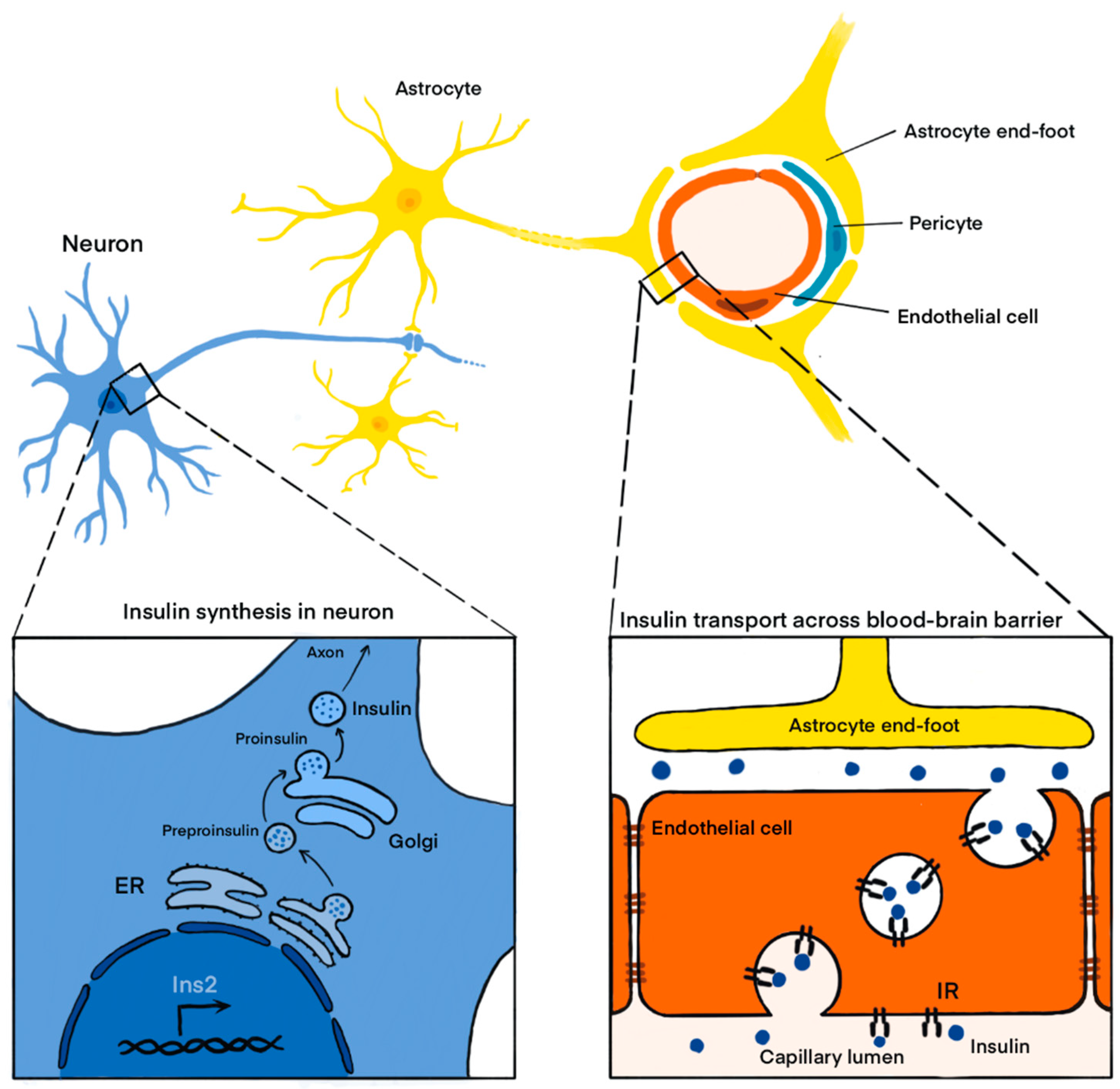
1. Introduction
2. Overview of Studies Demonstrating Insulin Expression in the Central Nervous System (CNS)
2.1. In Vitro Studies Confirming the Expression of Insulin in Neurons
2.2. In Vivo Studies Confirming the Expression of Insulin in the CNS
3. Actions of Insulin Expressed in the CNS
3.1. Brain-Derived Insulin Has Neuroprotective Effects
3.2. Fasting-Induced Increase in Hypothalamic Insulin Expression Appears Not to Be Associated with Facilitating Neuronal Glucose Uptake
3.3. Stimulation of Insulin-Producing Neurons in Dorsal Vagal Complex Results in Increased Appetite
3.4. Insulin Produced in the Paraventricular Nucleus Stimulates Growth Hormone Secretion
3.5. In Utero Alcohol Exposure Decreases Insulin Expression in the Cerebellum and Thus Impairs Insulin-Mediated Neuronal Glucose Uptake
3.6. The Impairment in Insulin Expression and Signaling in the Brain Is Associated with Memory Decline
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Lushchak, O.V.; Goergen, P.; Williams, M.J.; Nässel, D.R. Drosophila Insulin-Producing Cells Are Differentially Modulated by Serotonin and Octopamine Receptors and Affect Social Behavior. PLoS ONE 2014, 9, e99732. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H. The Internal Secretion of the Pancreas. J. Lab. Clin. Med. 1922, 7, 42–60. [Google Scholar] [CrossRef]
- Cori, C.F.; Cori, G.T. Insulin and Tissue Sugar. J. Pharmacol. Exp. Ther. 1925, 24, 465–478. [Google Scholar]
- Flatt, J.P.; Ball, E.G. Studies on the Metabolism of Adipose Tissue XV. An Evaluation of the Major Pathways of Glucose Catabolism as Influences by Insulin and Epinephrine. J. Biol. Chem. 1964, 239, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the Regulation of Hepatic Metabolism by Insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Havrankova, J.; Schmechel, D.; Roth, J.; Brownstein, M. Identification of Insulin in Rat Brain. Proc. Natl. Acad. Sci. USA 1978, 75, 5737–5741. [Google Scholar] [CrossRef]
- Baskin, D.G.; Stein, L.J.; Ikeda, H.; Woods, S.C.; Figlewicz, D.P.; Porte, D.; Greenwood, M.R.C.; Dorsa, D.M. Genetically Obese Zucker Rats Have Abnormally Low Brain Insulin Content. Life Sci. 1985, 36, 627–633. [Google Scholar] [CrossRef]
- Margolis, R.U.; Altszuler, N. Insulin in the Cerebrospinal Fluid. Nature 1967, 215, 1375–1376. [Google Scholar] [CrossRef]
- Baura, G.D.; Foster, D.M.; Porte, D.; Kahn, S.E.; Bergman, R.N.; Cobelli, C.; Schwartz, M.W. Saturable Transport of Insulin from Plasma into the Central Nervous System of Dogs in Vivo. A Mechanism for Regulated Insulin Delivery to the Brain. J. Clin. Investig. 1993, 92, 1824–1830. [Google Scholar] [CrossRef]
- King, G.L.; Johnson, S.M. Receptor-Mediated Transport of Insulin Across Endothelial Cells. Science 1985, 227, 1583–1586. [Google Scholar] [CrossRef]
- Weyhenmeyer, J.A.; Fellows, R.E. Presence of Immunoreactive Insulin in Neurons Cultured from Fetal Rat Brain. Cell. Mol. Neurobiol. 1983, 3, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Raizada, M.K. Localization of Insulin-like Immunoreactivity in the Neurons from Primary Cultures of Rat Brain. Exp. Cell Res. 1983, 143, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.P.; Christie, D.L.; Renwick, A.G.C. Proinsulin-like Material in Mouse Foetal Brain Cell Cultures. FEBS Lett. 1984, 168, 299–302. [Google Scholar] [CrossRef]
- Schechter, R.; Sadiq, H.F.; Devaskar, S.U. Insulin and Insulin MRNA Are Detected in Neuronal Cell Cultures Maintained in an Insulin-Free/Serum-Free Medium. J. Histochem. Cytochem. 1990, 38, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.S.; Rajakumar, P.A.; Eves, E.M.; Rosner, M.R.; Wainer, B.H.; Devaskar, S.U. Insulin Gene Expression in Immortalized Rat Hippocampal and Pheochromocytoma-12 Cell Lines. Regul. Pept. 1997, 69, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Schechter, R.; Holtzclaw, L.; Sadiq, F.; Kahn, A.; Devaskar, S. Insulin Synthesis by Isolated Rabbit Neurons. Endocrinology 1988, 123, 505–513. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Giddings, S.J.; Rajakumar, P.A.; Carnaghi, L.R.; Menon, R.K.; Zahm, D.S. Insulin Gene Expression and Insulin Synthesis in Mammalian Neuronal Cells. J. Biol. Chem. 1994, 269, 8445–8454. [Google Scholar] [CrossRef]
- Clarke, D.W.; Mudd, L.; Boyd, F.T.; Fields, M.; Raizada, M.K. Insulin Is Released from Rat Brain Neuronal Cells in Culture. J. Neurochem. 1986, 47, 831–836. [Google Scholar] [CrossRef]
- Molnár, G.; Faragó, N.; Kocsis, Á.K.; Rózsa, M.; Lovas, S.; Boldog, E.; Báldi, R.; Csajbók, É.; Gardi, J.; Puskás, L.G.; et al. GABAergic Neurogliaform Cells Represent Local Sources of Insulin in the Cerebral Cortex. J. Neurosci. 2014, 34, 1133–1137. [Google Scholar] [CrossRef]
- Csajbók, É.A.; Kocsis, Á.K.; Faragó, N.; Furdan, S.; Kovács, B.; Lovas, S.; Molnár, G.; Likó, I.; Zvara, Á.; Puskás, L.G.; et al. Expression of GLP-1 Receptors in Insulin-Containing Interneurons of Rat Cerebral Cortex. Diabetologia 2019, 62, 717–725. [Google Scholar] [CrossRef]
- Madadi, G.; Dalvi, P.S.; Belsham, D.D. Regulation of Brain Insulin MRNA by Glucose and Glucagon-like Peptide 1. Biochem. Biophys. Res. Commun. 2008, 376, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Yu, S.-W.; Kim, E.-K. Wnt3a Upregulates Brain-Derived Insulin by Increasing NeuroD1 via Wnt/β-Catenin Signaling in the Hypothalamus. Mol. Brain 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lu, Y.; Liu, X.; Huang, X.; Keller, E.T.; Qian, C.-N.; Zhang, J. Wnt3a: Functions and Implications in Cancer. Chin. J. Cancer 2015, 34, 50. [Google Scholar] [CrossRef]
- Dorn, A.; Bernstein, H.-G.; Hahn, H.-J.; Ziegler, M.; Rummelfnger, H. Insulin Immunohistochemistry of Rodent CNS: Apparent Species Differences but Good Correlation with Radioimmunological Data. Histochemistry 1981, 71, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Dorn, A.; Bernstein, H.-G.; Kostmann, G.; Hahn, H.-J.; Ziegler, M. An Immunofluorescent Reaction Appears to Insulin-Antiserum in Different CNS Regions of Two Rat Species. Acta Histochem. 1980, 66, 276–278. [Google Scholar] [CrossRef]
- Baskin, D.G.; Porte, D.; Guest, K.; Dorsa, D.M. Regional Concentrations of Insulin in the Rat Brain. Endocrinology 1983, 112, 898–903. [Google Scholar] [CrossRef]
- Dorn, A.; Rinne, A.; Bernstein, H.G.; Hahn, H.J.; Ziegler, M. Insulin and C-Peptide in Human Brain Neurons (Insulin/C-Peptide/Brain Peptides/Immunohistochemistry/Radioimmunoassay). J. Hirnforsch. 1983, 24, 495–499. [Google Scholar]
- Dorn, A.; Ziegler, M.; Bernstein, H.-G.; Dietz, H.; Rinne, A. Concerning the Presence of an Insulin-Related Peptide in the Human Brain: An Immunohistochemical Reinvestigation by Use of Monoclonal Insulin Antibodies. Acta Histochem. 1984, 74, 81–84. [Google Scholar] [CrossRef]
- Schechter, R.; Whitmire, J.; Holtzclaw, L.; George, M.; Harlow, R.; Devaskar, S.U. Developmental Regulation of Insulin in the Mammalian Central Nervous System. Brain Res. 1992, 582, 27–37. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Singh, B.S.; Carnaghi, L.R.; Rajakumar, P.A.; Giddings, S.J. Insulin II Gene Expression in Rat Central Nervous System. Regul. Pept. 1993, 48, 55–63. [Google Scholar] [CrossRef]
- Schechter, R.; Beju, D.; Gaffney, T.; Schaefer, F.; Whetsell, L. Preproinsulin I and II MRNAs and Insulin Electron Microscopic Immunoreaction Are Present within the Rat Fetal Nervous System. Brain Res. 1996, 736, 16–27. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.-S.; Liao, B.-Y.; Long, M.; Yu, H.-T. Adaptive Evolution of the Insulin Two-Gene System in Mouse. Genetics 2008, 178, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Leroux, L.; Desbois, P.; Lamotte, L.; Duvillié, B.; Cordonnier, N.; Jackerott, M.; Jami, J.; Bucchini, D.; Joshi, R.L. Compensatory Responses in Mice Carrying a Null Mutation for Ins1 or Ins2. Diabetes 2001, 50, S150. [Google Scholar] [CrossRef]
- Schechter, R.; Yanovitch, T.; Abboud, M.; Johnson, G.; Gaskins, J. Effects of Brain Endogenous Insulin on Neurofilament and MAPK in Fetal Rat Neuron Cell Cultures. Brain Res. 1998, 808, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Schechter, R.; Abboud, M.; Johnson, G. Brain Endogenous Insulin Effects on Neurite Growth within Fetal Rat Neuron Cell Cultures. Dev. Brain Res. 1999, 116, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Kagalwala, M.N.; Onuma, Y.; Ito, Y.; Warashina, M.; Terashima, K.; Sanosaka, T.; Nakashima, K.; Gage, F.H.; Asashima, M. Insulin Biosynthesis in Neuronal Progenitors Derived from Adult Hippocampus and the Olfactory Bulb. EMBO Mol. Med. 2011, 3, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Mazucanti, C.H.; Liu, Q.-R.; Lang, D.; Huang, N.; O’Connell, J.F.; Camandola, S.; Egan, J.M. Release of Insulin Produced by the Choroid Plexis Is Regulated by Serotonergic Signaling. JCI Insight 2019, 4, e131682. [Google Scholar] [CrossRef]
- Dorn, A.; Bernstein, H.-G.; Rinne, A.; Ziegler, M.; Hahn, H.-J.; Ansorge, S. Insulin- and Glucagonlike Peptides in the Brain. Anat. Rec. 1983, 207, 69–77. [Google Scholar] [CrossRef]
- Young, W.S. Periventricular Hypothalamic Cells in the Rat Brain Contain Insulin MRNA. Neuropeptides 1986, 8, 93–97. [Google Scholar] [CrossRef]
- Deltour, L.; Leduque, P.; Blume, N.; Madsen, O.; Dubois, P.; Jami, J.; Bucchini, D. Differential Expression of the Two Nonallelic Proinsulin Genes in the Developing Mouse Embryo. Proc. Natl. Acad. Sci. USA 1993, 90, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Frölich, L.; Blum-Degen, D.; Bernstein, H.-G.; Engelsberger, S.; Humrich, J.; Laufer, S.; Muschner, D.; Thalheimer, A.; Türk, A.; Hoyer, S.; et al. Brain Insulin and Insulin Receptors in Aging and Sporadic Alzheimer’s Disease. J. Neural Transm. 1998, 105, 423. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, D.S.; Gersham, B.; Tu, M.-P.; Palmer, M.; Tatar, M. Drosophila DFOXO Controls Lifespan and Regulates Insulin Signalling in Brain and Fat Body. Nature 2004, 429, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired Insulin and Insulin-like Growth Factor Expression and Signaling Mechanisms in Alzheimer’s Disease—Is This Type 3 Diabetes? JAD 2005, 7, 63–80. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Xu, X.J.; Wands, J.R. Ethanol Inhibits Insulin Expression and Actions in the Developing Brain. CMLS Cell. Mol. Life Sci. 2005, 62, 1131–1145. [Google Scholar] [CrossRef]
- Grünblatt, E.; Salkovic-Petrisic, M.; Osmanovic, J.; Riederer, P.; Hoyer, S. Brain Insulin System Dysfunction in Streptozotocin Intracerebroventricularly Treated Rats Generates Hyperphosphorylated Tau Protein: Alzheimer’s Disease Is an Insulin Resistant Brain State. J. Neurochem. 2007, 101, 757–770. [Google Scholar] [CrossRef]
- Hrytsenko, O.; Wright, J.R.; Morrison, C.M.; Pohajdak, B. Insulin Expression in the Brain and Pituitary Cells of Tilapia (Oreochromis Niloticus). Brain Res. 2007, 1135, 31–40. [Google Scholar] [CrossRef]
- Osmanovic, J.; Plaschke, K.; Salkovic-Petrisic, M.; Grünblatt, E.; Riederer, P.; Hoyer, S. Chronic Exogenous Corticosterone Administration Generates an Insulin-Resistant Brain State in Rats. Stress 2010, 13, 123–131. [Google Scholar] [CrossRef]
- Nemoto, T.; Toyoshima-Aoyama, F.; Yanagita, T.; Maruta, T.; Fujita, H.; Koshida, T.; Yonaha, T.; Wada, A.; Sawaguchi, A.; Murakami, M. New Insights Concerning Insulin Synthesis and Its Secretion in Rat Hippocampus and Cerebral Cortex: Amyloid-Β1–42-Induced Reduction of Proinsulin Level via Glycogen Synthase Kinase-3β. Cell Signal. 2014, 26, 253–259. [Google Scholar] [CrossRef]
- Dakic, T.B.; Jevdjovic, T.V.; Peric, M.I.; Bjelobaba, I.M.; Markelic, M.B.; Milutinovic, B.S.; Lakic, I.V.; Jasnic, N.I.; Djordjevic, J.D.; Vujovic, P.Z. Short-Term Fasting Promotes Insulin Expression in Rat Hypothalamus. Eur. J. Neurosci. 2017, 46, 1730–1737. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Cho, J.H.; Bae, J.Y.; O’Leary, T.P.; Johnson, J.D.; Bae, Y.C.; Kim, E.-K. Insulin Synthesized in the Paraventricular Nucleus of the Hypothalamus Regulates Pituitary Growth Hormone Production. JCI Insight 2020, 5, e135412. [Google Scholar] [CrossRef] [PubMed]
- Dakic, T.B.; Markelic, M.B.; Ruzicic, A.A.; Jevdjovic, T.V.; Lakic, I.V.; Djordjevic, J.D.; Vujovic, P.Z. Hypothalamic Insulin Expression Remains Unaltered after Short-Term Fasting in Female Rats. Endocrine 2022, 78, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin Receptors Are Widely Distributed in the Central Nervous System of the Rat. Nature 1978, 272, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.; McNeill, T.H.; Moxley, R.T.; White, M.; Moss, A.; Livingston, J.N. Distribution of Insulin Receptor-like Immunoreactivity in the Rat Forebrain. Neuroscience 1989, 31, 143–157. [Google Scholar] [CrossRef]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.-P.; Ponomarev, E.D.; Strekalova, T. Insulin Receptor in the Brain: Mechanisms of Activation and the Role in the CNS Pathology and Treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.-A.; Wells, D.G.; Fallon, J.R. The Insulin Receptor Tyrosine Kinase Substrate P58/53 and the Insulin Receptor Are Components of CNS Synapses. J. Neurosci. 1999, 19, 7300–7308. [Google Scholar] [CrossRef]
- Garwood, C.J.; Ratcliffe, L.E.; Morgan, S.V.; Simpson, J.E.; Owens, H.; Vazquez-Villaseñor, I.; Heath, P.R.; Romero, I.A.; Ince, P.G.; Wharton, S.B. Insulin and IGF1 Signalling Pathways in Human Astrocytes in Vitro and in Vivo; Characterisation, Subcellular Localisation and Modulation of the Receptors. Mol. Brain 2015, 8, 51. [Google Scholar] [CrossRef]
- Roh, E.; Song, D.K.; Kim, M.-S. Emerging Role of the Brain in the Homeostatic Regulation of Energy and Glucose Metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef]
- Mamik, M.K.; Asahchop, E.L.; Chan, W.F.; Zhu, Y.; Branton, W.G.; McKenzie, B.A.; Cohen, E.A.; Power, C. Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration. J. Neurosci. 2016, 36, 10683–10695. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.-U. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef]
- Suzuki, R.; Lee, K.; Jing, E.; Biddinger, S.B.; McDonald, J.G.; Montine, T.J.; Craft, S.; Kahn, C.R. Diabetes and Insulin in Regulation of Brain Cholesterol Metabolism. Cell Metab. 2010, 12, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Carpentier, A.C.; Pereira, S.; Hahn, M.; Giacca, A. Direct and Indirect Control of Hepatic Glucose Production by Insulin. Cell Metab. 2021, 33, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Filatova, N.; Lindtner, C.; Chi, T.; Degann, S.; Oberlin, D.; Buettner, C. Insulin Receptor Signaling in POMC, but Not AgRP, Neurons Controls Adipose Tissue Insulin Action. Diabetes 2017, 66, 1560–1571. [Google Scholar] [CrossRef]
- Manaserh, I.H.; Chikkamenahalli, L.; Ravi, S.; Dube, P.R.; Park, J.J.; Hill, J.W. Ablating Astrocyte Insulin Receptors Leads to Delayed Puberty and Hypogonadism in Mice. PLoS Biol. 2019, 17, e3000189. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mudher, A. Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Schechter, R.; Abboud, M. Neuronal Synthesized Insulin Roles on Neural Differentiation within Fetal Rat Neuron Cell Cultures. Dev. Brain Res. 2001, 127, 41–49. [Google Scholar] [CrossRef]
- Hernández-Sánchez, C.; Rubio, E.; Serna, J.; de la Rosa, E.J.; de Pablo, F. Unprocessed Proinsulin Promotes Cell Survival during Neurulation in the Chick Embryo. Diabetes 2002, 51, 770–777. [Google Scholar] [CrossRef]
- Corpas, R.; Hernández-Pinto, A.M.; Porquet, D.; Hernández-Sánchez, C.; Bosch, F.; Ortega-Aznar, A.; Comellas, F.; de la Rosa, E.J.; Sanfeliu, C. Proinsulin Protects against Age-Related Cognitive Loss through Anti-Inflammatory Convergent Pathways. Neuropharmacology 2017, 123, 221–232. [Google Scholar] [CrossRef]
- Orosco, M.; Rouch, C.; Gerozissis, K. Activation of Hypothalamic Insulin by Serotonin Is the Primary Event of the Insulin–Serotonin Interaction Involved in the Control of Feeding. Brain Res. 2000, 872, 64–70. [Google Scholar] [CrossRef]
- Dakic, T.; Jevdjovic, T.; Lakic, I.; Djurasevic, S.F.; Djordjevic, J.; Vujovic, P. Food for Thought: Short-Term Fasting Upregulates Glucose Transporters in Neurons and Endothelial Cells, But Not in Astrocytes. Neurochem. Res. 2019, 44, 388–399. [Google Scholar] [CrossRef]
- Dakic, T.; Jevdjovic, T.; Djordjevic, J.; Vujovic, P. Short-Term Fasting Differentially Regulates PI3K/AkT/MTOR and ERK Signalling in the Rat Hypothalamus. Mech. Ageing Dev. 2020, 192, 111358. [Google Scholar] [CrossRef] [PubMed]
- Eerola, K.; Longo, F.; Reinbothe, T.M.; Richard, J.E.; Shevchouk, O.T.; López-Ferreras, L.; Mishra, D.; Asker, M.; Tolö, J.; Miranda, C.; et al. Hindbrain Insulin Controls Feeding Behavior. Mol. Metab. 2022, 66, 101614. [Google Scholar] [CrossRef]
- Zhong, D.; Reid, B.M.; Donzella, B.; Miller, B.S.; Gunnar, M.R. Early-life Stress and Current Stress Predict BMI and Height Growth Trajectories in Puberty. Dev. Psychobiol. 2022, 64, e22342. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yeon, J.E.; Chang, H.; Tison, G.; Chen, G.J.; Wands, J.; de la Monte, S. Ethanol Impairs Insulin-Stimulated Neuronal Survival in the Developing Brain: Role of PTEN Phosphatase. J. Biol. Chem. 2003, 278, 26929–26937. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, L.V.; Chen, H.; Li, Y.; Quon, M.J. Phosphorylation of PTP1B at Ser(50) by Akt Impairs Its Ability to Dephosphorylate the Insulin Receptor. Mol. Endocrinol. 2001, 15, 1768–1780. [Google Scholar] [CrossRef]
- Olson, A.L. Regulation of GLUT4 and Insulin-Dependent Glucose Flux. ISRN Mol. Biol. 2012, 2012, 856987. [Google Scholar] [CrossRef] [PubMed]
- Kellar, D.; Craft, S. Brain Insulin Resistance in Alzheimer’s Disease and Related Disorders: Mechanisms and Therapeutic Approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Yoon, J.H.; Hwang, J.; Son, S.U.; Choi, J.; You, S.-W.; Park, H.; Cha, S.-Y.; Maeng, S. How Can Insulin Resistance Cause Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3506. [Google Scholar] [CrossRef]
- Leclerc, M.; Bourassa, P.; Tremblay, C.; Caron, V.; Sugère, C.; Emond, V.; Bennett, D.A.; Calon, F. Cerebrovascular Insulin Receptors Are Defective in Alzheimer’s Disease. Brain 2023, 146, 75–90. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Busquets, O.; Sánchez-López, E.; Castro-Torres, R.; Verdaguer, E.; Manzine, P.; Poor, S.; García, M.; Olloquequi, J.; et al. The Implication of the Brain Insulin Receptor in Late Onset Alzheimer’s Disease Dementia. Pharmaceuticals 2018, 11, 11. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bea, F.J.; Solas, M.; Solomon, A.; Mugueta, C.; Winblad, B.; Kivipelto, M.; Ramirez, M.J.; Cedazo-Mínguez, A. Insulin Levels Are Decreased in the Cerebrospinal Fluid of Women with Prodomal Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 22, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Shpakov, A.O.; Zorina, I.I.; Derkach, K.V. Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium. Int. J. Mol. Sci. 2023, 24, 3278. [Google Scholar] [CrossRef] [PubMed]
| In Vitro Studies | ||||
|---|---|---|---|---|
| Cell Culture Type | Species | Method | Reference | |
| Primary fetal brain culture | Rat | ICC | Weyhenmeyer and Fellows, 1983 [11] | |
| Neuron-enriched primary cultures | Rat | ICC | Raizada et al., 1983 [12] | |
| Fetal brain cell cultures | Mouse | HPLC and gel filtration | Birch et al., 1984 [13] | |
| Neuronal cell primary culture | Rat | RIA and HPLC | Clarke et al., 1986 [18] | |
| Neuronal cell primary culture | Rabbit | ICC and In Situ Hybridization | Schechter et al., 1988 [16] | |
| Neuronal cell primary culture | Rabbit | ICC, In Situ Hybridization and ELISA | Schechter et al., 1990 [14] | |
| Neuronal cell primary culture | Rabbit | RT-PCR and In Situ Hybridization | Devaskar et al., 1994 [17] | |
| Immortalized embryonic rat hippocampal clonal cell line | Rat | ICC and Southern blot | Singh et al., 1997 [15] | |
| Neuron cell cultures | Rat | RT-PCR and ICC | Schechter et al., 1998 [35] | |
| Neuron cell cultures | Rat | qRT-PCR | Schechter et al., 1999 [36] | |
| mHypoE-39 and mHypoE-46 | Mouse | RT-PCR and ICC | Madadi et al., 2008 [21] | |
| Adult neuronal cells derived from hippocampus and olfactory bulbs | Rat | Microarray and ICC | Kuwabara et al., 2011 [37] | |
| mHypoE-39 | Mouse | qRT-PCR and IF | Lee et al., 2016 [22] | |
| Primary culture of epithelial cells of the choroid plexus | Mouse | qRT-PCR and IF | Mazucanti et al., 2019 [38] | |
| In vivo studies | ||||
| Brain Region | Species | Age | Method | Reference |
| Whole brain extract; Hypothalamus, olfactory bulb, cerebellum, brainstem, cerebral cortex | Rat | Adult | RIA and IHC | Havrankova et al., 1978 [6] |
| Neocortex, hippocampus, hypothalamus, thalamus, | Rat | Adult | IF | Dorn et al., 1980 [25] |
| Cerebellum, brain stem, cortex, hippocampus, thalamus and hypothalamus | Rat and mouse | Adult | IF and RIA | Dorn et al., 1981 [24] |
| Hypothalamus, hippocampus, and olfactory bulbs | Rat (female) | Adult | RIA | Baskin et al., 1983 [26] |
| Hypothalamus | Human | Adult | IF | Dorn et al., 1983 [27] |
| Whole brain | Human, rat, mouse, tortoises, and frogs | Adult | RIA and IHC | Dorn et al., 1983 [39] |
| Hippocampus, hypothalamus and brainstem | Human | 20–25-week embryos and adult | IHC | Dorn et al., 1984 [28] |
| Hypothalamus, olfactory bulbs, hippocampus, amygdale, cortex, midbrain, and hindbrain | Rat | 2–3 months old | RIA | Baskin et al., 1985 [7] |
| Hypothalamu (PEV) | Rat | Adult | In Situ Hybridization | Young, 1989 [40] |
| Whole brain | Rabbit | Fetal, neonatal and adult | ELISA, Western blot, RIA, Northern blot and HPLC | Schechter et al., 1992 [29] |
| Whole brain | Rat | Fetal, neonatal and adult | RT-PCR | Devaskar et al., 1993 [30] |
| Whole brain | Rat | Embryos | RT-PCR | Deltour et al., 1993 [41] |
| Hippocampus and olfactory bulbs | Rabbit | / | RT-PCR and In Situ Hybridization | Devaskar et al., 1994 [17] |
| Whole brain | Rat | Embryos | PCR and electron microscopy | Schechter et al., 1996 [31] |
| Cortex | Human (male and female) | Middle-aged, aged and Alzheimer’s | RIA and IHC | Frölich et al., 1998 [42] |
| Whole brain | Drosophila | / | qRT-PCR | Hwangbo et al., 2004 [43] |
| Hypothalamus, hippocampus, cortex | Human | Adult and Alzheimer’s | qRT-PCR | Steen et al., 2005 [44] |
| Cerebellum | Rat | Neonatal | qRT-PCR | de la Monte et al., 2005 [45] |
| Front parietal cortex, hippocampus and hypothalamus | Rat | 3–4 months | qRT-PCR | Grünblatt et al., 2007 [46] |
| Hypothalamus, telencephalon, thalamus, brainstem, visual cortex, and cerebellum | Nile Tilapia | Adult | qRT-PCR, In situ hybridization and IHC | Hrytsenko et al., 2007 [47] |
| Cortex | Rat | 12 months | qRT-PCR | Osmanovic et al., 2010 [48] |
| Hippocampus | Rat (female) | 7–12 weeks | In situ hybridization and IHC | Kuwabara et al., 2011 [37] |
| Cerebellum, cortex, olfactory bulb, hippocampus | Mouse | 3 and 6 months | qRT-PCR | Mehran et al., 2012 [32] |
| Cortex (GABAergic interneurons) | Rat | 3–5 weeks | single-cell digital PCR | Molnár et al., 2012 [19] |
| Hippocampus and cortex | Rat | / | RT-PCR, Western blot and IHC | Nemoto et al., 2014 [49] |
| Hypothalamus | Mouse | 8 weeks | qRT-PCR and IF | Lee et al., 2016 [22] |
| Hypothalamus (PEV) | Rat | 2 months | qRT-PCR, RIA, IHC, IF and Western blot | Dakic et al., 2017 [50] |
| Cortex | Rat | 3–5 weeks | single-cell digital PCR | Csajbók et al., 2019 [20] |
| Choroid plexus | Mouse | / | FISH, IF, qPCR and IHC | Mazucanti et al., 2019 [38] |
| Hypothalamus (PVN) | Mouse | 8–12 weeks | In situ hybridization and IF | Lee et al., 2020 [51] |
| Hypothalamus (PEV) | Rat (female) | 2 months | qRT-PCR, RIA and IHC | Dakic et al., 2022 [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dakic, T.; Jevdjovic, T.; Lakic, I.; Ruzicic, A.; Jasnic, N.; Djurasevic, S.; Djordjevic, J.; Vujovic, P. The Expression of Insulin in the Central Nervous System: What Have We Learned So Far? Int. J. Mol. Sci. 2023, 24, 6586. https://doi.org/10.3390/ijms24076586
Dakic T, Jevdjovic T, Lakic I, Ruzicic A, Jasnic N, Djurasevic S, Djordjevic J, Vujovic P. The Expression of Insulin in the Central Nervous System: What Have We Learned So Far? International Journal of Molecular Sciences. 2023; 24(7):6586. https://doi.org/10.3390/ijms24076586
Chicago/Turabian StyleDakic, Tamara, Tanja Jevdjovic, Iva Lakic, Aleksandra Ruzicic, Nebojsa Jasnic, Sinisa Djurasevic, Jelena Djordjevic, and Predrag Vujovic. 2023. "The Expression of Insulin in the Central Nervous System: What Have We Learned So Far?" International Journal of Molecular Sciences 24, no. 7: 6586. https://doi.org/10.3390/ijms24076586
APA StyleDakic, T., Jevdjovic, T., Lakic, I., Ruzicic, A., Jasnic, N., Djurasevic, S., Djordjevic, J., & Vujovic, P. (2023). The Expression of Insulin in the Central Nervous System: What Have We Learned So Far? International Journal of Molecular Sciences, 24(7), 6586. https://doi.org/10.3390/ijms24076586







