Genome-Wide Analysis of the Odorant Receptor Gene Family in Solenopsis invicta, Ooceraea biroi, and Monomorium pharaonis (Hymenoptera: Formicidae)
Abstract
:1. Introduction
2. Results
2.1. Annotation of the ORs in the Genomes of Three Formicidae Species
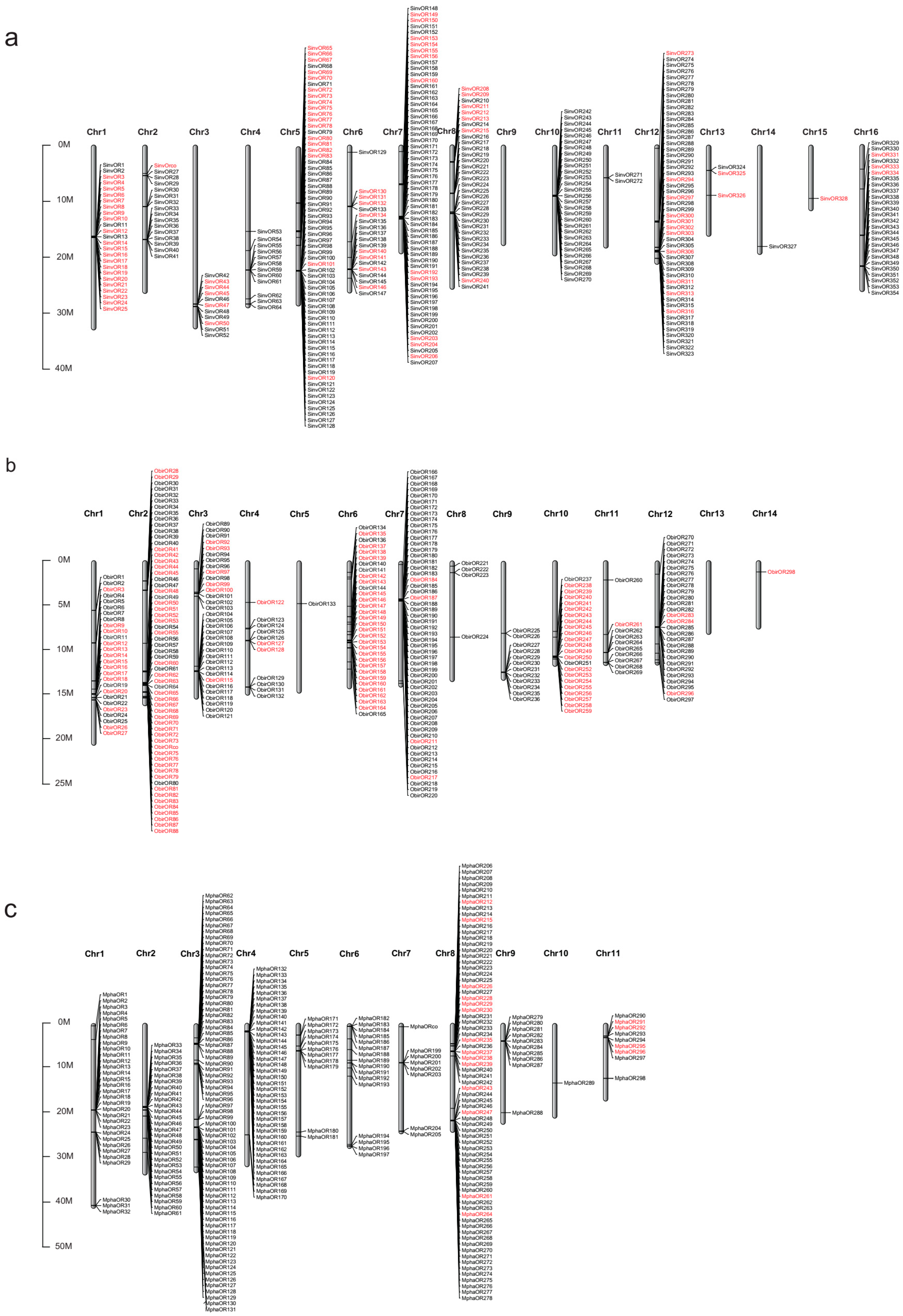
2.2. Genomic Organization of ORs
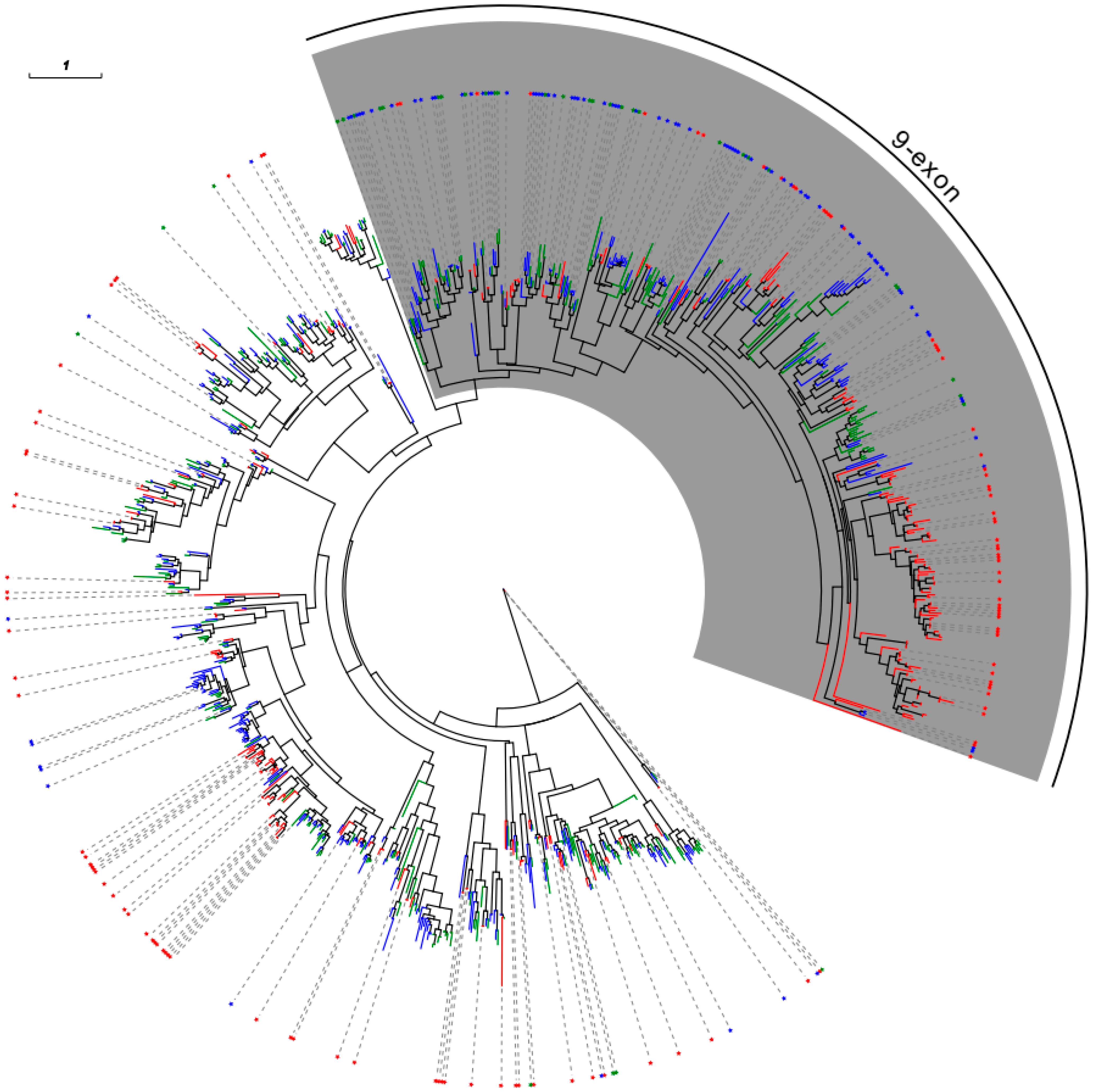
2.3. The 9-Exon OR Subfamily Expanded in Formicidae Species
2.4. Genome Synteny Analyses
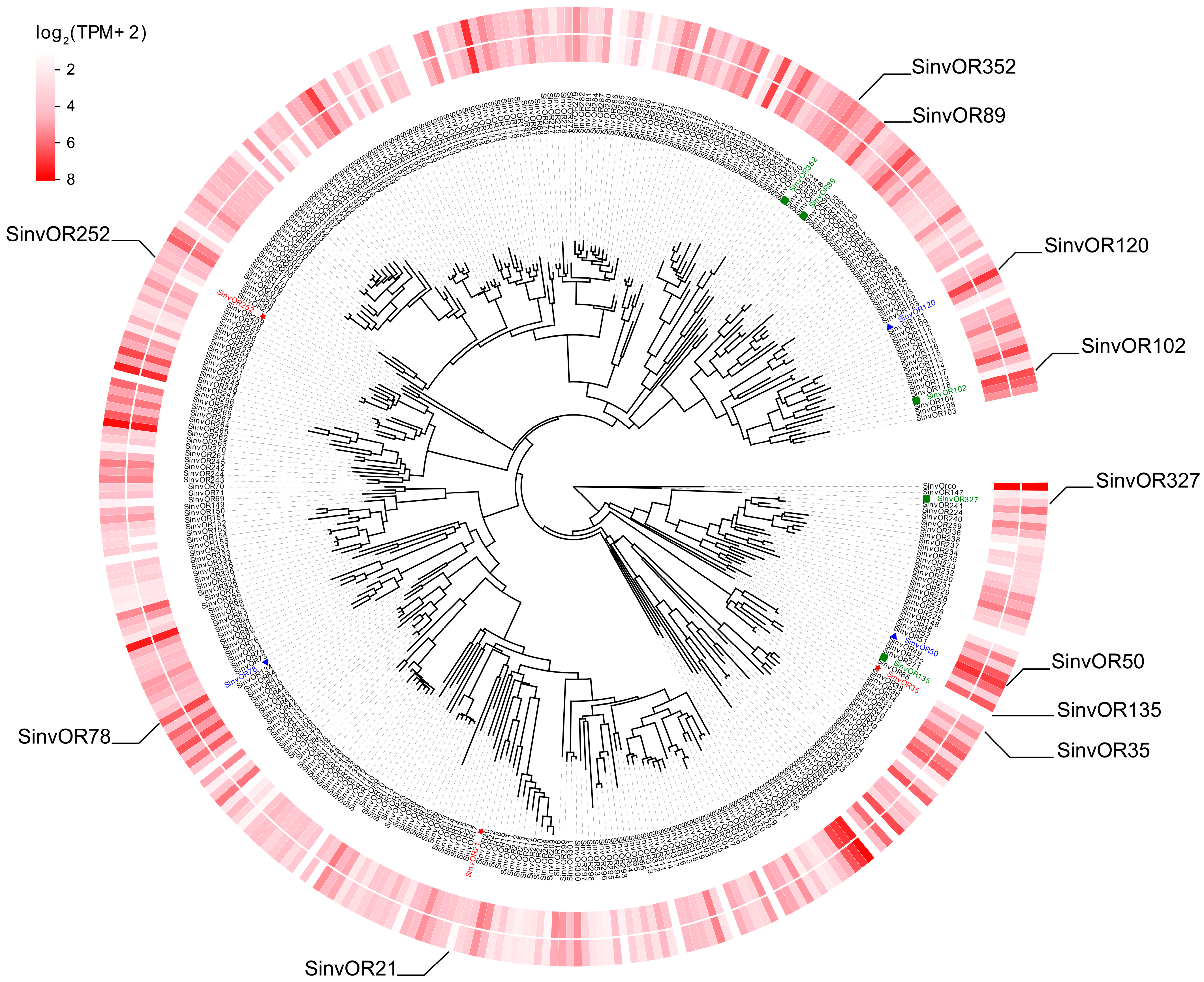
2.5. Phylogenetic Analysis of ORs
2.6. Analysis of Differentially Expressed OR Genes in S. invicta Workers from Different Societies
3. Discussion
4. Materials and Methods
4.1. Insect Rearing and Sample Preparation
4.2. Data Sources
4.3. Phylogenetic Analysis
4.4. Analysis of the OR Gene Family
4.5. Tandem Replication of the ORs in S. invicta
4.6. Genome Synteny Analysis
4.7. Analysis of Evolutionary Selective Pressure
4.8. Analysis of Differential Expression of the ORs in the Antennae of S. invicta Workers from Different Societies
4.9. Expression Analysis Using Semi-Quantitative RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demuth, J.P.; Hahn, M.W. The life and death of gene families. Bioessays 2009, 31, 29–39. [Google Scholar] [CrossRef]
- Long, M.; VanKuren, N.W.; Chen, S.; Vibranovski, M.D. New gene evolution: Little did we know. Annu. Rev. Genet. 2013, 47, 307–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Krinsky, B.H.; Long, M. New genes as drivers of phenotypic evolution. Nat. Rev. Genet. 2013, 14, 645–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soucy, S.; Olendzenski, L.; Gogarten, J.P. Orthologues, Paralogues and Horizontal Gene Transfer in the Human Holobiont. eLS 2013. [Google Scholar] [CrossRef]
- Mendivil Ramos, O.; Ferrier, D.E. Mechanisms of gene duplication and translocation and progress towards understanding their relative contributions to animal genome evolution. Int. J. Evol. Biol. 2012, 2012, 846421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayhoff, M.O. The origin and evolution of protein superfamilies. Fed. Proc. 1976, 35, 2132–2138. [Google Scholar]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [Green Version]
- Rutzler, M.; Zwiebel, L.J. Molecular biology of insect olfaction: Recent progress and conceptual models. J. Comp. Physiol. A 2005, 191, 777–790. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in Insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Smith, C.R.; Smith, C.D.; Robertson, H.M.; Helmkampf, M.; Zimin, A.; Yandell, M.; Holt, C.; Hu, H.; Abouheif, E.; Benton, R.; et al. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc. Natl. Acad. Sci. USA 2011, 108, 5667–5672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Slone, J.D.; Rokas, A.; Berger, S.L.; Liebig, J.; Ray, A.; Reinberg, D.; Zwiebel, L.J. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 2012, 8, e1002930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, S.K.; Fetter-Pruneda, I.; Ruta, V.; Kronauer, D.J. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl. Acad. Sci. USA 2016, 113, 14091–14096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branstetter, M.G.; Danforth, B.N.; Pitts, J.P.; Faircloth, B.C.; Ward, P.S.; Buffington, M.L.; Gates, M.W.; Kula, R.R.; Brady, S.G. Phylogenomic Insights into the Evolution of Stinging Wasps and the Origins of Ants and Bees. Curr. Biol. 2017, 27, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engsontia, P.; Sangket, U.; Robertson, H.M.; Satasook, C. Diversification of the ant odorant receptor gene family and positive selection on candidate cuticular hydrocarbon receptors. BMC Res. Notes 2015, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Sprenger, P.; Menzel, F. Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: How and why they differ among individuals, colonies, and species. Myrmecol. News 2020, 30, 1–26. [Google Scholar] [CrossRef]
- Zweden, J.S.V.; D’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. Insect Hydrocarb. Biol. Biochem. Chem. Ecol. 2010, 11, 222–243. [Google Scholar] [CrossRef]
- Ferguson, S.T.; Bakis, I.; Zwiebel, L.J. Advances in the study of olfaction in eusocial ants. Insects 2021, 12, 252. [Google Scholar] [CrossRef]
- Pask, G.M.; Slone, J.D.; Millar, J.G.; Das, P.; Moreira, J.A.; Zhou, X.; Bello, J.; Berger, S.L.; Bonasio, R.; Desplan, C.; et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun. 2017, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Legan, A.W.; Jernigan, C.M.; Miller, S.E.; Fuchs, M.F.; Sheehan, M.J. Expansion and accelerated evolution of 9-exon odorant receptors in polistes paper wasps. Mol. Biol. Evol. 2021, 38, 3832–3846. [Google Scholar] [CrossRef]
- Deheer, C.; Krieger, M.; Ross, K. Population genetics of the invasive fire ant Solenopsis invicta (Hymenoptera: Formicidae) in the United States. Ann. Entomol. Soc. Am. 2009, 99, 1213–1233. [Google Scholar] [CrossRef]
- Ross, K.G.; Shoemaker, D.D. Estimation of the number of founders of an invasive pest insect population: The fire ant Solenopsis invicta in the USA. Proc. R. Soc. B Biol. Sci. 2008, 275, 2231–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascunce, M.S.; Valles, S.M.; Oi, D.H.; Shoemaker, D.; Plowes, R.; Gilbert, L.; LeBrun, E.G.; Sanchez-Arroyo, H.; Sanchez-Pena, S. Molecular diversity of the microsporidium Kneallhazia solenopsae reveals an expanded host range among fire ants in North America. J. Invertebr. Pathol. 2010, 105, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Ross, K.G. Phenotypic plasticity and “cultural transmission” of alternative social organizations in the fire ant Solenopsis invicta. Behav. Ecol. Sociobiol. 2004, 33, 121–129. [Google Scholar] [CrossRef]
- Wills, B.D.; Powell, S.; Rivera, M.D.; Suarez, A.V. Correlates and consequences of worker polymorphism in ants. Annu. Rev. Entomol. 2018, 63, 575–598. [Google Scholar] [CrossRef]
- Dang, V.D.; Cohanim, A.B.; Fontana, S.; Privman, E.; Wang, J. Has gene expression neofunctionalization in the fire ant antennae contributed to queen discrimination behavior? Ecol. Evol. 2019, 9, 12754–12766. [Google Scholar] [CrossRef] [Green Version]
- Kjeldgaard, M.K.; Eyer, P.-A.; McMichael, C.C.; Bockoven, A.A.; King, J.T.; Hyodo, A.; Boutton, T.W.; Vargo, E.L.; Eubanks, M.D. Distinct colony boundaries and larval discrimination in polygyne red imported fire ants (Solenopsis invicta). Mol. Ecol. 2022, 31, 1007–1020. [Google Scholar] [CrossRef]
- Krieger, M.J.B.; Ross, K.G. Identification of a major gene regulating complex social behavior. Science 2002, 295, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Pracana, R.; Levantis, I.; Martínez-Ruiz, C.; Stolle, E.; Priyam, A.; Wurm, Y. Fire ant social chromosomes: Differences in number, sequence and expression of odorant binding proteins. Evol. Lett. 2017, 1, 199–210. [Google Scholar] [CrossRef]
- Lucas, C.; Nicolas, M.; Keller, L. Expression of foraging and Gp-9 are associated with social organization in the fire ant Solenopsis invicta. Insect Mol. Biol. 2015, 24, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.; Ross, K.G. Selfish genes: A green beard in the red fire ant. Nature 1998, 394, 573–575. [Google Scholar] [CrossRef]
- Wurm, Y.; Wang, J.; Riba-Grognuz, O.; Corona, M.; Nygaard, S.; Hunt, B.G.; Ingram, K.K.; Falquet, L.; Nipitwattanaphon, M.; Gotzek, D.; et al. The genome of the fire ant Solenopsis invicta. Proc. Natl. Acad. Sci. USA 2011, 108, 5679–5684. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, S.K.; Kronauer, D.J.C. The genomic architecture and molecular evolution of ant odorant receptors. Genome Res. 2018, 28, 1757–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, H.M.; Wanner, K.W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006, 16, 1395–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, A.; Alenius, M.; Dickson, B.J. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005, 15, 1535–1547. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Kim, J. Molecular evolution of Drosophila odorant receptor genes. Mol. Biol. Evol. 2007, 24, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Nozawa, M.; Nei, M. Evolutionary dynamics of olfactory receptor genes in Drosophila species. Proc. Natl. Acad. Sci. USA 2007, 104, 7122–7127. [Google Scholar] [CrossRef] [Green Version]
- Niimura, Y.; Nei, M. Comparative evolutionary analysis of olfactory receptor gene clusters between humans and mice. Gene 2005, 346, 13–21. [Google Scholar] [CrossRef]
- McKenzie, S.K.; Winston, M.E.; Grewe, F.; Vargas Asensio, G.; Rodriguez-Hernandez, N.; Rubin, B.E.R.; Murillo-Cruz, C.; von Beeren, C.; Moreau, C.S.; Suen, G.; et al. The genomic basis of army ant chemosensory adaptations. Mol. Ecol. 2021, 30, 6627–6641. [Google Scholar] [CrossRef]
- Funaro, C.F.; Boroczky, K.; Vargo, E.L.; Schal, C. Identification of a queen and king recognition pheromone in the subterranean termite Reticulitermes flavipes. Proc. Natl. Acad. Sci. USA 2018, 115, 3888–3893. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Zhang, N.; Xu, M.; Glauser, G.; Turlings, T.C.J.; Chen, L. Revisiting the trail pheromone components of the red imported fire ant, Solenopsis invicta Buren. Insect Sci. 2022, 30, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Cavill, G.; Williams, P.J.; Whitfield, F.B. α-Farnesene, Dufour’s gland secretion in the ant Aphaenogaster longiceps (F.Sm.). Tetrahedron Lett. 1967, 8, 2201–2205. [Google Scholar] [CrossRef]
- Bergström, G.; Löfqvist, J. Odour similarities between the slave-keeping ants Formica sanguinea and Polyergus rufescens and their slaves Formica fusca and Formica rufibarbis. J. Insect Physiol. 1968, 14, 995–1011. [Google Scholar] [CrossRef]
- Li, Y.Y.; Lu, Y.Y.; Lu, M.; Wei, H.Y.; Chen, L. HPLC separation of 2-ethyl-5(6)-methylpyrazine and its electroantennogram and alarm activities on fire ants (Solenopsis invicta Buren). Molecules 2018, 23, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, R.G.; Silverstein, R.M. Synthesis of S-(+)-4-methyl-3-heptanone, the principal alarm pheromone of Atta texana, and its enantiomer. Tetrahedron 1974, 30, 1171–1174. [Google Scholar] [CrossRef]
- Pasteels, J.M.; Verhaeghe, J.C.; Ottinger, R.; Braekman, J.C.; Daloze, D. Absolute configuration of (3R,4S)-4-methyl-3-hexanol—A pheromone from the head of the ant Tetramorium impurum foerster. Insect Biochem. 1981, 11, 675–678. [Google Scholar] [CrossRef]
- Bento, J.M.S.; Della Lucia, T.M.C.; Do Nascimento, R.R.; Bergmann, J.; Morgan, E.D. Response of workers of Atta sexdens rubropilosa (Hymenoptera: Formicidae) to mandibular gland compounds of virgin males and females. Physiol. Entomol. 2010, 32, 283–286. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Liebig, J. Genetic basis of chemical communication in eusocial insects. Genes Dev. 2021, 35, 470–482. [Google Scholar] [CrossRef] [PubMed]
- D’Ettorre, P.; Heinze, J.R.; Schulz, C.; Francke, W.; Ayasse, M. Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 2004, 207, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Holman, L.; Jørgensen, C.G.; Nielsen, J.; d’Ettorre, P. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. B Biol. Sci. 2010, 277, 3793–3800. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B.; et al. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Slone, J.D.; Pask, G.M.; Ferguson, S.T.; Millar, J.G.; Berger, S.L.; Reinberg, D.; Liebig, J.; Ray, A.; Zwiebel, L.J. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. Proc. Natl. Acad. Sci. USA 2017, 114, 8586–8591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoemaker, D.D.; Ross, K.G. Effects of social organization on gene flow in the fire ant Solenopsis invicta. Nature 1996, 383, 613–616. [Google Scholar] [CrossRef]
- Simão, F.; Waterhouse, R.M.; Panagiotis, I.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [Green Version]
- Emms, D.M.; Kelly, S. OrthoFinder2: Fast and accurate phylogenomic orthology analysis from gene sequences. bioRxiv 2018, 466201. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.-H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Altschul, S.F. Basic local alignment search tool (BLAST). J. Mol. Biol. 2012, 215, 403–410. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C.; Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H. Synteny and Collinearity in Plant Genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef] [Green Version]
- Sudhir, K.; Glen, S.; Michael, L.; Christina, K.; Koichiro, T. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinson, S.B. Invasion of the red imported fire ant (Hymenoptera: Formicidae): Spread, biology, and impact. Am. Entomol. 1997, 43, 23–39. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Dong, S.; Cao, D.; Dong, J.; Walker, W.B.; Liu, Y.; Wang, G. Antennal transcriptome analysis and comparison of chemosensory gene families in two closely related noctuidae moths, Helicoverpa armigera and H-assulta. PLoS ONE 2015, 10, e0117054. [Google Scholar] [CrossRef] [PubMed]




| Chromosome | Cluster | Ka/Ks | LnL | 2Δl (df = 1) | p-Value | |
|---|---|---|---|---|---|---|
| Null Model | Alternative Model | |||||
| Chr1 | SinvOR4–8 | 1.32 | −24,468.25 | −24,462.34 | 11.82 | 5.860 × 10−4 ** |
| SinvOR18–22 | 0.25 | −24,468.25 | −24,462.34 | 11.82 | 5.860 × 10−4 ** | |
| Chr3 | SinvOR42–47 | 0.13 | −17,005.03 | −17,000.66 | 8.74 | 3.113 × 10−3 * |
| Chr5 | SinvOR65–84 | 3.10 | −82,751.81 | −82,751.00 | 1.62 | 2.031 × 10−1 |
| Chr6 | SinvOR130–132 | 0.17 | −14,756.41 | −14,742.66 | 27.5 | 1.571 × 10−7 ** |
| Chr7 | SinvOR149–156 | 0.27 | −42,691.96 | −42,687.27 | 9.38 | 2.194 × 10−3 * |
| Chr8 | SinvOR208–215 | 0.01 | −33,648.31 | −33,643.12 | 10.38 | 1.274 × 10−3 * |
| Chr16 | SinvOR331–336 | 0.10 | −25,746.66 | −25,745.18 | 2.96 | 8.535 × 10−2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Yang, R.-R.; Jiang, X.-C.; Xu, X.-X.; Wang, B.; Wang, G.-R. Genome-Wide Analysis of the Odorant Receptor Gene Family in Solenopsis invicta, Ooceraea biroi, and Monomorium pharaonis (Hymenoptera: Formicidae). Int. J. Mol. Sci. 2023, 24, 6624. https://doi.org/10.3390/ijms24076624
Zhang B, Yang R-R, Jiang X-C, Xu X-X, Wang B, Wang G-R. Genome-Wide Analysis of the Odorant Receptor Gene Family in Solenopsis invicta, Ooceraea biroi, and Monomorium pharaonis (Hymenoptera: Formicidae). International Journal of Molecular Sciences. 2023; 24(7):6624. https://doi.org/10.3390/ijms24076624
Chicago/Turabian StyleZhang, Bo, Rong-Rong Yang, Xing-Chuan Jiang, Xiao-Xia Xu, Bing Wang, and Gui-Rong Wang. 2023. "Genome-Wide Analysis of the Odorant Receptor Gene Family in Solenopsis invicta, Ooceraea biroi, and Monomorium pharaonis (Hymenoptera: Formicidae)" International Journal of Molecular Sciences 24, no. 7: 6624. https://doi.org/10.3390/ijms24076624
APA StyleZhang, B., Yang, R.-R., Jiang, X.-C., Xu, X.-X., Wang, B., & Wang, G.-R. (2023). Genome-Wide Analysis of the Odorant Receptor Gene Family in Solenopsis invicta, Ooceraea biroi, and Monomorium pharaonis (Hymenoptera: Formicidae). International Journal of Molecular Sciences, 24(7), 6624. https://doi.org/10.3390/ijms24076624






