Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient’s Loss of Consciousness
Abstract
1. Introduction
2. Summary of Case Report
3. Discussion
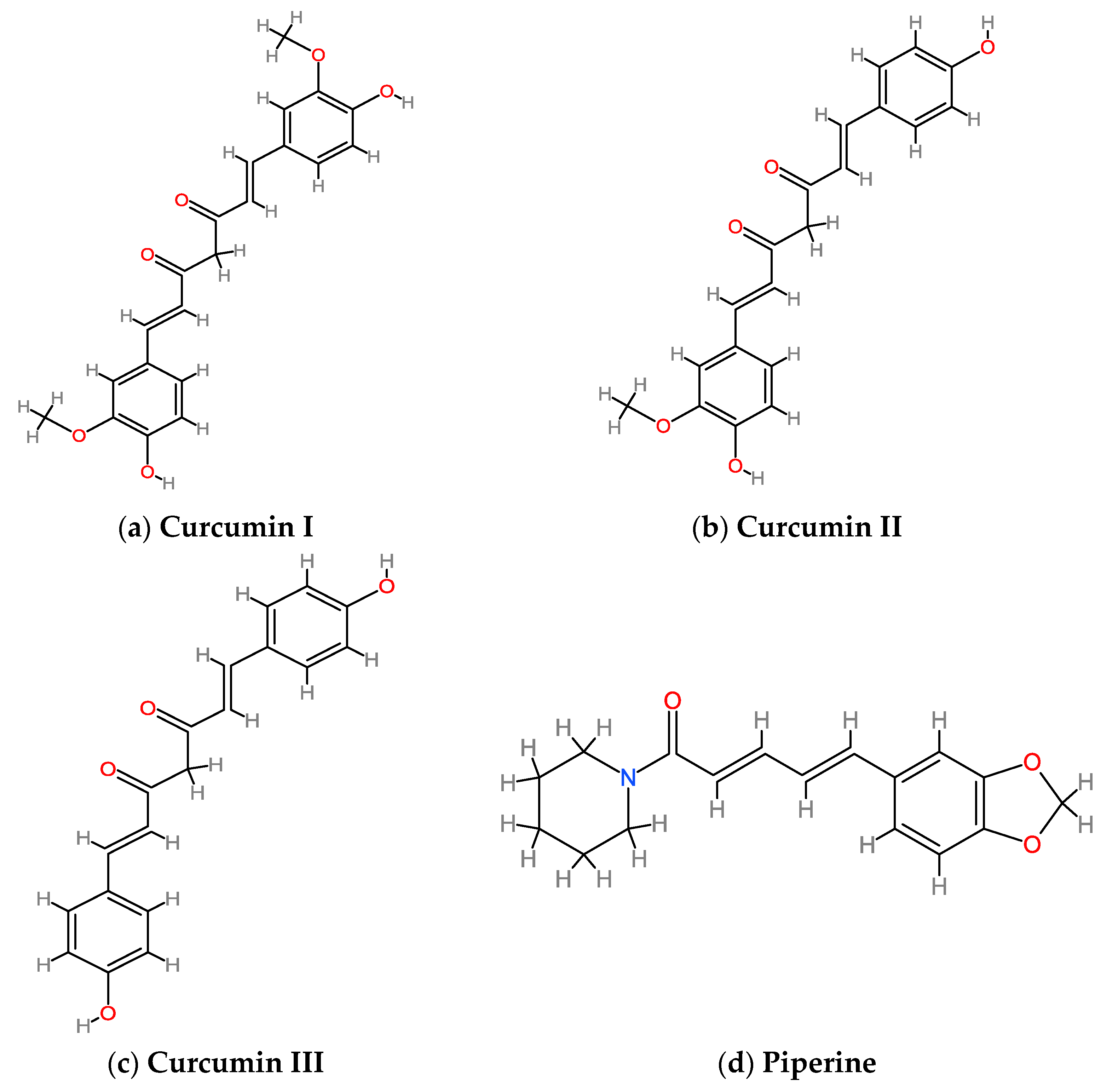
3.1. Curcumin and Piperine: Structure, Metabolism, Bioavailability and Safety
3.2. Curcumin: Mechanism of Action on Insulin Secretion/Activity and Glycemic Homeostasis
3.2.1. In Vitro Studies
3.2.2. In Vivo Animal and Human Studies
3.3. Piperine: Mechanism of Action on Insulin Secretion/Activity and Glycemic Homeostasis
3.3.1. In Vitro Studies
3.3.2. In Vivo Animal and Human Studies
3.4. Curcumin and Piperine Combined: Mechanism of Action on Insulin Secretion/Activity and Glycemic Homeostasis
In Vitro and In Vivo Studies
3.5. TLOC and Curcumin plus Piperine Supplementation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Curcumin (CUR) | Piperine (PIP) | Complex Curcumin + Piperine (CUR + PIP) | |
|---|---|---|---|
| Bioavailability and pharmacokinetics | Low bioavailability Poor absorption [2] | Low bioavailability Poor absorption [18]
| PIP causes higher adsorption, plasma concentration of CUR [2,4,28,44,51,60,61,62,72,73] |
| ↑ bioavailability in formulations, including micelles, nanoparticles, nano-emulsion liposomes, phospholipid complexes [48], β-casein-micelles [49], phytosomal formulations [50] | Excreted in urine and bile in form of metabolites [18,65] | ↑ PIP reduces CUR glucuronidation and sulfation (red UGTs and SULTs activity) [75] | |
| ↑ bioavailability with fresh or dried powder compared to supplements due to food matrix [53] | ↑ bioavailability in the formulation including liposomes, polymeric micelles, nanoparticles, nanofibers and solid-lipid nanoparticles. [18,65] | ↑ PIP enhances enzymes for the intracellular transport of nutrients (γ-glutamil transpeptidase) and inhibits drug-transporter (P-glycoprotein and CYP3A4) in enterocytes and hepatocytes [68] | |
| ↑ intestinal microbiota enhances metabolism and biotransformation into derivates with specific biological activities [1,54] Modulation of beneficial bacteria [1,56] | ↑ PIP enhances gut beneficial bacteria [66] | ↑ PIP increases intestinal permeability [28] | |
| ↑ PIP increases blood supply to the gastrointestinal tract and HCl secretion [74] | |||
| ↑ bioavailability nano-emulsion of CUR plus PIP [72] | |||
| no significant differences were observed between the piperine-curcuminoid combination and the standard extract of single curcumin. [52] | |||
| Adverse side effect | Flatulence and gastric irritation, stimulation of bile secretion, cholangitis. No effects on glycemia [68]. | Hemorrhagic stomach ulceration, mild to moderate enteritis, increased serum gonadotropins and decreased intratesticular testosterone levels. No effects on glycemia [33]. | No specific study, but PIP enhanced the risk for hepatotoxicity [68,69,70,71] |
| Plasma peak after oral administration | 1–2 h Undetectable within 12 h [46] | 2–4 h Undetectable within 48 h [18,63] | No study |
| Safety: ADI/ | 0.3 mg/kg/day [67] | 5 mg/day (max dose 50 mg/kg/day) [33] | No study |
References
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between gut microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrition 2020, 12, 2499. [Google Scholar] [CrossRef]
- Sahebkar, A.; Gandolfo, V.; Bianconi, V.; Mannarino, M.R.; Pirro, M. Effetti della curcumina con possibile impatto sul danno vascolare aterosclerotico. G. Ital. Dell’aterosclerosi 2017, 8, 90–102. [Google Scholar]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of curcumin I: Evidence from In Vitro Studies. Nutrients 2020, 12, 118. [Google Scholar] [CrossRef]
- Peijian, W.; Hui, L.; Hongjun, L.; Wenhong, L. Comparing the Effect of Piperine Ilepcimide on the Pharmacokinetics of curcumin in SD Rats. Front. Pharmacol. 2021, 12, 725362. [Google Scholar]
- Altobelli, E.; Angeletti, P.M.; Marziliano, C.; Mastrodomenico, M.; Giuliani, A.R.; Petrocelli, R. Potential Therapeutic Effects of Curcumin on Glycemic and Lipid Profile in Uncomplicated Type 2 Diabetes-A Meta-Analysis of Randomized Controlled Trial. Nutrients 2021, 13, 404. [Google Scholar] [CrossRef]
- Tian, J.; Feng, B.; Tian, Z. The Effect of Curcumin on Lipid Profile and Glycemic Status of Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 827874. [Google Scholar] [CrossRef]
- Pratò, S.; Didonna, V.; Garletti, F.; Marfia, G.; Barbaresi, A.; Palumbo, F.; Garzia, E.; Ciniglio Appiani, G.; Riboldi, L.; Vigna, L. Loss of consciousness in a helicopter pilot as plausible first sign of insulinoma: A case report. Med. Lav. 2022, 113, e2022007. [Google Scholar]
- Shanmugan, M.K.; Rana, G.; Mathi Kanchi, M.; Arfuso, F.; Cinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Krumar, A.P.; Sethi, G. Multifaced Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Cavaleri, F. The 3 Curcuminoid Analogs Comprising the Curcumin Extract Comparably Inhibit Nuclear Factor kappa-light-chain-enhancer Activation. Prog. Prev. Med. 2019, 4, e0023. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; He, B.; Zhang, C.; Rodriguez, E.; Hage, D.S.; Moreau, R. Piperine potentiates curcumin-mediated repression of mTORC1 signalling in human intestinal epithelial cells: Implication for the inhibition of protein synthesis and TNFα signalling. J. Nutr. Biochem. 2018, 57, 276–286. [Google Scholar] [CrossRef]
- Kang, C.; Kim, E. Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem. Toxicol. 2010, 48, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Tamrakar, A.K.; Mahajan., S.; Prasad, G.B.K.S. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycaemic function. Life Sci. 2018, 213, 226–235. [Google Scholar] [CrossRef]
- Maeda, A.; Shirao, T.; Shirasava, D.; Yoshioka, Y.; Yamashita, Y.; Akagawa, M.; Ashida, H. Piperine Promotes Glucose Uptake through ROS-Dependent Activation of the CAMKK/AMPK Signalling Pathway in Skeletal Muscle. Mol. Nutr. Food Res. 2018, 62, e1800086. [Google Scholar] [CrossRef]
- Priyanka, A.; Shyni, G.L.; Anupama, N.; Raj, P.S.; Anusree, S.S.; Raghu, K.G. Development of insulin resistance through sprouting of inflammatory markers during hypoxia in 3T3-L1 adipocytes and amelioration with curcumin. Eur. J. Pharmacol. 2017, 812, 73–81. [Google Scholar] [CrossRef]
- Quijia, C.R.; Araujo, V.H.; Chorilli, M. Piperine: Chemical, biological and nanotechnological applications. Acta Pharm. 2021, 71, 185–213. [Google Scholar] [CrossRef]
- Park, U.H.; Jeong, H.S.; Jo, E.Y.; Park, T.; Yoon, S.K.; Kim, E.J.; Jeong, J.C.; Um, S.J. Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPAR-g activity in 3T3-L1 cells. J. Agric. Food Chem. 2012, 60, 3853–3860. [Google Scholar] [CrossRef]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hosokawa, M.; Zhou, X.; Fujimoto, S.; Fukuda, K.; Toyoda, K.; Nishi, Y.; Fujita, Y.; Yamada, K.; Yamada, Y.; et al. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res. Clin. Pract. 2008, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.K.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Weng, C.F. Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Sci. Rep. 2019, 9, 15585. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Kurimoto, Y.; Tsuda, T. Curcumin stimulates glucagon-like peptide-1 secretion in GLUT cells via Ca2+/calmodulin-dependent kinase II activation. Biochem. Biophys. Res. Commun. 2013, 435, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xun, W.; Peng, W.; Hu, H.; Cao, T.; Hou, G. Effect of the Single and Combined Use of Curcumin and Piperine on Growth Performance, Intestinal Barrier Function, and Antioxidant Capacity of Weaned Wuzhishan Piglets Front. Vet. Sci. 2020, 7, 418. [Google Scholar] [CrossRef]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin restores mitochondrial functions and decreases lipid peroxidation in liver and kidneys of diabetic db/db mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Ali, Y.; Dhulap, A.; Alam, P.; Pasha, M.A. Novel Piperine Derivatives with Antidiabetic Effect as PPAR-γ Agonists. Chem. Biol. Drug Des. 2016, 88, 354–362. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kim, S.H.; Thomas, M.J.; Paul, L.; Zingg, J.M.; Dolnikowski, G.G.; Roberts, S.B.; Kiura, F.; Miyazawa, T.; et al. Curcumin and piperine supplementation of obese mice under caloric restriction modulates body fat and interleukin-1β. Nutr. Metab. 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signalling. Biomed. Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, H.; Ghavidel, F.; Panhai, G.; Majeed, M.; Sahebcar, A. A systematic review and meta-analysis of randomized controlled trials investigating the effect of the curcumin and piperine combination on lipid profile in patients with metabolic syndrome and related disorders. Phytother. Res. 2023, 37, 1212–1224. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef] [PubMed]
- Kelany, M.E.; Hakami, T.M.; Omar, A.H. Curcumin improves the metabolic syndrome in high-fructose-diet-fed rats: Role of TNF-α, NF-κB, and oxidative stress. Can. J. Physiol. Pharmacol. 2017, 95, 140–150. [Google Scholar] [CrossRef]
- Choi, S.; Choi, Y.; Choi, Y.; Kim, S.; Jang, J.; Park, T. Piperine reverses high Ft diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013, 141, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Valizadegan, G.; Ahamdi, N.; Ganjali, S.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell. Biochem. 2019, 120, 15989–15996. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Hu, R.; Zou, L.; Ji, L.; Jiang, G. Piperine protects against pancreatic β-cell dysfunction by alleviating macrophage inflammation in obese mice. Life Sci. 2021, 274, 119312. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effect of phytosomial curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices; a double bind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Embuscado, M.E. Bioactives From Spices and Herbs. In Encyclopedia of Food Chemestry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 497–514. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Mimica, B.; Bučević Popović, V.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Methods Used for Enhancing the Bioavailability of Oral Curcumin in Randomized Controlled Trials: A Meta-Research Study. Pharmaceuticals 2022, 15, 939. [Google Scholar] [CrossRef]
- Fança-Berthon, P.; Tenon, M.; Le Bouter-Banon, S.; Manfré, A.; Maudet, C.; Dion, A. Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study. J. Nutr. 2021, 151, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Nasef, N.; Loveday, S.M.; Golding, M.; Martins, R.N.; Shah, T.M.; Clarke, M.; Coad, J.; Moughan, P.J.; Garg, M.L.; Singh, H. Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans. Food Funct. 2019, 10, 4584–4592. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin and Its Potential Impact on Microbiota. Nutrients 2021, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signalling, and organization of tight junctions. Am. J. Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. -Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joi, D.; Joseph, T. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Suresh, M.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Sharma, V.; Nehru, B.; Munshi, A. Antioxidant potential of curcumin against oxidative insult induced by pentylenetetrazol in epileptic rats. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 227–232. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Q.; Li, C.; Yang, M.; Zuo, Z. Efficient brain uptake of piperine and its pharmacokinetics characterization after oral administration. Xenobiotica 2018, 48, 1249–1257. [Google Scholar] [CrossRef]
- Itharat, A.; Kanokkangsadal, P.; Khemawoot, P.; Wanichsetakul, P.; Davies, N.M. Pharmacokinetics of piperine after oral administration of Sahastara remedy capsules in healthy volunteers. Res. Pharm. Sci. 2020, 15, 410–417. [Google Scholar] [CrossRef]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, N.B.; Hemalatha, R.; Uppala, S.; Yathapu, S.R.; Mohammed, S.; Venkata Surekha, M.; Rajendran, A.; Bharadwaj, D.K. Ocimum sanctum, Zingiber officinale, and Piper nigrum extracts and their effects on gut microbiota modulations (prebiotic potential), basal inflammatory markers and lipid levels: Oral supplementation study in healthy rats. Pharm. Biol. 2022, 60, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Menniti-Ippolito, F.; Ippoliti, I.; Pastorelli, A.A.; Altieri, I.; Scalise, F.; De Santis, B.; Debegnach, F.; Brera, C.; Pacifici, R.; Pichini, S.; et al. Turmeric (Curcuma longa L.) food supplements and hepatotoxicity: An integrated evaluation approach. Ann. Ist. Super Sanita 2020, 56, 462–469. [Google Scholar] [CrossRef]
- Bucchini, L. The 2019 curcumin crisis in Italy: What we know so far, and early lessons. Planta Med. 2019, 85, 1401-0. [Google Scholar] [CrossRef]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef]
- Ministero della Salute, Direzione Generale per l’Igiene e la Sicurezza degli Alimenti e la Nutrizione. Integratori Alimentari Contenenti Estratti e Preparati di Piante di Curcuma longa e spp: Modifica All’allegato 1 del DM 10 Agosto 2018. Available online: https://www.cna.it/wp-content/uploads/2022/08/01082022-cambio-decreto-curcuma-associazioni-rev-2.pdf (accessed on 2 March 2023).
- Bundesinstitut fur Risikobewertung. Curcumin in Food Supplements: Acceptable Daily Intake May be Exceeded. BfR Opinion No 040/2021 Issued 14 December 2021. Available online: https://www.bfr.bund.de/cm/349/curcumin-in-food-supplements-acceptable-daily-intake-may-be-exceeded.pdf (accessed on 2 March 2023).
- Freitas, E.; Silva-Santana, N.C.; Rodrigues, H.C.N.; Pereira Martins, T.F.; Braga, C.C.; Silva, M.A.C.; Carlos da Cunha, L.; de Souza Freitas, A.T.V.; Costa, N.A.; Peixoto, M.D.R.G. Turmeric supplementation with piperine is more effective than turmeric alone in attenuating oxidative stress and inflammation in hemodialysis patients: A randomized, double-blind clinical trial. Free Radic. Biol. Med. 2022, 193 Pt 2, 648–655. [Google Scholar] [CrossRef]
- Racz, Z.R.; Pop, L.C.; Tomoaia, G.; Mounca, A.; Barbu, I.; Sarkozi, M.; Racz, C.P.; Roman, I.; Avram, A.; Tomoaia-Cotisel, M. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behaviour of Curcumin. Molecules 2022, 27, 6854. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Alqahtani, T.; Alqahtani, A.; Lakshmiraj, R.S.; Singh, G.; Rupeshkumar, M.; Thangathirupathi, A.; Sundram, K. A Unifying Perspective in Blunting the Limited Oral Bioavailability of Curcumin: A Succinct Look. Curr. Drug Metab. 2022, 23, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Green, A.; Kranse, J.; Rumemerger, J.M. Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomedicine 2014, 21, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.; Zhang, Y.; Ye, M.; Din, Y.; Tang, Z.; Li, M.; Zhan, Y.; Wangh, C. Interference with AKT signalling pathway contributes curcumin-induced adipocyte insulin resistance. Mol. Cell. Endocrinol. 2016, 429, 1–9. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, A. Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase. Br. J. Pharmacol. 2010, 161, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef]
- Mohammadi, E.; Behnam, B.; Mohammadinejad, R.; Guest, P.C.; Simental-Mendía, L.E.; Sahebkar, A. Antidiabetic Properties of Curcumin: Insights on New Mechanisms. Adv. Exp. Med. Biol. 2021, 1291, 151–164. [Google Scholar] [CrossRef]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell. Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin II: Evidence from in Vivo Studies. Nutrients 2020, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Ali Hussain, H.E. Hypoglycemic, hypolipidemic and antioxidant properties of combination of Curcumin from Curcuma longa, Linn, and partially purified product from Abroma augusta, Linn. in streptozotocin induced diabetes. Indian J. Clin. Biochem. 2002, 17, 33–43. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, Y.; Wang, Z.; Fang, Q.; Sun, Y.; Tong, C.; Peng, K.; Wang, Y.; Miao, L.; Cai, L.; et al. Inhibition of MAPK-mediated ACE expression by compound C66 prevents STZ-induced diabetic nephropathy. J. Cell. Mol. Med. 2014, 18, 231–241. [Google Scholar] [CrossRef]
- Kanitkar, M.; Gokhale, K.; Galande, S.; Bhonde, R.R. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br. J. Pharmacol. 2008, 155, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Ghayour Mobarhan, M.; Kazemi Oskuee, R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. [Google Scholar] [PubMed]
- Shah, S.S.; Shah, G.B.; Singh, S.D.; Gohil, P.V.; Chauhan, K.; Shah, K.A.; Chorawala, M. Effect of piperine in the regulation of obesity-induced dyslipidemia in high-fat diet rats. Indian J. Pharmacol. 2011, 43, 296–299. [Google Scholar] [CrossRef] [PubMed]
| Curcumin | Piperine | Complex Curcumin + Piperine | |
|---|---|---|---|
| In vitro studies | ↓ mTORC1 signal in human intestinal epithelium cells [13] | ↓ TORC1 signal in human intestinal epithelium cells [13] | ↓ mTORC1 signal in human intestinal epithelium cells more efficiently than CUR alone [13] |
| ↑ GLUT4 translocation in skeletal muscle cells, adipocytes and hepatocytes [14,15] | ↑ intracellular Ca2+ level with activation of CaMKKβ and consequent increase of GLUT4 translocation in L6 myotubes [16] | ↓ TNFα gene expression [13] | |
| ↑ GLUT4 expression and ↓ GLUT1 expression in hypoxic adipocytes [17] | ↑ anti-diabetic activity by PPAR-gamma and ↑ insulin-sensibility in 3T3-L1 cells. [18,19] | ||
| ↑ Akt phosphorylation [20] | |||
| ↓pro-inflammatory cytokines in skeletal muscle cells, adipocytes and hepatocytes cells [21] | |||
| ↓ gluconeogenesis and glycogenolysis in hepatocytes cells [22] | |||
| ↓ G6Pase and PECK activity [22] | |||
| ↓ mRNA expression of 11 PDE isoenzymes (PDE3B, PDE8A, PDE10A) in pancreatic islets, in dose-dependent [23] | |||
| ↓ DPP IV in Caco2 cells [24] | |||
| ↑ GLP-1 secretion via Ca2+/calmodulin-dependent Kinase pathway [25] | |||
| ↑ insulin secretion in pancreatic islets via PDE/cAMP regulation and ↑ recovery of pancreatic islets [23] | |||
| In vivo animal and human studies | ↓ glucose serum and HbA1c levels [26,27] | ↑ Ca2+ level with consequent translocation by APMK phosphorylation in Wistar rats [16] | ↑ recovery of intestinal permeability and integrity and ↓ oxidative stress in weaned Wuzhishan piglets [28]↓ |
| ↓ hyperlipidemia and hyperglycemia [29] | ↓ body weight, hepatotoxicity and peroxidation in diabetic animal models induced by streptozotocin [18,30] | inflammation index levels and ↓ weight in high-fat-diet-RC-induced mice [31] | |
| ↓ Inhibition of NLP3 inflammasome activation in genetic diabetes animals [32] | ↑ improved lipid profile in high-fat diets induced rats [33] | ↓ total cholesterol, LDL with no effect on TG in a patient with metabolic syndrome [34] | |
| ↓ serum glucose and leptin, ↑ adiponectin and ↓insulin resistance in diet-induced diabetes models [35,36] | ↑ improves insulin signal in HFD-induced hepatic steatosis, ↓plasma adiponectin and glucose levels [33,37] | ↓ albumin level and improve glycemic profile in patients with NAFLD [38] | |
| ↓ serum glucose, TG, LDL and HbA1c levels in human studies [26] | ↓ β-cell-dysfunction in pre-diabetic mice and reduced LPS level [39] | ↓ FPI, HOMA index, TG, LDL, hepatic transaminases, γ-GT, cortisol, blood pressure, steatosis index and waist circumference in overweight patients [40] | |
| ↓ insulin secretion and HOMA-IR in pre-diabetic and diabetic individuals [41,42] | ↓ glucose, HbA1c, C-peptide, alanine and aspartase aminotransferase in T2D patients [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servida, S.; Panzeri, E.; Tomaino, L.; Marfia, G.; Garzia, E.; Ciniglio Appiani, G.; Moroncini, G.; De Gennaro Colonna, V.; La Vecchia, C.; Vigna, L. Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient’s Loss of Consciousness. Int. J. Mol. Sci. 2023, 24, 6621. https://doi.org/10.3390/ijms24076621
Servida S, Panzeri E, Tomaino L, Marfia G, Garzia E, Ciniglio Appiani G, Moroncini G, De Gennaro Colonna V, La Vecchia C, Vigna L. Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient’s Loss of Consciousness. International Journal of Molecular Sciences. 2023; 24(7):6621. https://doi.org/10.3390/ijms24076621
Chicago/Turabian StyleServida, Simona, Elena Panzeri, Laura Tomaino, Giovanni Marfia, Emanuele Garzia, Giuseppe Ciniglio Appiani, Gianluca Moroncini, Vito De Gennaro Colonna, Carlo La Vecchia, and Luisella Vigna. 2023. "Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient’s Loss of Consciousness" International Journal of Molecular Sciences 24, no. 7: 6621. https://doi.org/10.3390/ijms24076621
APA StyleServida, S., Panzeri, E., Tomaino, L., Marfia, G., Garzia, E., Ciniglio Appiani, G., Moroncini, G., De Gennaro Colonna, V., La Vecchia, C., & Vigna, L. (2023). Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient’s Loss of Consciousness. International Journal of Molecular Sciences, 24(7), 6621. https://doi.org/10.3390/ijms24076621








