Migratory Response of Cells in Neurogenic Niches to Neuronal Death: The Onset of Harmonic Repair?
Abstract
1. Introduction
2. Neurogenic Niches and Migration
3. Alzheimer’s Disease
4. Parkinson’s Disease
5. Huntington’s Disease
6. Epilepsy and Adult Neurogenesis
7. Neurogenesis in Stroke
8. Neurogenesis in Traumatic Brain Injury
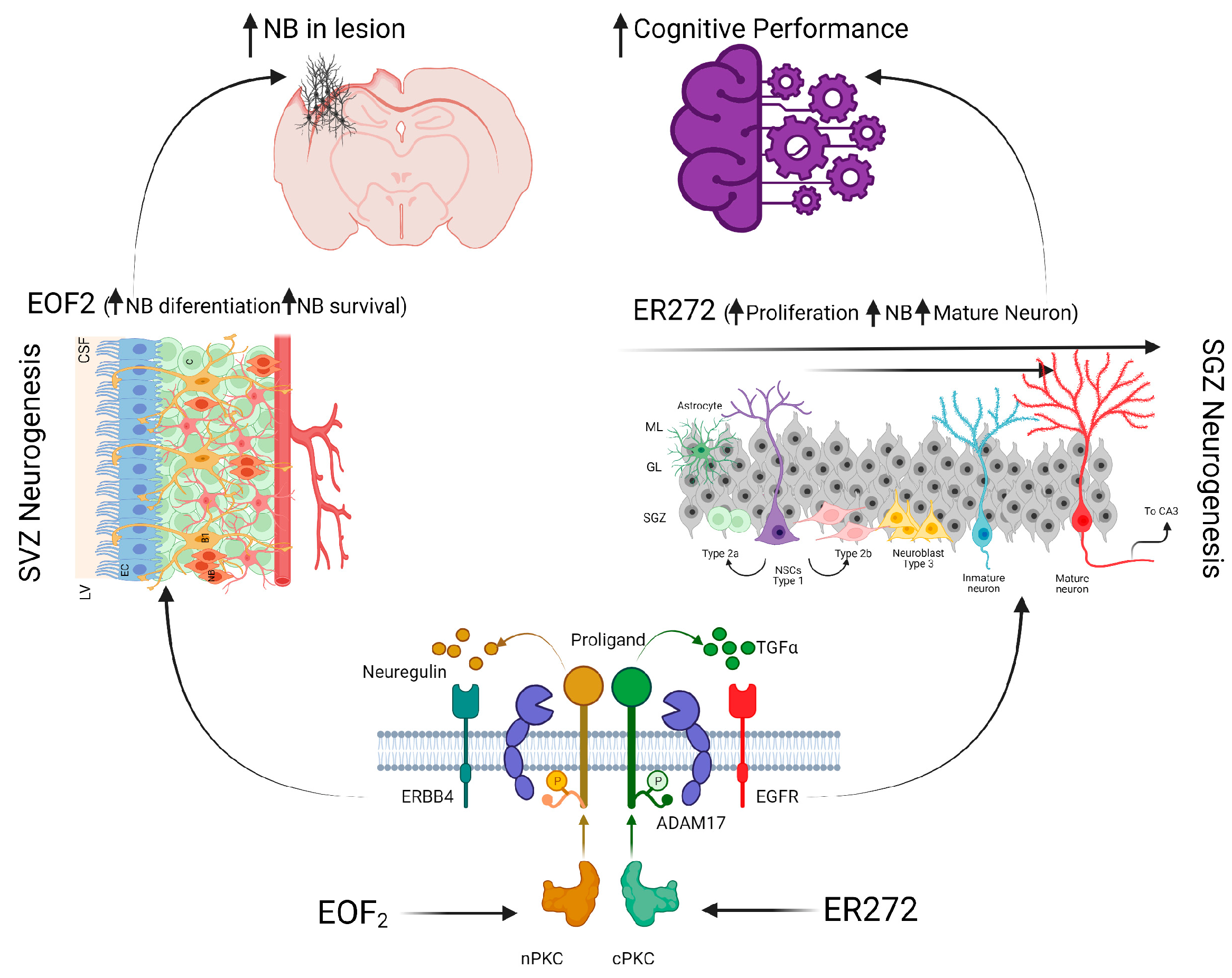
9. Strategies to Promote Neurogenesis in the Damaged Brain Regions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wingo, T.S.; Liu, Y.; Gerasimov, E.S.; Vattathil, S.M.; Wynne, M.E.; Liu, J.; Lori, A.; Faundez, V.; Bennett, D.A.; Seyfried, N.T.; et al. Shared mechanisms across the major psychiatric and neurodegenerative diseases. Nat. Commun. 2022, 13, 4314. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, F.; Zhang, S.; Xin, R.; Sun, Y. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Park. Dis. 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E. The therapeutic importance of understanding mechanisms of neuronal cell death in neurodegenerative disease. Mol. Neurodegener. 2009, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Pardillo-Díaz, R.; Carrascal, L.; Ayala, A.; Nunez-Abades, P. Oxidative stress induced by cumene hydroperoxide evokes changes in neuronal excitability of rat motor cortex neurons. Neuroscience 2015, 289, 85–98. [Google Scholar] [CrossRef]
- Pardillo-Díaz, R.; Pérez-García, P.; Castro, C.; Nunez-Abades, P.; Carrascal, L. Oxidative Stress as a Potential Mechanism Underlying Membrane Hyperexcitability in Neurodegenerative Diseases. Antioxidants 2022, 11, 1511. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.-H.; Zhang, H.; Pang, X.-W.; Chen, L.; Zhou, L.-Q.; Chen, M.; Tian, D.-S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal. Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Tuo, Q.-Z.; Zhang, S.-T.; Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 2022, 42, 259–305. [Google Scholar] [CrossRef]
- Morrison, S.J.; Kimble, J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006, 441, 1068–1074. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef]
- Gould, E.; Vail, N.; Wagers, M.; Gross, C.G. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl. Acad. Sci. USA 2001, 98, 10910–10917. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Abrous, D.N.; Koehl, M.; Le Moal, M. Adult neurogenesis: From precursors to network and physiology. Physiol. Rev. 2005, 85, 523–569. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef]
- Lledo, P.M.; Saghatelyan, A. Integrating new neurons into the adult olfactory bulb: Joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005, 28, 248–254. [Google Scholar] [CrossRef]
- Defterali, C.; Moreno-Estelles, M.; Crespo, C.; Diaz-Guerra, E.; Diaz-Moreno, M.; Vergano-Vera, E.; Nieto-Estevez, V.; Hurtado-Chong, A.; Consiglio, A.; Mira, H.; et al. Neural stem cells in the adult olfactory bulb core generate mature neurons in vivo. Stem Cells 2021, 39, 1253–1269. [Google Scholar] [CrossRef]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Seri, B.; Garcia-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef]
- Butti, E.; Cusimano, M.; Bacigaluppi, M.; Martino, G. Neurogenic and non-neurogenic functions of endogenous neural stem cells. Front. Neurosci. 2014, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Zhou, Y.; Stadel, R.P.; Moss, J.; Yong, J.H.; Ito, S.; Kawasaki, N.K.; Phan, A.T.; Oh, J.H.; Modak, N.; et al. Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 2015, 112, 9484–9489. [Google Scholar] [CrossRef] [PubMed]
- Dayer, A.G.; Cleaver, K.M.; Abouantoun, T.; Cameron, H.A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell. Biol. 2005, 168, 415–427. [Google Scholar] [CrossRef]
- Luzzati, F.; Nato, G.; Oboti, L.; Vigna, E.; Rolando, C.; Armentano, M.; Bonfanti, L.; Fasolo, A.; Peretto, P. Quiescent neuronal progenitors are activated in the juvenile guinea pig lateral striatum and give rise to transient neurons. Development 2014, 141, 4065–4075. [Google Scholar] [CrossRef]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisen, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef]
- Recabal, A.; Caprile, T.; Garcia-Robles, M.L.A. Hypothalamic Neurogenesis as an Adaptive Metabolic Mechanism. Front. Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef]
- Bergmann, O.; Spalding, K.L.; Frisen, J. Adult Neurogenesis in Humans. Cold Spring Harb. Perspect. Biol. 2015, 7, a018994. [Google Scholar] [CrossRef]
- Bergmann, O.; Liebl, J.; Bernard, S.; Alkass, K.; Yeung, M.S.; Steier, P.; Kutschera, W.; Johnson, L.; Landen, M.; Druid, H.; et al. The age of olfactory bulb neurons in humans. Neuron 2012, 74, 634–639. [Google Scholar] [CrossRef]
- Leuner, B.; Kozorovitskiy, Y.; Gross, C.G.; Gould, E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. USA 2007, 104, 17169–17173. [Google Scholar] [CrossRef]
- Ben Abdallah, N.M.; Slomianka, L.; Lipp, H.P. Reversible effect of X-irradiation on proliferation, neurogenesis, and cell death in the dentate gyrus of adult mice. Hippocampus 2007, 17, 1230–1240. [Google Scholar] [CrossRef]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599 e585. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Flor-Garcia, M.; Terreros-Roncal, J.; Moreno-Jimenez, E.P.; Avila, J.; Rabano, A.; Llorens-Martin, M. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 2020, 15, 668–693. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.; Rabaneda, L.G.; Murillo-Carretero, M.; Ortega-Martinez, S.; Martinez-Chantar, M.L.; Woodhoo, A.; Luka, Z.; Wagner, C.; Lu, S.C.; Mato, J.M.; et al. Glycine N-methyltransferase expression in the hippocampus and its role in neurogenesis and cognitive performance. Hippocampus 2014, 24, 840–852. [Google Scholar] [CrossRef]
- Rabaneda, L.G.; Geribaldi-Doldan, N.; Murillo-Carretero, M.; Carrasco, M.; Martinez-Salas, J.M.; Verastegui, C.; Castro, C. Altered regulation of the Spry2/Dyrk1A/PP2A triad by homocysteine impairs neural progenitor cell proliferation. Biochim. Biophys. Acta 2016, 1863, 3015–3026. [Google Scholar] [CrossRef]
- Gomez-Oliva, R.; Geribaldi-Doldan, N.; Dominguez-Garcia, S.; Carrascal, L.; Verastegui, C.; Nunez-Abades, P.; Castro, C. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: A role for Wnt/beta-catenin signaling. Aging (Albany N. Y.) 2020, 12, 13824–13844. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.I.; de la Parra, J.; Pérez-Ruiz, A.; Bravo-Ferrer, I.; Durán-Laforet, V.; García-Culebras, A.; García-Segura, J.M.; Dhaliwal, J.; Frankland, P.W.; Lizasoain, I.; et al. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J. Clin. Investig. 2019, 129, 1536–1550. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Vega, G.; Arias-Gil, G.; López-Fernández, A.; Artegiani, B.; Wasielewska, J.M.; Lee, C.C.; Lippert, M.T.; Kempermann, G.; Takagaki, K.; Calegari, F. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 2020, 11, 135. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jimenez, E.P.; Flor-Garcia, M.; Rodriguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rabano, A.; Llorens-Martin, M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hardy, J.; Zetterberg, H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The Role of SVZ Stem Cells in Glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef]
- Fabel, K.; Kempermann, G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008, 10, 59–66. [Google Scholar] [CrossRef]
- Ding, W.Y.; Huang, J.; Wang, H. Waking up quiescent neural stem cells: Molecular mechanisms and implications in neurodevelopmental disorders. PLoS Genet. 2020, 16, e1008653. [Google Scholar] [CrossRef]
- Fontan-Lozano, A.; Morcuende, S.; Davis-Lopez de Carrizosa, M.A.; Benitez-Temino, B.; Mejias, R.; Matarredona, E.R. To Become or Not to Become Tumorigenic: Subventricular Zone Versus Hippocampal Neural Stem Cells. Front. Oncol. 2020, 10, 602217. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef] [PubMed]
- Young, C.C.; Brooks, K.J.; Buchan, A.M.; Szele, F.G. Cellular and molecular determinants of stroke-induced changes in subventricular zone cell migration. Antioxid. Redox Signal. 2011, 14, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Bressan, C.; Saghatelyan, A. Intrinsic Mechanisms Regulating Neuronal Migration in the Postnatal Brain. Front. Cell. Neurosci. 2020, 14, 620379. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Sawada, M.; Sawamoto, K. Mechanisms of neuronal migration in the adult brain. J. Neurochem. 2017, 141, 835–847. [Google Scholar] [CrossRef]
- Bellion, A.; Baudoin, J.P.; Alvarez, C.; Bornens, M.; Metin, C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: Forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 2005, 25, 5691–5699. [Google Scholar] [CrossRef]
- Schaar, B.T.; McConnell, S.K. Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. USA 2005, 102, 13652–13657. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.W.; Bremner, K.H.; Vallee, R.B. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 2007, 10, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, R.; Thumkeo, D.; Kamijo, H.; Kaneko, N.; Sawamoto, K.; Watanabe, K.; Takebayashi, H.; Kiyonari, H.; Ishizaki, T.; Furuyashiki, T.; et al. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat. Neurosci. 2012, 15, 373–380. [Google Scholar] [CrossRef]
- Lois, C.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Chain migration of neuronal precursors. Science 1996, 271, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Chopp, M.; Gregg, S.R.; Toh, Y.; Roberts, C.; Letourneau, Y.; Buller, B.; Jia, L.; Siamak, P.N.D.; Zhang, Z.G. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J. Cereb. Blood Flow. Metab. 2009, 29, 1240–1250. [Google Scholar] [CrossRef]
- Fujioka, T.; Kaneko, N.; Ajioka, I.; Nakaguchi, K.; Omata, T.; Ohba, H.; Fassler, R.; Garcia-Verdugo, J.M.; Sekiguchi, K.; Matsukawa, N.; et al. beta1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine 2017, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Breunig, J.J.; Haydar, T.F.; Rakic, P. Neural stem cells: Historical perspective and future prospects. Neuron 2011, 70, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Barkho, B.Z.; Munoz, A.E.; Li, X.; Li, L.; Cunningham, L.A.; Zhao, X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 2008, 26, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Geribaldi-Doldan, N.; Carrasco, M.; Murillo-Carretero, M.; Dominguez-Garcia, S.; Garcia-Cozar, F.J.; Munoz-Miranda, J.P.; Del Rio-Garcia, V.; Verastegui, C.; Castro, C. Specific inhibition of ADAM17/TACE promotes neurogenesis in the injured motor cortex. Cell Death Dis. 2018, 9, 862. [Google Scholar] [CrossRef]
- Yagita, Y.; Sakurai, T.; Tanaka, H.; Kitagawa, K.; Colman, D.R.; Shan, W. N-cadherin mediates interaction between precursor cells in the subventricular zone and regulates further differentiation. J. Neurosci. Res. 2009, 87, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Belvindrah, R.; Hankel, S.; Walker, J.; Patton, B.L.; Muller, U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J. Neurosci. 2007, 27, 2704–2717. [Google Scholar] [CrossRef] [PubMed]
- Anton, E.S.; Ghashghaei, H.T.; Weber, J.L.; McCann, C.; Fischer, T.M.; Cheung, I.D.; Gassmann, M.; Messing, A.; Klein, R.; Schwab, M.H.; et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat. Neurosci. 2004, 7, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, H.T.; Weber, J.; Pevny, L.; Schmid, R.; Schwab, M.H.; Lloyd, K.C.; Eisenstat, D.D.; Lai, C.; Anton, E.S. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 2006, 103, 1930–1935. [Google Scholar] [CrossRef]
- Kaneko, N.; Marin, O.; Koike, M.; Hirota, Y.; Uchiyama, Y.; Wu, J.Y.; Lu, Q.; Tessier-Lavigne, M.; Alvarez-Buylla, A.; Okano, H.; et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron 2010, 67, 213–223. [Google Scholar] [CrossRef]
- Snapyan, M.; Lemasson, M.; Brill, M.S.; Blais, M.; Massouh, M.; Ninkovic, J.; Gravel, C.; Berthod, F.; Gotz, M.; Barker, P.A.; et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 2009, 29, 4172–4188. [Google Scholar] [CrossRef]
- Bovetti, S.; Hsieh, Y.C.; Bovolin, P.; Perroteau, I.; Kazunori, T.; Puche, A.C. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 2007, 27, 5976–5980. [Google Scholar] [CrossRef]
- Whitman, M.C.; Fan, W.; Rela, L.; Rodriguez-Gil, D.J.; Greer, C.A. Blood vessels form a migratory scaffold in the rostral migratory stream. J. Comp. Neurol. 2009, 516, 94–104. [Google Scholar] [CrossRef]
- Wittko, I.M.; Schanzer, A.; Kuzmichev, A.; Schneider, F.T.; Shibuya, M.; Raab, S.; Plate, K.H. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J. Neurosci. 2009, 29, 8704–8714. [Google Scholar] [CrossRef] [PubMed]
- Bozoyan, L.; Khlghatyan, J.; Saghatelyan, A. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J. Neurosci. 2012, 32, 1687–1704. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Herranz-Perez, V.; Otsuka, T.; Sano, H.; Ohno, N.; Omata, T.; Nguyen, H.B.; Thai, T.Q.; Nambu, A.; Kawaguchi, Y.; et al. New neurons use Slit-Robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration. Sci. Adv. 2018, 4, eaav0618. [Google Scholar] [CrossRef] [PubMed]
- Ohab, J.J.; Fleming, S.; Blesch, A.; Carmichael, S.T. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006, 26, 13007–13016. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2006, 26, 125–134. [Google Scholar] [CrossRef]
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006, 24, 739–747. [Google Scholar] [CrossRef]
- Grade, S.; Weng, Y.C.; Snapyan, M.; Kriz, J.; Malva, J.O.; Saghatelyan, A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS ONE 2013, 8, e55039. [Google Scholar] [CrossRef]
- Ng, K.L.; Li, J.D.; Cheng, M.Y.; Leslie, F.M.; Lee, A.G.; Zhou, Q.Y. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 2005, 308, 1923–1927. [Google Scholar] [CrossRef]
- Murase, S.; Horwitz, A.F. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J. Neurosci. 2002, 22, 3568–3579. [Google Scholar] [CrossRef]
- Paratcha, G.; Ibanez, C.F.; Ledda, F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Mol. Cell. Neurosci. 2006, 31, 505–514. [Google Scholar] [CrossRef]
- Garzotto, D.; Giacobini, P.; Crepaldi, T.; Fasolo, A.; De Marchis, S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J. Neurosci. 2008, 28, 5901–5909. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Zhang, H.; Gyetko, M.R.; Parent, J.M. Hepatocyte growth factor acts as a mitogen and chemoattractant for postnatal subventricular zone-olfactory bulb neurogenesis. Mol. Cell. Neurosci. 2011, 48, 38–50. [Google Scholar] [CrossRef]
- Yan, Y.P.; Lang, B.T.; Vemuganti, R.; Dempsey, R.J. Osteopontin is a mediator of the lateral migration of neuroblasts from the subventricular zone after focal cerebral ischemia. Neurochem. Int. 2009, 55, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.P.; Sailor, K.A.; Lang, B.T.; Park, S.W.; Vemuganti, R.; Dempsey, R.J. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J. Cereb. Blood Flow. Metab. 2007, 27, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Gotz, M.; Stricker, S.H. Go with the flow: Signaling from the ventricle directs neuroblast migration. Nat. Neurosci. 2006, 9, 470–472. [Google Scholar] [CrossRef]
- Nguyen-Ba-Charvet, K.T.; Picard-Riera, N.; Tessier-Lavigne, M.; Baron-Van Evercooren, A.; Sotelo, C.; Chedotal, A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J. Neurosci. 2004, 24, 1497–1506. [Google Scholar] [CrossRef]
- Hurtado-Chong, A.; Yusta-Boyo, M.J.; Vergano-Vera, E.; Bulfone, A.; de Pablo, F.; Vicario-Abejon, C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur. J. Neurosci. 2009, 30, 742–755. [Google Scholar] [CrossRef]
- Bolteus, A.J.; Bordey, A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J. Neurosci. 2004, 24, 7623–7631. [Google Scholar] [CrossRef]
- Sawamoto, K.; Wichterle, H.; Gonzalez-Perez, O.; Cholfin, J.A.; Yamada, M.; Spassky, N.; Murcia, N.S.; Garcia-Verdugo, J.M.; Marin, O.; Rubenstein, J.L.; et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 2006, 311, 629–632. [Google Scholar] [CrossRef]
- Wu, N.; Sun, X.; Zhou, C.; Yan, J.; Cheng, C. Neuroblasts migration under control of reactive astrocyte-derived BDNF: A promising therapy in late neurogenesis after traumatic brain injury. Stem Cell. Res. 2023, 14, 2. [Google Scholar] [CrossRef]
- Battista, D.; Rutishauser, U. Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues. J. Neurosci. 2010, 30, 3995–4003. [Google Scholar] [CrossRef]
- Collaborators, G.D.F. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Hohman, T.J.; Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Gifford, K.A.; Bush, W.S.; Chibnik, L.B.; Mukherjee, S.; et al. Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurol. 2018, 75, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, N.; Sierra, A.; Valero, J. Rewiring of Memory Circuits: Connecting Adult Newborn Neurons With the Help of Microglia. Front. Cell. Dev. Biol. 2019, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e3. [Google Scholar] [CrossRef]
- Jin, K.; Peel, A.L.; Mao, X.O.; Xie, L.; Cottrell, B.A.; Henshall, D.C.; Greenberg, D.A. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Boekhoorn, K.; Joels, M.; Lucassen, P.J. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 2006, 24, 1–14. [Google Scholar] [CrossRef]
- Moser, M.B.; Moser, E.I.; Forrest, E.; Andersen, P.; Morris, R.G. Spatial learning with a minislab in the dorsal hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 9697–9701. [Google Scholar] [CrossRef]
- Henke, P.G. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res. Bull. 1990, 25, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Valadez, B.; Rabano, A.; Llorens-Martin, M. Progression of Alzheimer’s disease parallels unusual structural plasticity of human dentate granule cells. Acta Neuropathol. Commun. 2022, 10, 125. [Google Scholar] [CrossRef]
- Haughey, N.J.; Nath, A.; Chan, S.L.; Borchard, A.C.; Rao, M.S.; Mattson, M.P. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J. Neurochem. 2002, 83, 1509–1524. [Google Scholar] [CrossRef]
- Ermini, F.V.; Grathwohl, S.; Radde, R.; Yamaguchi, M.; Staufenbiel, M.; Palmer, T.D.; Jucker, M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am. J. Pathol. 2008, 172, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.H.; Yazdani, U.; Norris, R.D.; Games, D.; German, D.C.; Eisch, A.J. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J. Comp. Neurol. 2006, 495, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; de Barreda, E.G.; Dawson, H.N.; Vitek, M.P.; Avila, J.; Hernandez, F. Function of tau protein in adult newborn neurons. FEBS Lett. 2009, 583, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Pallas-Bazarra, N.; Jurado-Arjona, J.; Navarrete, M.; Esteban, J.A.; Hernandez, F.; Avila, J.; Llorens-Martin, M. Novel function of Tau in regulating the effects of external stimuli on adult hippocampal neurogenesis. EMBO J. 2016, 35, 1417–1436. [Google Scholar] [CrossRef]
- Esteve, D.; Molina-Navarro, M.M.; Giraldo, E.; Martinez-Varea, N.; Blanco-Gandia, M.C.; Rodriguez-Arias, M.; Garcia-Verdugo, J.M.; Vina, J.; Lloret, A. Adult Neural Stem Cell Migration Is Impaired in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 1168–1182. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Mortality, G.B.D.; Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Blauwendraat, C.; Heilbron, K.; Vallerga, C.L.; Bandres-Ciga, S.; von Coelln, R.; Pihlstrom, L.; Simon-Sanchez, J.; Schulte, C.; Sharma, M.; Krohn, L.; et al. Parkinson’s disease age at onset genome-wide association study: Defining heritability, genetic loci, and alpha-synuclein mechanisms. Mov. Disord. 2019, 34, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Olanow, C.W.; Greenamyre, J.T.; Bezard, E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 2014, 384, 545–555. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Bezard, E.; Gross, C.E. Compensatory mechanisms in experimental and human parkinsonism: Towards a dynamic approach. Prog. Neurobiol. 1998, 55, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef]
- Boger, H.A.; Granholm, A.C.; McGinty, J.F.; Middaugh, L.D. A dual-hit animal model for age-related parkinsonism. Prog. Neurobiol. 2010, 90, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Sturm, E.; Stefanova, N. Multiple system atrophy: Genetic or epigenetic? Exp. Neurobiol. 2014, 23, 277–291. [Google Scholar] [CrossRef]
- Hope, A.D.; Myhre, R.; Kachergus, J.; Lincoln, S.; Bisceglio, G.; Hulihan, M.; Farrer, M.J. Alpha-synuclein missense and multiplication mutations in autosomal dominant Parkinson’s disease. Neurosci. Lett. 2004, 367, 97–100. [Google Scholar] [CrossRef]
- Salmina, A.B.; Kapkaeva, M.R.; Vetchinova, A.S.; Illarioshkin, S.N. Novel Approaches Used to Examine and Control Neurogenesis in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 9608. [Google Scholar] [CrossRef] [PubMed]
- Novosadova, E.V.; Nenasheva, V.V.; Makarova, I.V.; Dolotov, O.V.; Inozemtseva, L.S.; Arsenyeva, E.L.; Chernyshenko, S.V.; Sultanov, R.I.; Illarioshkin, S.N.; Grivennikov, I.A.; et al. Parkinson’s Disease-Associated Changes in the Expression of Neurotrophic Factors and their Receptors upon Neuronal Differentiation of Human Induced Pluripotent Stem Cells. J. Mol. Neurosci. 2020, 70, 514–521. [Google Scholar] [CrossRef]
- Jellinger, K.A. Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy1. J. Alzheimers Dis. 2018, 62, 1141–1179. [Google Scholar] [CrossRef]
- Arzate, D.M.; Guerra-Crespo, M.; Covarrubias, L. Induction of typical and atypical neurogenesis in the adult substantia nigra after mouse embryonic stem cells transplantation. Neuroscience 2019, 408, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.A.; Baker, K.A.; Hagg, T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004, 20, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Farzanehfar, P. Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci. Res. 2018, 134, 1–9. [Google Scholar] [CrossRef]
- Hermann, A.; Storch, A. Endogenous regeneration in Parkinson’s disease: Do we need orthotopic dopaminergic neurogenesis? Stem Cells 2008, 26, 2749–2752. [Google Scholar] [CrossRef] [PubMed]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Cossetti, C.; D’Adamo, P.; Zardini, E.; Andreoni, L.; Ihekwaba, A.E.; et al. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol. Dis. 2011, 41, 508–527. [Google Scholar] [CrossRef]
- Bender, H.; Fietz, S.A.; Richter, F.; Stanojlovic, M. Alpha-Synuclein Pathology Coincides With Increased Number of Early Stage Neural Progenitors in the Adult Hippocampus. Front. Cell. Dev. Biol. 2021, 9, 691560. [Google Scholar] [CrossRef]
- Farzanehfar, P. Towards a Better Treatment Option for Parkinson’s Disease: A Review of Adult Neurogenesis. Neurochem. Res. 2016, 41, 3161–3170. [Google Scholar] [CrossRef]
- Ermine, C.M.; Wright, J.L.; Frausin, S.; Kauhausen, J.A.; Parish, C.L.; Stanic, D.; Thompson, L.H. Modelling the dopamine and noradrenergic cell loss that occurs in Parkinson’s disease and the impact on hippocampal neurogenesis. Hippocampus 2018, 28, 327–337. [Google Scholar] [CrossRef]
- Aponso, P.M.; Faull, R.L.; Connor, B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience 2008, 151, 1142–1153. [Google Scholar] [CrossRef]
- Brown, S.J.; Boussaad, I.; Jarazo, J.; Fitzgerald, J.C.; Antony, P.; Keatinge, M.; Blechman, J.; Schwamborn, J.C.; Kruger, R.; Placzek, M.; et al. PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Sci. Rep. 2021, 11, 6617. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.K.; Shen, R.; Li, J.; Gao, X.; Bueler, H. Loss of PINK1 leads to metabolic deficits in adult neural stem cells and impedes differentiation of newborn neurons in the mouse hippocampus. FASEB J. 2017, 31, 2839–2853. [Google Scholar] [CrossRef] [PubMed]
- Brodski, C.; Blaess, S.; Partanen, J.; Prakash, N. Crosstalk of Intercellular Signaling Pathways in the Generation of Midbrain Dopaminergic Neurons In Vivo and from Stem Cells. J. Dev. Biol. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Arenas, E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2010, 11, 77–86. [Google Scholar] [CrossRef]
- Maiese, K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen. Res. 2015, 10, 518–528. [Google Scholar] [CrossRef]
- Marchetti, B. Wnt/beta-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3743. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Deleidi, M.; Serapide, M.F.; Pluchino, S.; Marchetti, B. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/beta-catenin signaling pathways: Functional consequences for neuroprotection and repair. J. Neurosci. 2012, 32, 2062–2085. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Impagnatiello, F.; Pluchino, S.; Marchetti, B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/beta-catenin dysregulation. J. Neurosci. 2013, 33, 1462–1485. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Serapide, M.F.; Pluchino, S.; Marchetti, B. Wnt/beta-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson’s disease. Stem Cells 2014, 32, 2147–2163. [Google Scholar] [CrossRef]
- Bjugstad, K.B.; Teng, Y.D.; Redmond, D.E., Jr.; Elsworth, J.D.; Roth, R.H.; Cornelius, S.K.; Sladek, J.R., Jr. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson’s disease. Exp. Neurol. 2008, 211, 362–369. [Google Scholar] [CrossRef]
- Van den Berge, S.A.; van Strien, M.E.; Hol, E.M. Resident adult neural stem cells in Parkinson’s disease—The brain’s own repair system? Eur. J. Pharm. 2013, 719, 117–127. [Google Scholar] [CrossRef]
- Jiang, M.; Tu, H.T.; Zhang, K.; Zhang, W.; Yu, W.P.; Xu, J.; Tan, E.K.; Guo, K.H.; Zeng, L. Impaired neurogenesis in the hippocampus of an adult VPS35 mutant mouse model of Parkinson’s disease through interaction with APP. Neurobiol. Dis. 2021, 153, 105313. [Google Scholar] [CrossRef] [PubMed]
- Gusella, J.F.; Wexler, N.S.; Conneally, P.M.; Naylor, S.L.; Anderson, M.A.; Tanzi, R.E.; Watkins, P.C.; Ottina, K.; Wallace, M.R.; Sakaguchi, A.Y.; et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 1983, 306, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Bates, G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet 2003, 361, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Moily, N.S.; Kota, L.N.; Anjanappa, R.M.; Venugopal, S.; Vaidyanathan, R.; Pal, P.; Purushottam, M.; Jain, S.; Kandasamy, M. Trinucleotide repeats and haplotypes at the huntingtin locus in an Indian sample overlaps with European haplogroup a. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef]
- Bates, G.P. History of genetic disease: The molecular genetics of Huntington disease—A history. Nat. Rev. Genet. 2005, 6, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.A.; Jiang, H.; Ross, C.A. A structure-based analysis of huntingtin mutant polyglutamine aggregation and toxicity: Evidence for a compact beta-sheet structure. Hum. Mol. Genet. 2005, 14, 765–774. [Google Scholar] [CrossRef]
- Graveland, G.A.; Williams, R.S.; DiFiglia, M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science 1985, 227, 770–773. [Google Scholar] [CrossRef]
- Kandasamy, M.; Aigner, L. Reactive Neuroblastosis in Huntington’s Disease: A Putative Therapeutic Target for Striatal Regeneration in the Adult Brain. Front. Cell. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Crowell, V.; Houghton, R.; Tomar, A.; Fernandes, T.; Squitieri, F. Modeling Manifest Huntington’s Disease Prevalence Using Diagnosed Incidence and Survival Time. Neuroepidemiology 2021, 55, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Connor, B.; Faull, R.L. Neurogenesis in the diseased adult human brain—New therapeutic strategies for neurodegenerative diseases. Cell. Cycle 2003, 2, 428–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohl, Z.; Regensburger, M.; Aigner, R.; Kandasamy, M.; Winner, B.; Aigner, L.; Winkler, J. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington’s disease. BMC Neurosci. 2010, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Rosskopf, M.; Wagner, K.; Klein, B.; Couillard-Despres, S.; Reitsamer, H.A.; Stephan, M.; Nguyen, H.P.; Riess, O.; Bogdahn, U.; et al. Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington’s disease is accompanied by striatal invasion of neuroblasts. PLoS ONE 2015, 10, e0116069. [Google Scholar] [CrossRef]
- Kandasamy, M.; Couillard-Despres, S.; Raber, K.A.; Stephan, M.; Lehner, B.; Winner, B.; Kohl, Z.; Rivera, F.J.; Nguyen, H.P.; Riess, O.; et al. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J. Neuropathol. Exp. Neurol. 2010, 69, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.E.; Grote, H.; Armstrong, R.J.; Blakemore, C.; Hannan, A.J.; van Dellen, A.; Barker, R.A. Decreased hippocampal cell proliferation in R6/1 Huntington’s mice. Neuroreport 2004, 15, 811–813. [Google Scholar] [CrossRef]
- Lazic, S.E.; Grote, H.E.; Blakemore, C.; Hannan, A.J.; van Dellen, A.; Phillips, W.; Barker, R.A. Neurogenesis in the R6/1 transgenic mouse model of Huntington’s disease: Effects of environmental enrichment. Eur. J. Neurosci. 2006, 23, 1829–1838. [Google Scholar] [CrossRef]
- Gil, J.M.; Mohapel, P.; Araujo, I.M.; Popovic, N.; Li, J.Y.; Brundin, P.; Petersen, A. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol. Dis. 2005, 20, 744–751. [Google Scholar] [CrossRef]
- Aigner, L.; Bogdahn, U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell. Tissue Res. 2008, 331, 225–241. [Google Scholar] [CrossRef]
- Pitkanen, A.; Lukasiuk, K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009, 14 (Suppl. 1), 16–25. [Google Scholar] [CrossRef]
- Kuruba, R.; Hattiangady, B.; Shetty, A.K. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009, 14 (Suppl. 1), 65–73. [Google Scholar] [CrossRef]
- Cho, K.O.; Lybrand, Z.R.; Ito, N.; Brulet, R.; Tafacory, F.; Zhang, L.; Good, L.; Ure, K.; Kernie, S.G.; Birnbaum, S.G.; et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015, 6, 6606. [Google Scholar] [CrossRef]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef]
- Luo, C.; Koyama, R.; Ikegaya, Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia 2016, 64, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Martin-Suarez, S.; Valcarcel-Martin, R.; Pascual-Brazo, J.; Aelvoet, S.A.; Abiega, O.; Deudero, J.J.; Brewster, A.L.; Bernales, I.; Anderson, A.E.; et al. Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell 2015, 16, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Wu, Y.; Zhou, B. New Insights Into the Role of Aberrant Hippocampal Neurogenesis in Epilepsy. Front. Neurol. 2021, 12, 727065. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Lue, M.Y.; Hsu, K.C.; Fan, H.A. Identification of novel genes that are differentially expressed in human colorectal carcinoma. Biochim. Biophys. Acta 1998, 1407, 200–204. [Google Scholar] [CrossRef]
- Huttmann, K.; Sadgrove, M.; Wallraff, A.; Hinterkeuser, S.; Kirchhoff, F.; Steinhauser, C.; Gray, W.P. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: Functional and immunocytochemical analysis. Eur. J. Neurosci. 2003, 18, 2769–2778. [Google Scholar] [CrossRef]
- Jessberger, S.; Romer, B.; Babu, H.; Kempermann, G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 2005, 196, 342–351. [Google Scholar] [CrossRef]
- Deisseroth, K.; Singla, S.; Toda, H.; Monje, M.; Palmer, T.D.; Malenka, R.C. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 2004, 42, 535–552. [Google Scholar] [CrossRef]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Gotz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef]
- Blumcke, I.; Schewe, J.C.; Normann, S.; Brustle, O.; Schramm, J.; Elger, C.E.; Wiestler, O.D. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus 2001, 11, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Sollas, A.L.; Smith, K.L.; Jackson, M.B.; Goodman, J.H. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J. Comp. Neurol. 2002, 454, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Gray, W.P. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia 2007, 48 (Suppl. 2), 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wang, T.W.; Huang, H.S.; Parent, J.M. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J. Neurosci. 2007, 27, 1803–1811. [Google Scholar] [CrossRef]
- Bielefeld, P.; Dura, I.; Danielewicz, J.; Lucassen, P.J.; Baekelandt, V.; Abrous, D.N.; Encinas, J.M.; Fitzsimons, C.P. Insult-induced aberrant hippocampal neurogenesis: Functional consequences and possible therapeutic strategies. Behav. Brain Res. 2019, 372, 112032. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Goodman, J.H.; Sollas, A.L. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J. Neurosci. 2000, 20, 6144–6158. [Google Scholar] [CrossRef]
- Ribak, C.E.; Tran, P.H.; Spigelman, I.; Okazaki, M.M.; Nadler, J.V. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J. Comp. Neurol. 2000, 428, 240–253. [Google Scholar] [CrossRef]
- Dashtipour, K.; Tran, P.H.; Okazaki, M.M.; Nadler, J.V.; Ribak, C.E. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001, 890, 261–271. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Kim, M.; Jeong, S.W.; Song, Y.M.; Lee, S.T.; Kim, J.Y.; Lee, S.K.; Roh, J.K. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur. J. Neurosci. 2004, 19, 3219–3226. [Google Scholar] [CrossRef]
- Parent, J.M.; Elliott, R.C.; Pleasure, S.J.; Barbaro, N.M.; Lowenstein, D.H. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 2006, 59, 81–91. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Kron, M.M.; Masachs, N.; Zhang, H.; Lagace, D.C.; Martinez, A.; Reillo, I.; Duan, X.; Bosch, C.; Pujadas, L.; et al. Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J. Neurosci. 2012, 32, 12051–12065. [Google Scholar] [CrossRef] [PubMed]
- Pun, R.Y.; Rolle, I.J.; Lasarge, C.L.; Hosford, B.E.; Rosen, J.M.; Uhl, J.D.; Schmeltzer, S.N.; Faulkner, C.; Bronson, S.L.; Murphy, B.L.; et al. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron 2012, 75, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Valentin, V.V.; Lowenstein, D.H. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J. Neurosci. 2002, 22, 3174–3188. [Google Scholar] [CrossRef]
- Jessberger, S.; Parent, J.M. Epilepsy and Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020677. [Google Scholar] [CrossRef]
- Araki, T.; Ikegaya, Y.; Koyama, R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef] [PubMed]
- Lybrand, Z.R.; Goswami, S.; Zhu, J.; Jarzabek, V.; Merlock, N.; Aktar, M.; Smith, C.; Zhang, L.; Varma, P.; Cho, K.O.; et al. A critical period of neuronal activity results in aberrant neurogenesis rewiring hippocampal circuitry in a mouse model of epilepsy. Nat. Commun. 2021, 12, 1423. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Nemes, A.D.; Lee, D.; Ro, E.J.; Zhang, J.; Nowacki, A.S.; Dymecki, S.M.; Najm, I.M.; Suh, H. Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J. Clin. Investig. 2019, 129, 310–323. [Google Scholar] [CrossRef]
- Varma, P.; Brulet, R.; Zhang, L.; Hsieh, J. Targeting Seizure-Induced Neurogenesis in a Clinically Relevant Time Period Leads to Transient But Not Persistent Seizure Reduction. J. Neurosci. 2019, 39, 7019–7028. [Google Scholar] [CrossRef]
- Hattiangady, B.; Rao, M.S.; Shetty, A.K. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol. Dis. 2004, 17, 473–490. [Google Scholar] [CrossRef]
- Bonde, S.; Ekdahl, C.T.; Lindvall, O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur. J. Neurosci. 2006, 23, 965–974. [Google Scholar] [CrossRef]
- Pineda, J.R.; Encinas, J.M. The Contradictory Effects of Neuronal Hyperexcitation on Adult Hippocampal Neurogenesis. Front. Neurosci. 2016, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Kralic, J.E.; Ledergerber, D.A.; Fritschy, J.M. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur. J. Neurosci. 2005, 22, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Hattiangady, B.; Shetty, A.K. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus 2010, 20, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Muro-Garcia, T.; Martin-Suarez, S.; Espinosa, N.; Valcarcel-Martin, R.; Marinas, A.; Zaldumbide, L.; Galbarriatu, L.; Sierra, A.; Fuentealba, P.; Encinas, J.M. Reactive Disruption of the Hippocampal Neurogenic Niche After Induction of Seizures by Injection of Kainic Acid in the Amygdala. Front. Cell. Dev. Biol. 2019, 7, 158. [Google Scholar] [CrossRef]
- Mathern, G.W.; Leiphart, J.L.; De Vera, A.; Adelson, P.D.; Seki, T.; Neder, L.; Leite, J.P. Seizures decrease postnatal neurogenesis and granule cell development in the human fascia dentata. Epilepsia 2002, 43 (Suppl. 5), 68–73. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, A.; Kann, G.; Flubacher, A.; Heinrich, C.; Freiman, T.M.; Zentner, J.; Frotscher, M.; Haas, C.A. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp. Neurol. 2007, 203, 320–332. [Google Scholar] [CrossRef]
- Ammothumkandy, A.; Ravina, K.; Wolseley, V.; Tartt, A.N.; Yu, P.N.; Corona, L.; Zhang, N.; Nune, G.; Kalayjian, L.; Mann, J.J.; et al. Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nat. Neurosci. 2022, 25, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Martin-Suarez, S.; Abiega, O.; Ricobaraza, A.; Hernandez-Alcoceba, R.; Encinas, J.M. Alterations of the Hippocampal Neurogenic Niche in a Mouse Model of Dravet Syndrome. Front. Cell. Dev. Biol. 2020, 8, 654. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 2016, 94, 634A. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T.; Molnar, Z.; Nakano-Doi, A.; Taguchi, A.; Saino, O.; Kubo, S.; Clausen, M.; Yoshikawa, H.; Nakagomi, N.; Matsuyama, T. Ischemia-induced neural stem/progenitor cells in the pia mater following cortical infarction. Stem Cells Dev. 2011, 20, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.S.; Washington, P.M.; Kernie, S.G. Injury-Induced Neurogenesis: Mechanisms and Relevance. Neuroscientist 2016, 22, 61–71. [Google Scholar] [CrossRef]
- Rahman, A.A.; Amruta, N.; Pinteaux, E.; Bix, G.J. Neurogenesis After Stroke: A Therapeutic Perspective. Transl. Stroke Res. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Jones, T.A.; Adkins, D.L. Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection. Physiology 2015, 30, 358–370. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Hirota, Y.; Ema, M.; Takahashi, S.; Miyoshi, I.; Okano, H.; Sawamoto, K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 2010, 28, 545–554. [Google Scholar] [CrossRef]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef]
- Komitova, M.; Mattsson, B.; Johansson, B.B.; Eriksson, P.S. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke 2005, 36, 1278–1282. [Google Scholar] [CrossRef]
- Marlier, Q.; Verteneuil, S.; Vandenbosch, R.; Malgrange, B. Mechanisms and Functional Significance of Stroke-Induced Neurogenesis. Front. Neurosci. 2015, 9, 458. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, Z.G.; Lu, M.; Wang, Y.; Yang, J.J.; Chopp, M. Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J. Cereb. Blood Flow. Metab. 2006, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Zhang, Z.G.; Roberts, C.; LeTourneau, Y.; Lu, M.; Zhang, L.; Wang, Y.; Chopp, M. Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J. Cereb. Blood Flow. Metab. 2008, 28, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Zhang, Z.G.; Chopp, M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology 2008, 55, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lichtenwalner, R.J.; Parent, J.M. Adult neurogenesis and the ischemic forebrain. J. Cereb. Blood Flow. Metab. 2006, 26, 1–20. [Google Scholar] [CrossRef]
- Woitke, F.; Ceanga, M.; Rudolph, M.; Niv, F.; Witte, O.W.; Redecker, C.; Kunze, A.; Keiner, S. Adult hippocampal neurogenesis poststroke: More new granule cells but aberrant morphology and impaired spatial memory. PLoS ONE 2017, 12, e0183463. [Google Scholar] [CrossRef]
- Niv, F.; Keiner, S.; Krishna; Witte, O.W.; Lie, D.C.; Redecker, C. Aberrant neurogenesis after stroke: A retroviral cell labeling study. Stroke 2012, 43, 2468–2475. [Google Scholar] [CrossRef]
- Faiz, M.; Sachewsky, N.; Gascon, S.; Bang, K.W.; Morshead, C.M.; Nagy, A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell 2015, 17, 624–634. [Google Scholar] [CrossRef]
- He, T.; Yang, G.Y.; Zhang, Z. Crosstalk of Astrocytes and Other Cells during Ischemic Stroke. Life 2022, 12, 910. [Google Scholar] [CrossRef]
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke. Int. J. Mol. Sci. 2021, 22, 4280. [Google Scholar] [CrossRef]
- Wang, L.; Chopp, M.; Zhang, R.L.; Zhang, L.; Letourneau, Y.; Feng, Y.F.; Jiang, A.; Morris, D.C.; Zhang, Z.G. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 2009, 158, 1356–1363. [Google Scholar] [CrossRef]
- Carlen, M.; Meletis, K.; Goritz, C.; Darsalia, V.; Evergren, E.; Tanigaki, K.; Amendola, M.; Barnabe-Heider, F.; Yeung, M.S.; Naldini, L.; et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 2009, 12, 259–267. [Google Scholar] [CrossRef]
- Xiao, M.J.; Han, Z.; Shao, B.; Jin, K. Notch signaling and neurogenesis in normal and stroke brain. Int. J. Physiol. Pathophysiol. Pharm. 2009, 1, 192–202. [Google Scholar]
- Li, Z.; Wang, J.; Zhao, C.; Ren, K.; Xia, Z.; Yu, H.; Jiang, K. Acute Blockage of Notch Signaling by DAPT Induces Neuroprotection and Neurogenesis in the Neonatal Rat Brain After Stroke. Transl. Stroke Res. 2016, 7, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Santopolo, G.; Magnusson, J.P.; Lindvall, O.; Kokaia, Z.; Frisen, J. Blocking Notch-Signaling Increases Neurogenesis in the Striatum after Stroke. Cells 2020, 9, 1732. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Urrutia, P.; Carrasco, C.M.; Lois, P.; Palma, V.; Roth, A.D. Shh Signaling through the Primary Cilium Modulates Rat Oligodendrocyte Differentiation. PLoS ONE 2015, 10, e0133567. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, B.; Xiong, X.; Xia, Z. The Neuroprotective Roles of Sonic Hedgehog Signaling Pathway in Ischemic Stroke. Neurochem. Res. 2018, 43, 2199–2211. [Google Scholar] [CrossRef]
- Dai, R.L.; Zhu, S.Y.; Xia, Y.P.; Mao, L.; Mei, Y.W.; Yao, Y.F.; Xue, Y.M.; Hu, B. Sonic hedgehog protects cortical neurons against oxidative stress. Neurochem. Res. 2011, 36, 67–75. [Google Scholar] [CrossRef]
- Jin, Y.; Raviv, N.; Barnett, A.; Bambakidis, N.C.; Filichia, E.; Luo, Y. The shh signaling pathway is upregulated in multiple cell types in cortical ischemia and influences the outcome of stroke in an animal model. PLoS ONE 2015, 10, e0124657. [Google Scholar] [CrossRef]
- Macas, J.; Nern, C.; Plate, K.H.; Momma, S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J. Neurosci. 2006, 26, 13114–13119. [Google Scholar] [CrossRef]
- Carroll, L.J.; Cassidy, J.D.; Holm, L.; Kraus, J.; Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 43, 113–125. [Google Scholar] [CrossRef]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I. Traumatic brain injury in India: A big problem in need of data. Neurol. India 2017, 65, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. What is the Relationship of Traumatic Brain Injury to Dementia? J. Alzheimers Dis. 2017, 57, 667–681. [Google Scholar] [CrossRef] [PubMed]
- van Gils, A.; Stone, J.; Welch, K.; Davidson, L.R.; Kerslake, D.; Caesar, D.; McWhirter, L.; Carson, A. Management of mild traumatic brain injury. Pr. Neurol. 2020, 20, 213–221. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef]
- Rosenfeld, J.V.; Maas, A.I.; Bragge, P.; Morganti-Kossmann, M.C.; Manley, G.T.; Gruen, R.L. Early management of severe traumatic brain injury. Lancet 2012, 380, 1088–1098. [Google Scholar] [CrossRef]
- Badner, A.; Cummings, B.J. The endogenous progenitor response following traumatic brain injury: A target for cell therapy paradigms. Neural Regen. Res. 2022, 17, 2351–2354. [Google Scholar] [CrossRef]
- Sun, D. Endogenous neurogenic cell response in the mature mammalian brain following traumatic injury. Exp. Neurol. 2016, 275, 405–410. [Google Scholar] [CrossRef]
- Fallon, J.; Reid, S.; Kinyamu, R.; Opole, I.; Opole, R.; Baratta, J.; Korc, M.; Endo, T.L.; Duong, A.; Nguyen, G.; et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 2000, 97, 14686–14691. [Google Scholar] [CrossRef]
- Jin, K.; Minami, M.; Lan, J.Q.; Mao, X.O.; Batteur, S.; Simon, R.P.; Greenberg, D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 2001, 98, 4710–4715. [Google Scholar] [CrossRef]
- Liu, J.; Solway, K.; Messing, R.O.; Sharp, F.R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998, 18, 7768–7778. [Google Scholar] [CrossRef] [PubMed]
- Romero-Grimaldi, C.; Murillo-Carretero, M.; Lopez-Toledano, M.A.; Carrasco, M.; Castro, C.; Estrada, C. ADAM-17/tumor necrosis factor-alpha-converting enzyme inhibits neurogenesis and promotes gliogenesis from neural stem cells. Stem Cells 2011, 29, 1628–1639. [Google Scholar] [CrossRef]
- Buffo, A.; Rite, I.; Tripathi, P.; Lepier, A.; Colak, D.; Horn, A.P.; Mori, T.; Gotz, M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA 2008, 105, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Garcia, S.; Geribaldi-Doldan, N.; Gomez-Oliva, R.; Ruiz, F.A.; Carrascal, L.; Bolivar, J.; Verastegui, C.; Garcia-Alloza, M.; Macias-Sanchez, A.J.; Hernandez-Galan, R.; et al. A novel PKC activating molecule promotes neuroblast differentiation and delivery of newborn neurons in brain injuries. Cell. Death Dis. 2020, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Villasana, L.E.; Kim, K.N.; Westbrook, G.L.; Schnell, E. Functional Integration of Adult-Born Hippocampal Neurons after Traumatic Brain Injury(1,2,3). eNeuro 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Blaiss, C.A.; Yu, T.S.; Zhang, G.; Chen, J.; Dimchev, G.; Parada, L.F.; Powell, C.M.; Kernie, S.G. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 2011, 31, 4906–4916. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.M.; Sun, D.; Bullock, M.R. Neurogenesis after traumatic brain injury. Neurosurg. Clin. N. Am. 2007, 18, 169–181. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J. Moderate traumatic brain injury promotes neural precursor proliferation without increasing neurogenesis in the adult hippocampus. Exp. Neurol. 2013, 239, 38–48. [Google Scholar] [CrossRef]
- Ortiz-Lopez, L.; Vega-Rivera, N.M.; Babu, H.; Ramirez-Rodriguez, G.B. Brain-Derived Neurotrophic Factor Induces Cell Survival and the Migration of Murine Adult Hippocampal Precursor Cells During Differentiation In Vitro. Neurotox. Res. 2017, 31, 122–135. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, H.L.; Gao, Y.; Zhang, F.; Ding, Y.Q.; Huang, Z.H. Brain-derived Neurotrophic Factor Promotes the Migration of Olfactory Ensheathing Cells Through TRPC Channels. Glia 2016, 64, 2154–2165. [Google Scholar] [CrossRef]
- Carabalona, A.; Hu, D.J.; Vallee, R.B. KIF1A inhibition immortalizes brain stem cells but blocks BDNF-mediated neuronal migration. Nat. Neurosci. 2016, 19, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Oudin, M.J.; Gajendra, S.; Sonego, M.; Falenta, K.; Williams, G.; Lalli, G.; Doherty, P. Regional effects of endocannabinoid, BDNF and FGF receptor signalling on neuroblast motility and guidance along the rostral migratory stream. Mol. Cell. Neurosci. 2015, 64, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, S.; Marei, O.; Elalfy, O.; Zaben, M. Neurogenesis after traumatic brain injury—The complex role of HMGB1 and neuroinflammation. Neuropharmacology 2021, 183, 108400. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; Schafer, K.; Hollt, V. BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J. Neurotrauma 2002, 19, 975–983. [Google Scholar] [CrossRef]
- Itoh, T.; Satou, T.; Hashimoto, S.; Ito, H. Isolation of neural stem cells from damaged rat cerebral cortex after traumatic brain injury. Neuroreport 2005, 16, 1687–1691. [Google Scholar] [CrossRef]
- Grade, S.; Götz, M. Neuronal replacement therapy: Previous achievements and challenges ahead. NPJ Regen. Med. 2017, 2, 29. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. Regenerative stem cell therapy for neurodegenerative diseases: An overview. Int. J. Mol. Sci. 2021, 22, 2153. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef]
- Niu, W.; Zang, T.; Smith, D.K.; Vue, T.Y.; Zou, Y.; Bachoo, R.; Johnson, J.E.; Zhang, C.L. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 2015, 4, 780–794. [Google Scholar] [CrossRef]
- Fon, D.; Al-Abboodi, A.; Chan, P.P.; Zhou, K.; Crack, P.; Finkelstein, D.I.; Forsythe, J.S. Effects of GDNF-loaded injectable gelatin-based hydrogels on endogenous neural progenitor cell migration. Adv. Heal. Mater. 2014, 3, 761–774. [Google Scholar] [CrossRef]
- Fon, D.; Zhou, K.; Ercole, F.; Fehr, F.; Marchesan, S.; Minter, M.R.; Crack, P.J.; Finkelstein, D.I.; Forsythe, J.S. Nanofibrous scaffolds releasing a small molecule BDNF-mimetic for the re-direction of endogenous neuroblast migration in the brain. Biomaterials 2014, 35, 2692–2712. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; Llorente, I.L.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow. Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Nakaguchi, K.; Jinnou, H.; Kaneko, N.; Sawada, M.; Hikita, T.; Saitoh, S.; Tabata, Y.; Sawamoto, K. Growth factors released from gelatin hydrogel microspheres increase new neurons in the adult mouse brain. Stem Cells Int. 2012, 2012, 915160. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ahlenius, H.; Thored, P.; Kobayashi, R.; Kokaia, Z.; Lindvall, O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 2006, 37, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Qiu, J.; Plumier, J.C.; Moskowitz, M.A. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J. Clin. Investig. 2003, 111, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef]
- Filippo, T.R.; Galindo, L.T.; Barnabe, G.F.; Ariza, C.B.; Mello, L.E.; Juliano, M.A.; Juliano, L.; Porcionatto, M.A. CXCL12 N-terminal end is sufficient to induce chemotaxis and proliferation of neural stem/progenitor cells. Stem Cell Res. 2013, 11, 913–925. [Google Scholar] [CrossRef]
- Lim, D.A.; Tramontin, A.D.; Fau–Trevejo, J.M.; Herrera, D.G.; García-Verdugo, J.M.; Alvarez-Buylla, A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 2000, 28, 713–726. [Google Scholar] [CrossRef]
- Tang, J.; Song, M.; Wang, Y.; Fan, X.; Xu, H.; Bai, Y. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APP(swe)/PS1(DeltaE9) transgenic mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2009, 385, 341–345. [Google Scholar] [CrossRef]
- Zhang, C.; Chopp, M.; Cui, Y.; Wang, L.; Zhang, R.; Zhang, L.; Lu, M.; Szalad, A.; Doppler, E.; Hitzl, M.; et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J. Neurosci. Res. 2010, 88, 3275–3281. [Google Scholar] [CrossRef]
- Cui, X.; Chen, J.; Zacharek, A.; Roberts, C.; Yang, Y.; Chopp, M. Nitric oxide donor up-regulation of SDF1/CXCR4 and Ang1/Tie2 promotes neuroblast cell migration after stroke. J. Neurosci. Res. 2009, 87, 86–95. [Google Scholar] [CrossRef]
- Calio, M.L.; Mosini, A.C.; Marinho, D.S.; Salles, G.N.; Massinhani, F.H.; Ko, G.M.; Porcionatto, M.A. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105219. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Ren, C.; Xing, L.; Guan, L.; Zhang, C.; Sun, X.; Wang, G.; Niu, H.; Qun, S. Ginkgo biloba extract improves cognitive function and increases neurogenesis by reducing Abeta pathology in 5xFAD mice. Am. J. Transl. Res. 2021, 13, 1471–1482. [Google Scholar] [PubMed]
- Han, J.; Pollak, J.; Yang, T.; Siddiqui, M.R.; Doyle, K.P.; Taravosh-Lahn, K.; Cekanaviciute, E.; Han, A.; Goodman, J.Z.; Jones, B.; et al. Delayed administration of a small molecule tropomyosin-related kinase B ligand promotes recovery after hypoxic-ischemic stroke. Stroke 2012, 43, 1918–1924. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Liu, Z.R.; Yang, J.J.; Gu, X.; Wei, Z.Z.; Liu, L.P.; Yu, S.P. Pyruvate Kinase M2 Increases Angiogenesis, Neurogenesis, and Functional Recovery Mediated by Upregulation of STAT3 and Focal Adhesion Kinase Activities After Ischemic Stroke in Adult Mice. Neurotherapeutics 2018, 15, 770–784. [Google Scholar] [CrossRef]
- Dominguez-Garcia, S.; Gomez-Oliva, R.; Geribaldi-Doldan, N.; Hierro-Bujalance, C.; Sendra, M.; Ruiz, F.A.; Carrascal, L.; Macias-Sanchez, A.J.; Verastegui, C.; Hernandez-Galan, R.; et al. Effects of classical PKC activation on hippocampal neurogenesis and cognitive performance: Mechanism of action. Neuropsychopharmacology 2021, 46, 1207–1219. [Google Scholar] [CrossRef]
- Geribaldi-Doldan, N.; Gomez-Oliva, R.; Dominguez-Garcia, S.; Nunez-Abades, P.; Castro, C. Protein Kinase C: Targets to Regenerate Brain Injuries? Front. Cell. Dev. Biol. 2019, 7, 39. [Google Scholar] [CrossRef]
- Garcia-Bernal, F.; Geribaldi-Doldan, N.; Dominguez-Garcia, S.; Carrasco, M.; Murillo-Carretero, M.; Delgado-Ariza, A.; Diez-Salguero, M.; Verastegui, C.; Castro, C. Protein Kinase C Inhibition Mediates Neuroblast Enrichment in Mechanical Brain Injuries. Front. Cell. Neurosci. 2018, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Geribaldi-Doldan, N.; Flores-Giubi, E.; Murillo-Carretero, M.; Garcia-Bernal, F.; Carrasco, M.; Macias-Sanchez, A.J.; Dominguez-Riscart, J.; Verastegui, C.; Hernandez-Galan, R.; Castro, C. 12-Deoxyphorbols Promote Adult Neurogenesis by Inducing Neural Progenitor Cell Proliferation via PKC Activation. Int. J. Neuropsychopharmacol. 2015, 19, pyv085. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Carretero, M.; Geribaldi-Doldan, N.; Flores-Giubi, E.; Garcia-Bernal, F.; Navarro-Quiroz, E.A.; Carrasco, M.; Macias-Sanchez, A.J.; Herrero-Foncubierta, P.; Delgado-Ariza, A.; Verastegui, C.; et al. ELAC (3,12-di-O-acetyl-8-O-tigloilingol), a plant-derived lathyrane diterpene, induces subventricular zone neural progenitor cell proliferation through PKCbeta activation. Br. J. Pharm. 2017, 174, 2373–2392. [Google Scholar] [CrossRef]
- Gómez-Oliva, R.; Martínez-Ortega, S.; Atienza-Navarro, I.; Domínguez-García, S.; Bernal, C.; Geribaldi-Doldán, N.; Verástegui, C.; Ezzanad, A.; Hernández-Galán, R.; Núnez-Abades, P.; et al. Rescue of neurogenesis and age-associated cognitive decline in SAMP8 mouse: Role of transforming growth factor alpha. bioRxiv 2023. [Google Scholar] [CrossRef]

| Molecule | Brain Location | Secretor | Receptor | Role | References |
|---|---|---|---|---|---|
| Prokineticin-2 (PK2) | OB | Granular and periglomerular layers of the OB | PRK1/PRK2 | Chemoattractant | Ng et al., 2005 [78]. |
| Netrin-1 | OB and RMS | Mitral cells (OB) and astrocytes (RMS) | Deleted in colorectal cancer (DCC) | Chemoattractant | Murase and Horwitz., 2002 [79]. |
| Glial cell line-derived neurotrophic factor (GDNF) | OB and RMS | OB cells | NCAM | Chemoattractant | Paratcha et al., 2006 [80]. |
| Hepatocyte growth factor (HGF) | SVZ and RMS | Astrocytes | Met | Chemoattractant (restricts cells to the RMS) | Garzotto et al., 2008 [81]; Wang et al., 2001 [82]. |
| Monocyte chemoattractant protein-1 (MCP-1) | Injury (focal ischemia) | Injury-activated microglia and astrocytes | CCR2 | Chemoattractant | Yan et al., 2007 [84]. |
| Osteopontin (OPN) | Injury (focal ischemia) | Activated microglia and macrophages | Beta1-integrins | Chemoattractant | Yan et al., 2009 [83]. |
| Slit1 | Septum, RMS and SVZ | Type A and C cells, migrating neuroblast (SVZ, RMS) | Robo2, Robo3/Rig-1 | Repellent | Nguyen-Ba-Charvet et al., 2004 [86]. |
| RMS | Migrating neurons | Robo2 (astrocytes) | Modifies glial tube thus promoting migration | Kaneko et al., 2010 [67]. | |
| Injured striatum (stroke) | Migrating neurons | Robo2 (astrocytes) | Promotes migration through astrocytes | Kaneko et al., 2018 [73]. | |
| Slit2 | Septum | Undefined | Robo2, Robo3/Rig-1 | Repellent | Nguyen-Ba-Charvet et al., 2004 [86]. |
| CSF of LV, creating a gradient with lower concentration at rostral position | Choroid plexus | Robo2 | Repellent | Sawamoto et al., 2006 [89]. | |
| Insulin growth factor 1 (IGF-1) | SVZ | Choroid plexus | IGF-IR | Repellent | Hurtado-Chong et al., 2009 [87]. |
| GABA | Anterior SVZ and RMS | Neuroblast | GABAAR | Reduce migration rate | Bolteus and Bordey, 2004 [88]. |
| Vascular endothelial growth factor (VEGF) | SVZ and RMS | Glial cells | VEGFR-2 | Migration along blood vessels | Wittko et al., 2009 [71]; Bozoyan et al., 2012 [72]. |
| Stromal-derived factor 1 (SDF-1) | Injury (stroke) | Stroke-activated cerebral endothelial cells | CXCR4 | Migration along blood vessels | Ohab et al., 2006 [74]; Robin et al., 2006 [75]. |
| Injury (stroke) | Reactive astrocytes and activates microglia | CXCR4 | Migration along blood vessels | Thored et al., 2006 [76]. | |
| Angiopoietin 1 (Ang1) | Injury (stroke) | Stroke-activated cerebral endothelial cells | Tie2 | Migration along blood vessels | Ohab et al., 2006 [74]. |
| Brain-derived neurotrophic factor (BDNF) | OB, RMS | Vascular endothelial cells | p75NTR | Migration along blood vessels | Snapyan et al., 2009 [68]. |
| Injury (stroke) | Endothelial cells | p75NTR | Migration along blood vessels | Grade et al., 2013 [77]. | |
| Injury (TBI) | Reactive astrocytes | - | Chemoattractant | Wu et al., 2023 [90]. | |
| Polyasilated form of neural cell adhesion molecule (PSA-NCAM) | SVZ and RMS | Migrating neuroblast | NCAM | Migration by creating neuroblast chains, maintenance of RMS structure | Battista and Rutishauer., 2010 [91]. |
| Laminin | RMS | ECM | Beta1-integrins | Migration by creating neuroblast chains | Belvindrah et al., 2007 [64]. |
| Blood vessels to injury (stroke) | ECM | Beta1-integrins | Migration by creating neuroblast chains along blood vessels | Fujioka et al., 2017 [59]. | |
| Neuregulin 1 (NRG1) | RMS | Undefined | ErbB4 | Permissive guidance cue, chemotropic agent or mitogen | Anton et al., 2004 [65]. |
| SVZ | Neuroblast | ErbB4 | Chemoattractant/Prevents neuroblast migration toward OB leading cells toward the source of NRG1 | Ghashghaei et al., 2005 [66]. | |
| Neuregulin 2 (NRG2) | SVZ | Neuroblast | ErbB4 | Chemoattractant. Attracts neuroblasts to the OB | Ghashghaei et al., 2005 [66]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geribaldi-Doldán, N.; Carrascal, L.; Pérez-García, P.; Oliva-Montero, J.M.; Pardillo-Díaz, R.; Domínguez-García, S.; Bernal-Utrera, C.; Gómez-Oliva, R.; Martínez-Ortega, S.; Verástegui, C.; et al. Migratory Response of Cells in Neurogenic Niches to Neuronal Death: The Onset of Harmonic Repair? Int. J. Mol. Sci. 2023, 24, 6587. https://doi.org/10.3390/ijms24076587
Geribaldi-Doldán N, Carrascal L, Pérez-García P, Oliva-Montero JM, Pardillo-Díaz R, Domínguez-García S, Bernal-Utrera C, Gómez-Oliva R, Martínez-Ortega S, Verástegui C, et al. Migratory Response of Cells in Neurogenic Niches to Neuronal Death: The Onset of Harmonic Repair? International Journal of Molecular Sciences. 2023; 24(7):6587. https://doi.org/10.3390/ijms24076587
Chicago/Turabian StyleGeribaldi-Doldán, Noelia, Livia Carrascal, Patricia Pérez-García, José M. Oliva-Montero, Ricardo Pardillo-Díaz, Samuel Domínguez-García, Carlos Bernal-Utrera, Ricardo Gómez-Oliva, Sergio Martínez-Ortega, Cristina Verástegui, and et al. 2023. "Migratory Response of Cells in Neurogenic Niches to Neuronal Death: The Onset of Harmonic Repair?" International Journal of Molecular Sciences 24, no. 7: 6587. https://doi.org/10.3390/ijms24076587
APA StyleGeribaldi-Doldán, N., Carrascal, L., Pérez-García, P., Oliva-Montero, J. M., Pardillo-Díaz, R., Domínguez-García, S., Bernal-Utrera, C., Gómez-Oliva, R., Martínez-Ortega, S., Verástegui, C., Nunez-Abades, P., & Castro, C. (2023). Migratory Response of Cells in Neurogenic Niches to Neuronal Death: The Onset of Harmonic Repair? International Journal of Molecular Sciences, 24(7), 6587. https://doi.org/10.3390/ijms24076587










