Towards a More Realistic In Vitro Meat: The Cross Talk between Adipose and Muscle Cells
Abstract
1. Introduction
2. The Cross Talk between Adipose and Muscle Cells
2.1. In Vitro Meat—The Challenge
2.2. Adipose and Muscle Cells—The Cross Talk
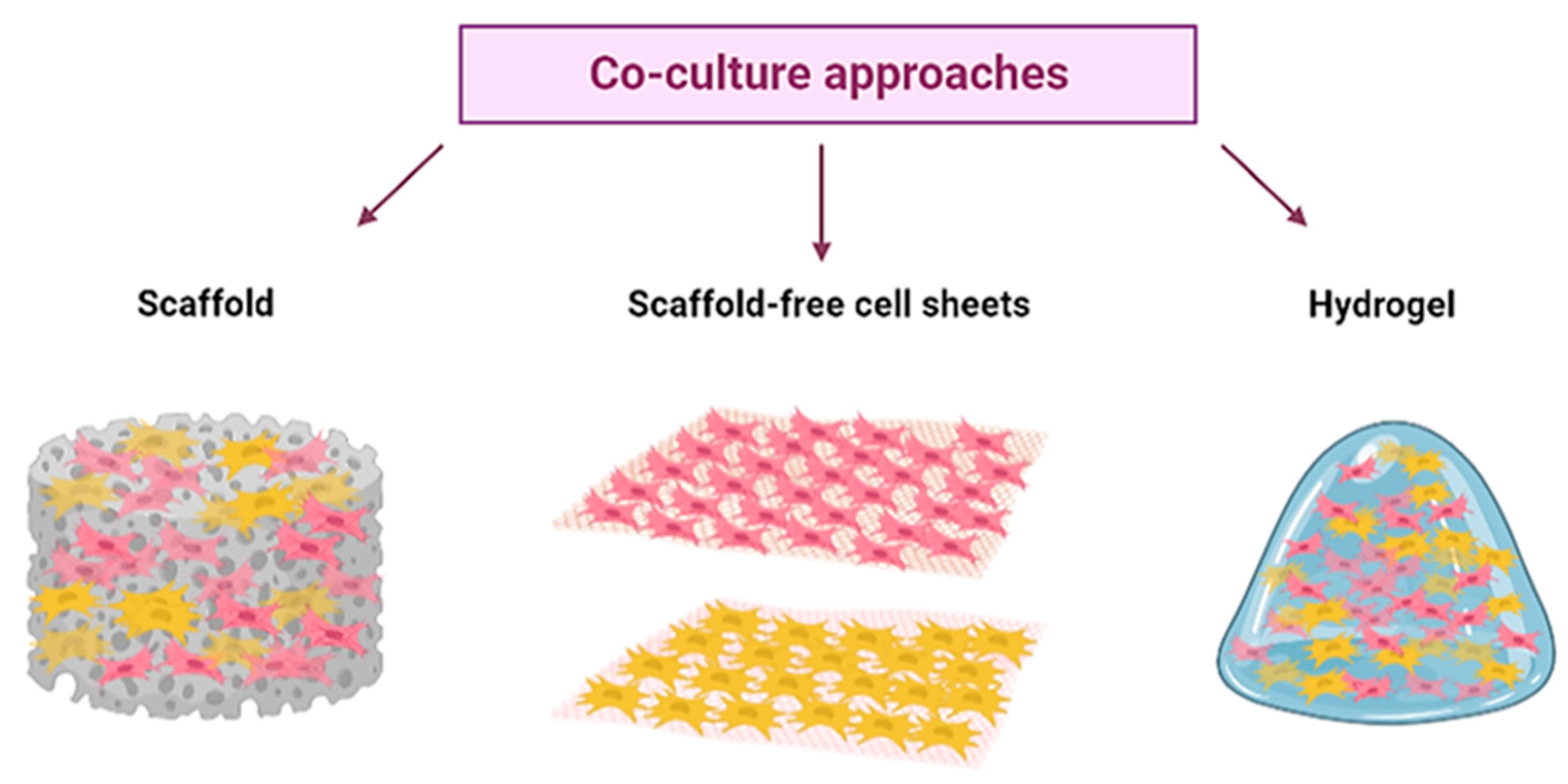
3. New Approaches and Strategies for In Vitro Meat Culturing
3.1. Scaffold: Edible Scaffolds
3.2. Scaffold-Free Cell Sheets
3.3. Hydrogel
3.3.1. Hydrogel Sheets
3.3.2. Fibrillar Hydrogel
3.3.3. Hydrogel Beads
3.3.4. Gelatin Microcarrier
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luis, A.D.; Hayman, D.T.S.; O’shea, T.J.; Cryan, P.M.; Gilbert, A.T.; Pulliam, J.R.C.; Mills, J.N.; Timonin, M.E.; Willis, C.K.R.; Cunningham, A.A.; et al. The Epic of In Vitro Meat Production—A Fiction into Reality. Foods 2021, 10, 1395. [Google Scholar] [CrossRef]
- Benton, T.; Bieg, C.; Harwatt, H.; Pudassaini, R.; Wellesley, L. Food System Impacts on Biodiversity Loss Three Levers for Food; Chatham House: London, UK, 2021; ISBN 9783030023188. [Google Scholar]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO Publications Catalogue 2022; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- International Energy Agency. Global Energy Review: CO2 Emissions in 2021. Global Emissions Rebound Sharply to Highest Ever Level. 2021. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 13 March 2023).
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H. Water Pollution from Agriculture: A Global Review. Executive Summary; FAO: Rome, Italy; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2017. [Google Scholar]
- Takhar, M. In Vitro Meat: An Ethical Solution to an Unsustainable Practice. UC Merced Undergrad. Res. J. 2018, 10. [Google Scholar] [CrossRef]
- Chodkowska, K.A.; Wódz, K.; Wojciechowski, J. Sustainable Future Protein Foods: The Challenges and the Future of Cultivated Meat. Foods 2022, 11, 4008. [Google Scholar] [CrossRef]
- Ross, R. The Smooth Muscle Cell. J. Cell Biol. 1971, 50, 172–186. [Google Scholar] [CrossRef]
- Jha, A. First lab-grown hamburger gets full marks for ‘mouth feel’. The Guardian, 6 August 2013. [Google Scholar]
- Handral, H.K.; Tay, S.H.; Chan, W.W.; Choudhury, D. 3D Printing of cultured meat products. Crit. Rev. Food Sci. Nutr. 2022, 62, 272–281. [Google Scholar] [CrossRef]
- Pandurangan, M.; Kim, D.H. A novel approach for in vitro meat production. Appl. Microbiol. Biotechnol. 2015, 99, 5391–5395. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef]
- Domeneghini, C.; Di Giancamillo, A.; Corino, C. Conjugated linoleic acids (CLAs) and white adipose tissue: How both in vitro and in vivo studies tell the story of a relationship. Histol. Histopathol. 2006, 21, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Ding, S.; Deng, L.; Zhang, Y.; Li, J.; Shi, Z.; Wu, Z.; Liang, K.; Yan, X.; et al. Engineered meatballs via scalable skeletal muscle cell expansion and modular micro-tissue assembly using porous gelatin micro-carriers. Biomaterials 2022, 287, 121615. [Google Scholar] [CrossRef]
- Kang, D.-H.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 2021, 12, 5059. [Google Scholar] [CrossRef]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. Engineering Murine Adipocytes and Skeletal Muscle Cells in Meat-like Constructs Using Self-Assembled Layer-by-Layer Biofabrication: A Platform for Development of Cultivated Meat. Cells Tissues Organs 2022, 211, 304–312. [Google Scholar] [CrossRef]
- Weaver, A.D.; Kauffman, R.G.; Greaser, M.L.; Guo, W. Part II Meat Science. In Handbook of Meat and Meat Processing; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Warner, R. Meat: Conversion of Muscle into Meat. Encycl. Food Health 2015, 677–684. [Google Scholar] [CrossRef]
- Purslow, P.P. The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front. Physiol. 2020, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.R.; Rogers, R.W.; Althen, T.G. The Role of Collagen in Meat Tenderness. Prof. Anim. Sci. 2002, 18, 107–111. [Google Scholar] [CrossRef]
- Listrat, A.; Gagaoua, M.; Normand, J.; Gruffat, D.; Andueza, D.; Mairesse, G.; Mourot, B.P.; Chesneau, G.; Gobert, C.; Picard, B. Contribution of connective tissue components, muscle fibres and marbling to beef tenderness variability in longissimus thoracis, rectus abdominis, semimembranosus and semitendinosus muscles. J. Sci. Food Agric. 2020, 100, 2502–2511. [Google Scholar] [CrossRef]
- Watanabe, G.; Motoyama, M.; Nakajima, I.; Sasaki, K. Relationship between water-holding capacity and intramuscular fat content in Japanese commercial pork loin. Asian-Australas. J. Anim. Sci. 2018, 31, 914. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee on Technological Options to Improve the Nutritional Attributes of Animal Products. The Role of Fat in the Palatability of Beef, Pork, and Lamb. 1988. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218173/ (accessed on 2 January 2023).
- Maltin, C.A.; Warkup, C.C.; Matthews, K.R.; Grant, C.M.; Porter, A.D.; Delday, M.I. Pig muscle fibre characteristics as a source of variation in eating quality. Meat Sci. 1997, 47, 237–248. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Kim, D.; Soundharrajan, I.; Hwang, I.; Choi, K.C. Adipose and Muscle Cell Co-Culture System: A Novel In Vitro Tool to Mimic the In Vivo Cellular Environment. Biology 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef]
- Shin, W.S.; Hemmi, C.; Toyo-oka, T. Co-culture and crosstalk between endothelial cells and vascular smooth muscle cells mediated by intracellular calcium. Methods Mol. Biol. 2002, 188, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.-G. Development of an Insert Co-culture System of Two Cellular Types in the Absence of Cell-Cell Contact. J. Vis. Exp. 2016, 113, e54356. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. In vitro co-culture model of medulloblastoma and human neural stem cells for drug delivery assessment. J. Biotechnol. 2015, 205, 3–13. [Google Scholar] [CrossRef]
- Dohle, E.; Scherrieble, A.; Doser, M.; Al-Maawi, S.; Hoss, M.; Dauner, M.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Co-culture Model for Cutaneous Wound Healing to Assess a Porous Fiber-Based Drug Delivery System. Tissue Eng. Part C Methods 2020, 26, 475–484. [Google Scholar] [CrossRef]
- Lonergan, S.M.; Topel, D.G.; Marple, D.N. Fat and fat cells in domestic animals. In The Science of Animal Growth and Meat Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 51–69. [Google Scholar]
- Pedersen, B.K. Muscle-to-fat interaction: A two-way street? J. Physiol. 2010, 588, 21. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Brunelli, A.; Saatchi, T.; Urbani, S.; Giani, A.; Rossi, A.P.; Zoico, E.; Fantin, F. The Role of Crosstalk between Adipose Cells and Myocytes in the Pathogenesis of Sarcopenic Obesity in the Elderly. Cells 2022, 11, 3361. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Seo, K.; Suzuki, T.; Kobayashi, K.; Nishimura, T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 2019, 90, 423–434. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Nakamura, K.; Takeuchi, S.; Hosoyama, T.; Matsuwaki, T.; Nishihara, M. Suppression of MyoD induces spontaneous adipogenesis in skeletal muscle progenitor cell culture. Anim. Sci. J. 2021, 92, e13573. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, F.; Wen, J.; Ye, S.; Wang, L.; Yang, Y.; Gong, P.; Jiang, S. The function of myostatin in the regulation of fat mass in mammals. Nutr. Metab. 2017, 14, 29. [Google Scholar] [CrossRef]
- Artaza, J.N.; Bhasin, S.; Magee, T.R.; Reisz-Porszasz, S.; Shen, R.; Groome, N.P.; Fareez, M.M.; Gonzalez-Cadavid, N.F. Myostatin Inhibits Myogenesis and Promotes Adipogenesis in C3H 10T(1/2) Mesenchymal Multipotent Cells. Endocrinology 2005, 146, 3547–3557. [Google Scholar] [CrossRef]
- Takegahara, Y.; Yamanouchi, K.; Nakamura, K.; Nakano, S.-I.; Nishihara, M. Myotube formation is affected by adipogenic lineage cells in a cell-to-cell contact-independent manner. Exp. Cell Res. 2014, 324, 105–114. [Google Scholar] [CrossRef]
- Choi, S.H.; Chung, K.Y.; Johnson, B.J.; Go, G.W.; Kim, K.H.; Choi, C.W.; Smith, S.B. Co-culture of bovine muscle satellite cells with preadipocytes increases PPARγ and C/EBPβ gene expression in differentiated myoblasts and increases GPR43 gene expression in adipocytes. J. Nutr. Biochem. 2013, 24, 539–543. [Google Scholar] [CrossRef]
- Guo, L.; Cui, H.; Zhao, G.; Liu, R.; Li, Q.; Zheng, M.; Guo, Y.; Wen, J. Intramuscular preadipocytes impede differentiation and promote lipid deposition of muscle satellite cells in chickens. BMC Genom. 2018, 19, 838. [Google Scholar] [CrossRef]
- McGeady, T.A.; Quinn, P.J.; Fitzpatrick, E.S.; Ryan, M.T.; Kilroy, D.; Lonergan, P. Veterinary Embriology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9781118940617. [Google Scholar]
- Essentials of Domestic Animal Embryology—9780702028991|Elsevier Health. Available online: https://www.eu.elsevierhealth.com/essentials-of-domestic-animal-embryology-9780702028991.html?gclid=Cj0KCQiAzeSdBhC4ARIsACj36uHEBb9v8cha9RIh4CudIiwb0zUYJUVi-bzz5fL-arMqFJSlTRBpZfEaAlpzEALw_wcB&gclsrc=aw.ds (accessed on 7 January 2023).
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Black, B.L.; Derynck, R. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001, 15, 2950–2966. [Google Scholar] [CrossRef]
- Tapscott, S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005, 132, 2685–2695. [Google Scholar] [CrossRef]
- Wang, D.-T.; Yang, Y.-J.; Huang, R.-H.; Zhang, Z.-H.; Lin, X. Myostatin Activates the Ubiquitin-Proteasome and Autophagy-Lysosome Systems Contributing to Muscle Wasting in Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2015, 2015, 684965. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef]
- Heras, G.; Namuduri, A.V.; Traini, L.; Shevchenko, G.; Falk, A.; Bergström Lind, S.; Jia, M.; Tian, G.; Gastaldello, S. Muscle RING-finger protein-1 (MuRF1) functions and cellular localization are regulated by SUMO1 post-translational modification. J. Mol. Cell Biol. 2019, 11, 356–370. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Price, S.R. Mechanisms activating proteolysis to cause muscle atrophy in catabolic conditions. J. Ren. Nutr. 2003, 13, 149–152. [Google Scholar] [CrossRef]
- Pelosi, M.; De Rossi, M.; Barberi, L.; Musarò, A. IL-6 Impairs Myogenic Differentiation by Downmodulation of p90RSK/eEF2 and mTOR/p70S6K Axes, without Affecting AKT Activity. BioMed Res. Int. 2014, 2014, 206026. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, F.; Yan, T.; Liu, Z.; Wang, C.; Ge, X.; Zhai, Q. C/EBPα regulates SIRT1 expression during adipogenesis. Cell Res. 2010, 20, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, C.; Luo, J.; Wei, Y.; Wang, W.; Zhong, Y. Adiponectin promotes preadipocyte differentiation via the PPARγ pathway. Mol. Med. Rep. 2017, 17, 428–435. [Google Scholar] [CrossRef]
- Nakajima, A.; Nakatani, A.; Hasegawa, S.; Irie, J.; Ozawa, K.; Tsujimoto, G.; Suganami, T.; Itoh, H.; Kimura, I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE 2017, 12, e0179696. [Google Scholar] [CrossRef]
- Hardie, D.G. Balancing Cellular Energy. Science 2007, 315, 1671–1672. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Sodhi, S.S. Differential Expression Profiling of Myogenic Regulatory Factor Genes in Postnatal Longissimus dorsi Muscle of Indigenous and Large White Yorkshire Breeds of Pigs. J. Anim. Res. 2021, 11, 11–17. [Google Scholar] [CrossRef]
- Ropka-Molik, K.; Eckert, R.; Piórkowska, K. The expression pattern of myogenic regulatory factors MyoD, Myf6 and Pax7 in postnatal porcine skeletal muscles. Gene Expr. Patterns 2011, 11, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Addison, O.; Drummond, M.J.; Lastayo, P.C.; Dibble, L.E.; Wende, A.R.; McClain, D.A.; Marcus, R.L. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J. Nutr. Health Aging 2014, 18, 532–538. [Google Scholar] [CrossRef]
- O’Leary, M.F.; Wallace, G.R.; Davis, E.T.; Murphy, D.P.; Nicholson, T.; Bennett, A.J.; Tsintzas, K.; Jones, S.W. Obese subcutaneous adipose tissue impairs human myogenesis, particularly in old skeletal muscle, via resistin-mediated activation of NFκB. Sci. Rep. 2018, 8, 15360. [Google Scholar] [CrossRef]
- Rahemi, H.; Nigam, N.; Wakeling, J.M. The effect of intramuscular fat on skeletal muscle mechanics: Implications for the elderly and obese. J. R. Soc. Interface 2015, 12, 20150365. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, V.; Singh, S.K.; Gupta, J.; Kumar, M.; Sarma, D.K.; Verma, V. Recent advances in bioengineered scaffold for in vitro meat production. Cell Tissue Res. 2022, 391, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Curras, D.; Mousa, J.; Hengsbach, S. Tissue engineering scaffolds for 3D cell culture. In Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer: Cham, Switzerland, 2016; Volume 18, pp. 249–268. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells 2015, 7, 728. [Google Scholar] [CrossRef]
- Gomillion, C.T.; Burg, K.J.L. Adipose Tissue Engineering. Compr. Biomater. 2011, 5, 529–539. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Chen, G.; Qi, Y.; Niu, L.; Di, T.; Zhong, J.; Fang, T.; Yan, W. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 2015, 3, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Cushing, M.C.; Anseth, K.S. Hydrogel Cell Cultures. Science 2007, 316, 1133–1134. [Google Scholar] [CrossRef]
- Thyden, R.; Perreault, L.R.; Jones, J.D.; Notman, H.; Varieur, B.M.; Patmanidis, A.A.; Dominko, T.; Gaudette, G.R. An Edible, Decellularized Plant Derived Cell Carrier for Lab Grown Meat. Appl. Sci. 2022, 12, 5155. [Google Scholar] [CrossRef]
- Song, W.J.; Liu, P.P.; Zheng, Y.Y.; Meng, Z.Q.; Zhu, H.Z.; Tang, C.B.; Li, H.X.; Ding, S.J.; Zhou, G.H. Production of cultured fat with peanut wire-drawing protein scaffold and quality evaluation based on texture and volatile compounds analysis. Food Res. Int. 2022, 160, 111636. [Google Scholar] [CrossRef]
- Xiang, N.; Yuen, J.S.K.; Stout, A.J.; Rubio, N.R.; Chen, Y.; Kaplan, D.L. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials 2022, 285, 121543. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakaguchi, K.; Yoshida, A.; Takahashi, H.; Haraguchi, Y.; Shimizu, T. Production of scaffold-free cell-based meat using cell sheet technology. npj Sci. Food 2022, 6, 41. [Google Scholar] [CrossRef]
- Li, C.-H.; Yang, I.-H.; Ke, C.-J.; Chi, C.-Y.; Matahum, J.; Kuan, C.-Y.; Celikkin, N.; Swieszkowski, W.; Lin, F.-H. The Production of Fat-Containing Cultured Meat by Stacking Aligned Muscle Layers and Adipose Layers Formed From Gelatin-Soymilk Scaffold. Front. Bioeng. Biotechnol. 2022, 10, 875069. [Google Scholar] [CrossRef] [PubMed]
- Zagury, Y.; Ianovici, I.; Landau, S.; Lavon, N.; Levenberg, S. Engineered marble-like bovine fat tissue for cultured meat. Commun. Biol. 2022, 5, 927. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Kumashiro, Y.; Kobayashi, J.; Yamato, M.; Sakai, K.; Okano, T. Characterization of Ultra-Thin Temperature-Responsive Polymer Layer and Its Polymer Thickness Dependency on Cell Attachment/Detachment Properties. Macromol. Biosci. 2010, 10, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, S.; Wang, L.; Zhang, X.; Gao, J.; Jiang, L.; Tang, D.; Zhang, L.; Midgley, A.; Kong, D.; et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Vuong, T.A.; Go, G.-Y.; Song, Y.-J.; Lee, S.; Lee, S.-Y.; Kim, S.-W.; Lee, J.; Kim, Y.K.; Seo, D.-W.; et al. An isoflavone compound daidzein elicits myoblast differentiation and myotube growth. J. Funct. Foods 2017, 38, 438–446. [Google Scholar] [CrossRef]
- Chien, K.B.; Shah, R.N. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012, 8, 694–703. [Google Scholar] [CrossRef]
- Chien, K.B.; Aguado, B.A.; Bryce, P.J.; Shah, R.N. In vivo acute and humoral response to three-dimensional porous soy protein scaffolds. Acta Biomater. 2013, 9, 8983–8990. [Google Scholar] [CrossRef]
- Goto, T.; Mori, A.; Nagaoka, S. Soluble soy protein peptic hydrolysate stimulates adipocyte differentiation in 3T3-L1 cells. Mol. Nutr. Food Res. 2013, 57, 1435–1445. [Google Scholar] [CrossRef]
- Ianovici, I.; Zagury, Y.; Redenski, I.; Lavon, N.; Levenberg, S. 3D-printable plant protein-enriched scaffolds for cultivated meat development. Biomaterials 2022, 284, 121487. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]

| Authors | Cell Types | Culture System | Outcome | Doi | Year |
|---|---|---|---|---|---|
| Seo et al. [36] | 3T3-L1 | Indirect co-culture | Inhibition of muscle cells differentiation | 10.1111/ asj.13145 | 2019 |
| C2C12 | |||||
| Artaza et al. [39] | 10T(1/2) | Monoculture: | 10.1210/en.2005-0362 | 2005 | |
| +Recombinant myostatin | Adipogenic differentiation | ||||
| +Recombinant anti-myostatin | Myogenic differentiation | ||||
| Takegahara et al. [40] | Rat muscle progenitors | Indirect co-culture: | 10.1016/j.yexcr.2014.03.021 | 2014 | |
| 2G11 rat preadipocytes | Preadipocytes + muscle progenitors | No inhibition of muscle differentiation | |||
| 2G11 rat mature adipocytes | Mature adipocytes + muscle progenitors | Inhibition of muscle differentiation | |||
| Choi et al. [41] | Bovine satellite cells | Indirect co-culture | Inhibition of muscle differentiation | 10.1016/j.jnutbio.2012.01.015 | 2013 |
| Bovine preadipocytes | |||||
| Guo et al. [42] | Chicken satellite cells | Indirect co-culture | Inhibition of muscle differentiation | 10.1186/s12864-018-5209-5 | 2018 |
| Chicken intramuscular adipocytes |
| Support | Author | Species | Cell Types | Approach | Doi | Year |
|---|---|---|---|---|---|---|
| Scaffold - Edible scaffold | Thyden et al. [77] | Bovine | Satellite cells | Decellularized broccoli floret + Rotating bioreactor | 10.3390/app12105155 | 2022 |
| Song et al. [78] | Pig | Adipose Mesenchymal Stromal Cells | Peanut Wire-drawing Protein scaffold | 10.1016/j.foodres.2022.111636 | 2022 | |
| Xiang et al. [79] | Mouse | C2C12 | Wheat gluten scaffold | 10.1016/j.biomaterials.2022.121543 | 2022 | |
| Bovine | Satellite cells | |||||
| Scaffold-free cell sheets | Shahin-Shamsabadi et al. [17] | Mouse | 3T3-L1 | ECM-free bio-fabricated cellular sheets | 10.1159/000511764 | 2022 |
| C2C12 | ||||||
| Tanaka et al. [80] | Bovine | Myoblasts | Scaffold-free cell-based sheets | 10.1038/s41538-022-00155-1 | 2022 | |
| Hydrogel sheets | Li et al. [81] | Mouse | 3T3-L1 | Soy milk gelatin sheets | 10.3389/fbioe.2022.875069 | 2022 |
| C2C12 | ||||||
| Fibrillar Hydrogel | Kang et al. [16] | Bovine | Adipose Mesenchymal Stromal Cells | Bath-assisted 3D printing + tendon gel integrated bioprinting | 10.1038/s41467-021-25236-9 | 2021 |
| Satellite cells | ||||||
| Hydrogel beads | Zagury et al. [82] | Bovine | Adipose Mesenchymal Stromal Cells | Alginate spherical Hydrogel | 10.1038/s42003-022-03852-5 | 2022 |
| Satellite cells | Alginate spherical Hydrogel | |||||
| Adipose Mesenchymal Stromal Cells | Alginate spherical Hydrogel | |||||
| Satellite cells | 3D-printed support | |||||
| Gelatin microcarrier | Liu et al. [15] | Mouse | 3T3-L1 | Microcarrier + spinner flasks Bioreactor + Mold | 10.1016/j.biomaterials.2022.121615 | 2022 |
| C2C12 | ||||||
| Pig | Satellite cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallaoro, M.; Modina, S.C.; Fiorati, A.; Altomare, L.; Mirra, G.; Scocco, P.; Di Giancamillo, A. Towards a More Realistic In Vitro Meat: The Cross Talk between Adipose and Muscle Cells. Int. J. Mol. Sci. 2023, 24, 6630. https://doi.org/10.3390/ijms24076630
Pallaoro M, Modina SC, Fiorati A, Altomare L, Mirra G, Scocco P, Di Giancamillo A. Towards a More Realistic In Vitro Meat: The Cross Talk between Adipose and Muscle Cells. International Journal of Molecular Sciences. 2023; 24(7):6630. https://doi.org/10.3390/ijms24076630
Chicago/Turabian StylePallaoro, Margherita, Silvia Clotilde Modina, Andrea Fiorati, Lina Altomare, Giorgio Mirra, Paola Scocco, and Alessia Di Giancamillo. 2023. "Towards a More Realistic In Vitro Meat: The Cross Talk between Adipose and Muscle Cells" International Journal of Molecular Sciences 24, no. 7: 6630. https://doi.org/10.3390/ijms24076630
APA StylePallaoro, M., Modina, S. C., Fiorati, A., Altomare, L., Mirra, G., Scocco, P., & Di Giancamillo, A. (2023). Towards a More Realistic In Vitro Meat: The Cross Talk between Adipose and Muscle Cells. International Journal of Molecular Sciences, 24(7), 6630. https://doi.org/10.3390/ijms24076630










