The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs
Abstract
:1. Introduction
2. The Melanocortin System
3. The Monoamine Hypothesis of Depression and Melanocortins
4. The Inflammatory Hypothesis of Depression and Anti-Inflammatory Effects of Melanocortins
5. The Neuroendocrine Hypothesis of Depression and Melanocortins
6. The Neurotrophic Hypothesis of Depression and Melanocortins
7. The Neurogenesis Hypothesis of Depression and Melanocortins
8. The Glutamate Hypothesis of Depression and Melanocortins
9. The Endocannabinoid Hypothesis of Depression and Melanocortins
10. The Effect of Melanocortins on Depression-like and Anxious Behavior
11. The Role of Melanocortins in Motivational and Hedonic Behavior
12. Some Features of Melanocortins and Their Possible Site of Action
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Depression. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 28 February 2023).
- Moitra, M.; Santomauro, D.; Collins, P.Y.; Vos, T.; Whiteford, H.; Saxena, S.; Ferrari, A.J. The Global Gap in Treatment Coverage for Major Depressive Disorder in 84 Countries from 2000–2019: A Systematic Review and Bayesian Meta-Regression Analysis. PLoS Med. 2022, 19, e1003901. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- van Loo, H.M.; de Jonge, P.; Romeijn, J.-W.; Kessler, R.C.; Schoevers, R.A. Data-Driven Subtypes of Major Depressive Disorder: A Systematic Review. BMC Med. 2012, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic Epidemiology of Major Depression: Review and Meta-Analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef]
- Kendler, K.S.; Gatz, M.; Gardner, C.O.; Pedersen, N.L. A Swedish National Twin Study of Lifetime Major Depression. Am. J. Psychiatry 2006, 163, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ripke, S.; Wray, N.R.; Lewis, C.M.; Hamilton, S.P.; Weissman, M.M.; Breen, G.; Byrne, E.M.; Blackwood, D.H.R.; Boomsma, D.I.; Cichon, S.; et al. A Mega-Analysis of Genome-Wide Association Studies for Major Depressive Disorder. Mol. Psychiatry 2013, 18, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-Wide Association Analyses Identify 44 Risk Variants and Refine the Genetic Architecture of Major Depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-Wide Meta-Analysis of Depression Identifies 102 Independent Variants and Highlights the Importance of the Prefrontal Brain Regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Flint, J. The Genetic Basis of Major Depressive Disorder. Mol. Psychiatry 2023. [Google Scholar] [CrossRef]
- Dall’Aglio, L.; Lewis, C.M.; Pain, O. Delineating the Genetic Component of Gene Expression in Major Depression. Biol. Psychiatry 2021, 89, 627–636. [Google Scholar] [CrossRef]
- Fabbri, C.; Pain, O.; Hagenaars, S.P.; Lewis, C.M.; Serretti, A. Transcriptome-Wide Association Study of Treatment-Resistant Depression and Depression Subtypes for Drug Repurposing. Neuropsychopharmacology 2021, 46, 1821–1829. [Google Scholar] [CrossRef]
- Li, X.; Su, X.; Liu, J.; Li, H.; Li, M.; Li, W.; Luo, X.-J. Transcriptome-Wide Association Study Identifies New Susceptibility Genes and Pathways for Depression. Transl. Psychiatry 2021, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Mariani, N.; Cattane, N.; Pariante, C.; Cattaneo, A. Gene Expression Studies in Depression Development and Treatment: An Overview of the Underlying Molecular Mechanisms and Biological Processes to Identify Biomarkers. Transl. Psychiatry 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Morrison, F.G.; Miller, M.W.; Wolf, E.J.; Logue, M.W.; Maniates, H.; Kwasnik, D.; Cherry, J.D.; Svirsky, S.; Restaino, A.; Hildebrandt, A.; et al. Reduced Interleukin 1A Gene Expression in the Dorsolateral Prefrontal Cortex of Individuals with PTSD and Depression. Neurosci. Lett. 2019, 692, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Leday, G.G.R.; Vértes, P.E.; Richardson, S.; Greene, J.R.; Regan, T.; Khan, S.; Henderson, R.; Freeman, T.C.; Pariante, C.M.; Harrison, N.A.; et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol. Psychiatry 2018, 83, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, A.; Ferrari, C.; Turner, L.; Mariani, N.; Enache, D.; Hastings, C.; Kose, M.; Lombardo, G.; McLaughlin, A.P.; Nettis, M.A.; et al. Whole-Blood Expression of Inflammasome- and Glucocorticoid-Related MRNAs Correctly Separates Treatment-Resistant Depressed Patients from Drug-Free and Responsive Patients in the BIODEP Study. Transl. Psychiatry 2020, 10, 232. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The Molecular Neurobiology of Depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Kamran, M.; Bibi, F.; ur Rehman, A.; Morris, D.W. Major Depressive Disorder: Existing Hypotheses about Pathophysiological Mechanisms and New Genetic Findings. Genes 2022, 13, 646. [Google Scholar] [CrossRef]
- Lv, S.; Yao, K.; Zhang, Y.; Zhu, S. NMDA Receptors as Therapeutic Targets for Depression Treatment: Evidence from Clinical to Basic Research. Neuropharmacology 2023, 225, 109378. [Google Scholar] [CrossRef]
- Sheffler, Z.M.; Patel, P.; Abdijadid, S. Antidepressants; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tian, H.; Hu, Z.; Xu, J.; Wang, C. The Molecular Pathophysiology of Depression and the New Therapeutics. MedComm 2022, 3. [Google Scholar] [CrossRef]
- Li, K.; Zhou, G.; Xiao, Y.; Gu, J.; Chen, Q.; Xie, S.; Wu, J. Risk of Suicidal Behaviors and Antidepressant Exposure Among Children and Adolescents: A Meta-Analysis of Observational Studies. Front. Psychiatry 2022, 13, 880496. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Davidson, K.G. Definition and epidemiology of treatment-resistant depression. Psychiatr. Clin. N. Am. 1996, 19, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.S.; Patterson, E.H.; Hirshbein, L. Treatment-Resistant Depression Reconsidered. SSM–Ment. Health 2022, 2, 100081. [Google Scholar] [CrossRef]
- Kirsch, I.; Deacon, B.J.; Huedo-Medina, T.B.; Scoboria, A.; Moore, T.J.; Johnson, B.T. Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration. PLoS Med. 2008, 5, e45. [Google Scholar] [CrossRef] [Green Version]
- Hengartner, M.P.; Jakobsen, J.C.; Sørensen, A.; Plöderl, M. Efficacy of New-Generation Antidepressants Assessed with the Montgomery-Asberg Depression Rating Scale, the Gold Standard Clinician Rating Scale: A Meta-Analysis of Randomised Placebo-Controlled Trials. PLoS ONE 2020, 15, e0229381. [Google Scholar] [CrossRef] [Green Version]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Baumann, J.; Wheeler-Castillo, C.; Latov, D.; Henter, I.; Salvadore, G.; Zarate, C. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals 2010, 3, 19–41. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant Effects of Ketamine in Depressed Patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- aan het Rot, M.; Zarate, C.A.; Charney, D.S.; Mathew, S.J. Ketamine for Depression: Where Do We Go from Here? Biol. Psychiatry 2012, 72, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Yavi, M.; Lee, H.; Henter, I.D.; Park, L.T.; Zarate, C.A. Ketamine Treatment for Depression: A Review. Discov. Ment. Health 2022, 2, 9. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Blasey, C.; Sudheimer, K.; Pannu, J.; Pankow, H.; Hawkins, J.; Birnbaum, J.; Lyons, D.M.; Rodriguez, C.I.; et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 2018, 175, 1205–1215. [Google Scholar] [CrossRef]
- Zhang, F.; Hillhouse, T.M.; Anderson, P.M.; Koppenhaver, P.O.; Kegen, T.N.; Manicka, S.G.; Lane, J.T.; Pottanat, E.; Van Fossen, M.; Rice, R.; et al. Opioid Receptor System Contributes to the Acute and Sustained Antidepressant-like Effects, but Not the Hyperactivity Motor Effects of Ketamine in Mice. Pharmacol. Biochem. Behav. 2021, 208, 173228. [Google Scholar] [CrossRef] [PubMed]
- Alldredge, B. Pathogenic Involvement of Neuropeptides in Anxiety and Depression. Neuropeptides 2010, 44, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Heilig, M.; Rupniak, N.M.J.; Steckler, T.; Griebel, G. Neuropeptide Systems as Novel Therapeutic Targets for Depression and Anxiety Disorders. Trends Pharmacol. Sci. 2003, 24, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaszner, B. Role of Neuropeptides in Anxiety, Stress, and Depression: From Animals to Humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef]
- Kupcova, I.; Danisovic, L.; Grgac, I.; Harsanyi, S. Anxiety and Depression: What Do We Know of Neuropeptides? Behav. Sci. 2022, 12, 262. [Google Scholar] [CrossRef]
- Lee, M. The Central Melanocortin System and the Regulation of Energy Balance. Front. Biosci. 2007, 12, 3994. [Google Scholar] [CrossRef] [Green Version]
- Ulrich-Lai, Y.M.; Ryan, K.K. Neuroendocrine Circuits Governing Energy Balance and Stress Regulation: Functional Overlap and Therapeutic Implications. Cell Metab. 2014, 19, 910–925. [Google Scholar] [CrossRef] [Green Version]
- Micioni Di Bonaventura, E.; Botticelli, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Romano, A.; Gaetani, S.; Micioni Di Bonaventura, M.V.; Cifani, C. Investigating the Role of the Central Melanocortin System in Stress and Stress-Related Disorders. Pharmacol. Res. 2022, 185, 106521. [Google Scholar] [CrossRef]
- Laiho, L.; Murray, J.F. The Multifaceted Melanocortin Receptors. Endocrinology 2022, 163, bqac083. [Google Scholar] [CrossRef]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.I.; Funder, J.W. Proopiomelanocortin Processing in the Pituitary, Central Nervous System, and Peripheral Tissues. Endocr. Rev. 1988, 9, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Jacobowitz, D.M.; O’Donohue, T.L. Alpha-Melanocyte Stimulating Hormone: Immunohistochemical Identification and Mapping in Neurons of Rat Brain. Proc. Natl. Acad. Sci. USA 1978, 75, 6300–6304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikberg, J.E. Melanocortin Receptors: Perspectives for Novel Drugs. Eur. J. Pharmacol. 1999, 375, 295–310. [Google Scholar] [CrossRef]
- Yang, Y. Structure, Function and Regulation of the Melanocortin Receptors. Eur. J. Pharmacol. 2011, 660, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Getting, S.J. Targeting Melanocortin Receptors as Potential Novel Therapeutics. Pharmacol. Ther. 2006, 111, 1–15. [Google Scholar] [CrossRef]
- Shukla, C.; Koch, L.G.; Britton, S.L.; Cai, M.; Hruby, V.J.; Bednarek, M.; Novak, C.M. Contribution of Regional Brain Melanocortin Receptor Subtypes to Elevated Activity Energy Expenditure in Lean, Active Rats. Neuroscience 2015, 310, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.; Almeida, H.; Gouveia, A.M. Intracellular Signaling Mechanisms of the Melanocortin Receptors: Current State of the Art. Cell Mol. Life Sci. 2015, 72, 1331–1345. [Google Scholar] [CrossRef]
- Chan, L.F.; Webb, T.R.; Chung, T.-T.; Meimaridou, E.; Cooray, S.N.; Guasti, L.; Chapple, J.P.; Egertová, M.; Elphick, M.R.; Cheetham, M.E.; et al. MRAP and MRAP2 Are Bidirectional Regulators of the Melanocortin Receptor Family. Proc. Natl. Acad. Sci. USA 2009, 106, 6146–6151. [Google Scholar] [CrossRef] [Green Version]
- Berruien, N.N.A.; Smith, C.L. Emerging Roles of Melanocortin Receptor Accessory Proteins (MRAP and MRAP2) in Physiology and Pathophysiology. Gene 2020, 757, 144949. [Google Scholar] [CrossRef]
- Novoselova, T.V.; Chan, L.F.; Clark, A.J.L. Pathophysiology of Melanocortin Receptors and Their Accessory Proteins. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Ericson, M.D.; Lensing, C.J.; Fleming, K.A.; Schlasner, K.N.; Doering, S.R.; Haskell-Luevano, C. Bench-Top to Clinical Therapies: A Review of Melanocortin Ligands from 1954 to 2016. Biochim. Biophys. Acta–Mol. Basis Dis. 2017, 1863, 2414–2435. [Google Scholar] [CrossRef]
- Montero-Melendez, T.; Boesen, T.; Jonassen, T.E.N. Translational Advances of Melanocortin Drugs: Integrating Biology, Chemistry and Genetics. Semin. Immunol. 2022, 59, 101603. [Google Scholar] [CrossRef] [PubMed]
- Ruhé, H.G.; Mason, N.S.; Schene, A.H. Mood Is Indirectly Related to Serotonin, Norepinephrine and Dopamine Levels in Humans: A Meta-Analysis of Monoamine Depletion Studies. Mol. Psychiatry 2007, 12, 331–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumeister, A.A.; Hawkins, M.F.; Uzelac, S.M. The Myth of Reserpine-Induced Depression: Role in the Historical Development of the Monoamine Hypothesis. J. Hist. Neurosci. 2003, 12, 207–220. [Google Scholar] [CrossRef]
- Strawbridge, R.; Javed, R.R.; Cave, J.; Jauhar, S.; Young, A.H. The Effects of Reserpine on Depression: A Systematic Review. J. Psychopharmacol. 2022. [Google Scholar] [CrossRef]
- Haase, J.; Brown, E. Integrating the Monoamine, Neurotrophin and Cytokine Hypotheses of Depression—A Central Role for the Serotonin Transporter? Pharmacol. Ther. 2015, 147, 1–11. [Google Scholar] [CrossRef]
- Sánchez, M.S.; Barontini, M.; Armando, I.; Celis, M.E. Correlation of Increased Grooming Behavior and Motor Activity with Alterations in Nigrostriatal and Mesolimbic Catecholamines after Alpha-Melanotropin and Neuropeptide Glutamine-Isoleucine Injection in the Rat Ventral Tegmental Area. Cell Mol. Neurobiol. 2001, 21, 523–533. [Google Scholar] [CrossRef]
- Davis, J.F.; Choi, D.L.; Shurdak, J.D.; Krause, E.G.; Fitzgerald, M.F.; Lipton, J.W.; Sakai, R.R.; Benoit, S.C. Central Melanocortins Modulate Mesocorticolimbic Activity and Food Seeking Behavior in the Rat. Physiol. Behav. 2011, 102, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Roseberry, A.G.; Stuhrman, K.; Dunigan, A.I. Regulation of the Mesocorticolimbic and Mesostriatal Dopamine Systems by α-Melanocyte Stimulating Hormone and Agouti-Related Protein. Neurosci. Biobehav. Rev. 2015, 56, 15–25. [Google Scholar] [CrossRef]
- Lindblom, J.; Opmane, B.; Mutulis, F.; Mutule, I.; Petrovska, R.; Klusa, V.; Bergström, L.; Wikberg, J.E.S. The MC4 Receptor Mediates α-MSH Induced Release of Nucleus Accumbens Dopamine. Neuroreport 2001, 12, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, J.; Kask, A.; Hägg, E.; Härmark, L.; Bergström, L.; Wikberg, J. Chronic Infusion of a Melanocortin Receptor Agonist Modulates Dopamine Receptor Binding in the Rat Brain. Pharmacol. Res. 2002, 45, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Florijn, W.J.; De Boer, T.; Tonnaer, J.A.; Versteeg, D.H. Characterization of the Inhibitory Effect of Adrenocorticotropin/Melanocyte-Stimulating Hormone-like Peptides on the Binding of Dopamine Receptor Ligands to the Dopamine D2 Receptor in Vitro. J. Pharmacol. Exp. Ther. 1992, 263, 787–792. [Google Scholar]
- Kawashima, N.; Chaki, S.; Okuyama, S. Electrophysiological Effects of Melanocortin Receptor Ligands on Neuronal Activities of Monoaminergic Neurons in Rats. Neurosci. Lett. 2003, 353, 119–122. [Google Scholar] [CrossRef]
- Markey, K.A.; Sze, P.Y. Influence of ACTH on Tyrosine Hydroxylase Activity in the Locus Coeruleus of Mouse Brain. Neuroendocrinology 1984, 38, 269–275. [Google Scholar] [CrossRef]
- Versteeg, D.H.G.; Wurtman, R.J. Effect of ACTH4–10 on the Rate of Synthesis of [3H]Catecholamines in the Brains of Intact, Hypophysectomized and Adrenalectomized Rats. Brain Res. 1975, 93, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Racca, S.; Spaccamiglio, A.; Esculapio, P.; Abbadessa, G.; Cangemi, L.; DiCarlo, F.; Portaleone, P. Effects of Swim Stress and α-MSH Acute Pre-Treatment on Brain 5-HT Transporter and Corticosterone Receptor. Pharmacol. Biochem. Behav. 2005, 81, 894–900. [Google Scholar] [CrossRef]
- Eremin, K.O.; Kudrin, V.S.; Saransaari, P.; Oja, S.S.; Grivennikov, I.A.; Myasoedov, N.F.; Rayevsky, K.S. Semax, An ACTH(4-10) Analogue with Nootropic Properties, Activates Dopaminergic and Serotoninergic Brain Systems in Rodents. Neurochem. Res. 2005, 30, 1493–1500. [Google Scholar] [CrossRef]
- Krishnadas, R.; Cavanagh, J. Depression: An Inflammatory Illness? Figure 1. J. Neurol. Neurosurg. Psychiatry 2012, 83, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Patel, A. Review: The Role of Inflammation in Depression. Psychiatr. Danub. 2013, 25 (Suppl. 2), S216–S223. [Google Scholar]
- Brás, J.P.; Pinto, S.; Almeida, M.I.; Prata, J.; von Doellinger, O.; Coelho, R.; Barbosa, M.A.; Santos, S.G. Peripheral Biomarkers of Inflammation in Depression: Evidence from Animal Models and Clinical Studies. In Psychiatric Disorders: Methods and Protocols; Humana: New York, NY, USA, 2019; pp. 467–492. [Google Scholar]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5,166 Patients and 5,083 Controls. Brain. Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Engelbrechta, M.A.; Gut, O.; Fiebich, B.L.; Bauer, J.; Schmidt, F.; Grunze, H.; Lieb, K. Interferon Alpha (IFNα) and Psychiatric Syndromes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 731–746. [Google Scholar] [CrossRef]
- Schedlowski, M.; Engler, H.; Grigoleit, J.-S. Endotoxin-Induced Experimental Systemic Inflammation in Humans: A Model to Disentangle Immune-to-Brain Communication. Brain. Behav. Immun. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmächer, T. Cytokine-Associated Emotional and Cognitive Disturbances in Humans. Arch. Gen. Psychiatry 2001, 58, 445. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Inagaki, T.K.; Rameson, L.T.; Mashal, N.M.; Irwin, M.R. An FMRI Study of Cytokine-Induced Depressed Mood and Social Pain: The Role of Sex Differences. Neuroimage 2009, 47, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Grigoleit, J.-S.; Kullmann, J.S.; Wolf, O.T.; Hammes, F.; Wegner, A.; Jablonowski, S.; Engler, H.; Gizewski, E.; Oberbeck, R.; Schedlowski, M. Dose-Dependent Effects of Endotoxin on Neurobehavioral Functions in Humans. PLoS ONE 2011, 6, e28330. [Google Scholar] [CrossRef] [Green Version]
- Draper, A.; Koch, R.M.; van der Meer, J.W.; AJ Apps, M.; Pickkers, P.; Husain, M.; van der Schaaf, M.E. Effort but Not Reward Sensitivity Is Altered by Acute Sickness Induced by Experimental Endotoxemia in Humans. Neuropsychopharmacology 2018, 43, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Benson, S.; Rebernik, L.; Wegner, A.; Kleine-Borgmann, J.; Engler, H.; Schlamann, M.; Forsting, M.; Schedlowski, M.; Elsenbruch, S. Neural Circuitry Mediating Inflammation-Induced Central Pain Amplification in Human Experimental Endotoxemia. Brain. Behav. Immun. 2015, 48, 222–231. [Google Scholar] [CrossRef]
- Lasselin, J.; Elsenbruch, S.; Lekander, M.; Axelsson, J.; Karshikoff, B.; Grigoleit, J.-S.; Engler, H.; Schedlowski, M.; Benson, S. Mood Disturbance during Experimental Endotoxemia: Predictors of State Anxiety as a Psychological Component of Sickness Behavior. Brain. Behav. Immun. 2016, 57, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Elsenbruch, S.; Maluck, J.; Grigoleit, J.-S.; Engler, H.; Jäger, M.; Spreitzer, I.; Schedlowski, M.; Benson, S. Inflammation-Induced Hyperalgesia: Effects of Timing, Dosage, and Negative Affect on Somatic Pain Sensitivity in Human Experimental Endotoxemia. Brain. Behav. Immun. 2014, 41, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Grigoleit, J.-S.; Kullmann, J.S.; Oberbeck, R.; Schedlowski, M.; Engler, H. Salivary α-Amylase Response to Endotoxin Administration in Humans. Psychoneuroendocrinology 2013, 38, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Engler, H.; Benson, S.; Wegner, A.; Spreitzer, I.; Schedlowski, M.; Elsenbruch, S. Men and Women Differ in Inflammatory and Neuroendocrine Responses to Endotoxin but Not in the Severity of Sickness Symptoms. Brain. Behav. Immun. 2016, 52, 18–26. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So Depression Is an Inflammatory Disease, but Where Does the Inflammation Come From? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Steptoe, A.; Kunz-Ebrecht, S.R.; Owen, N. Lack of Association between Depressive Symptoms and Markers of Immune and Vascular Inflammation in Middle-Aged Men and Women. Psychol. Med. 2003, 33, 667–674. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Szałach, Ł.P.; Lisowska, K.A.; Cubała, W.J. The Influence of Antidepressants on the Immune System. Arch. Immunol. Ther. Exp. 2019, 67, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Kenis, G.; Maes, M. Effects of Antidepressants on the Production of Cytokines. Int. J. Neuropsychopharmacol. 2002, 5, S1461145702003164. [Google Scholar] [CrossRef] [Green Version]
- Janssen, D.G.A.; Caniato, R.N.; Verster, J.C.; Baune, B.T. A Psychoneuroimmunological Review on Cytokines Involved in Antidepressant Treatment Response. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.; Lavretsky, H.; Kartika, J.; Qassim, A.; Baune, B. Modulatory Effects of Antidepressant Classes on the Innate and Adaptive Immune System in Depression. Pharmacopsychiatry 2016, 49, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, J.; Ormstad, H.; Aass, H.C.D.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The Plasma Levels of Various Cytokines Are Increased during Ongoing Depression and Are Reduced to Normal Levels after Recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Herane Vives, A.; Cleare, A.J. Inflammation and Clinical Response to Treatment in Depression: A Meta-Analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2017, 55, 4195–4206. [Google Scholar] [CrossRef] [Green Version]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of Antidepressant Treatment on Peripheral Inflammation Markers–A Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef]
- Köhler, O.; Benros, M.E.; Nordentoft, M.; Farkouh, M.E.; Iyengar, R.L.; Mors, O.; Krogh, J. Effect of Anti-Inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects. JAMA Psychiatry 2014, 71, 1381. [Google Scholar] [CrossRef]
- Eyre, H.A.; Air, T.; Proctor, S.; Rositano, S.; Baune, B.T. A Critical Review of the Efficacy of Non-Steroidal Anti-Inflammatory Drugs in Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 57, 11–16. [Google Scholar] [CrossRef]
- Baune, B.T. Are Non-Steroidal Anti-Inflammatory Drugs Clinically Suitable for the Treatment of Symptoms in Depression-Associated Inflammation? Curr. Top. Behav. Neurosci. 2016, 31, 303–319. [Google Scholar]
- Warner-Schmidt, J.L.; Vanover, K.E.; Chen, E.Y.; Marshall, J.J.; Greengard, P. Antidepressant Effects of Selective Serotonin Reuptake Inhibitors (SSRIs) Are Attenuated by Antiinflammatory Drugs in Mice and Humans. Proc. Natl. Acad. Sci. USA 2011, 108, 9262–9267. [Google Scholar] [CrossRef] [Green Version]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of Anti-inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, Cytokines and Brain Abnormalities in Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, D.-Y.; Lin, Y.-J.; Tao, Y.-X. Melanocortin Regulation of Inflammation. Front. Endocrinol. 2019, 10, 683. [Google Scholar] [CrossRef] [Green Version]
- Dinparastisaleh, R.; Mirsaeidi, M. Antifibrotic and Anti-Inflammatory Actions of α-Melanocytic Hormone: New Roles for an Old Player. Pharmaceuticals 2021, 14, 45. [Google Scholar] [CrossRef]
- Luger, T.A.; Scholzen, T.E.; Brzoska, T.; Böhm, M. New Insights into the Functions of α-MSH and Related Peptides in the Immune System. Ann. N. Y. Acad. Sci. 2003, 994, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Kannengiesser, K.; Kucharzik, T. Role of the Melanocortin System in Inflammation. Ann. N. Y. Acad. Sci. 2006, 1072, 123–134. [Google Scholar] [CrossRef]
- Brzoska, T.; Luger, T.A.; Maaser, C.; Abels, C.; Böhm, M. α-Melanocyte-Stimulating Hormone and Related Tripeptides: Biochemistry, Antiinflammatory and Protective Effects in Vitro and in Vivo, and Future Perspectives for the Treatment of Immune-Mediated Inflammatory Diseases. Endocr. Rev. 2008, 29, 581–602. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.W.; Lipton, J.M. Acute Phase Response to Endotoxin: Rise in Plasma Alpha-MSH and Effects of Alpha-MSH Injection. Am. J. Physiol. Integr. Comp. Physiol. 1990, 259, R768–R772. [Google Scholar] [CrossRef]
- Catania, A.; Suffredini, A.F.; Lipton, J.M. Endotoxin Causes Release of α-Melanocyte-Stimulating Hormone in Normal Human Subjects. Neuroimmunomodulation 1995, 2, 258–262. [Google Scholar] [CrossRef]
- Sergeyev, V.; Broberger, C.; Hökfelt, T. Effect of LPS Administration on the Expression of POMC, NPY, Galanin, CART and MCH MRNAs in the Rat Hypothalamus. Mol. Brain Res. 2001, 90, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Goelst, K.; Mitchell, D.; Laburn, H. Effects of Alpha-Melanocyte Stimulating Hormone on Fever Caused by Endotoxin in Rabbits. J. Physiol. 1991, 441, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.W.; Catania, A.; Hiltz, M.E.; Lipton, J.M. Neuropeptide α-MSH Antagonizes IL-6- and TNF-Induced Fever. Peptides 1991, 12, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Holdeman, M.; Lipton, J.M. Antipyretic Activity of a Potent α-MSH Analog. Peptides 1985, 6, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-H.; Entwistle, M.L.; Alvaro, J.D.; Duman, R.S.; Hruby, V.J.; Tatro, J.B. Antipyretic Role of Endogenous Melanocortins Mediated by Central Melanocortin Receptors during Endotoxin-Induced Fever. J. Neurosci. 1997, 17, 3343–3351. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.-H.; Hruby, V.J.; Tatro, J.B. Systemic α-MSH Suppresses LPS Fever via Central Melanocortin Receptors Independently of Its Suppression of Corticosterone and IL-6 Release. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R524–R530. [Google Scholar] [CrossRef]
- Sinha, P.S.; Schiöth, H.B.; Tatro, J.B. Roles of the Melanocortin-4 Receptor in Antipyretic and Hyperthermic Actions of Centrally Administered α-MSH. Brain Res. 2004, 1001, 150–158. [Google Scholar] [CrossRef]
- Lipton, J.M.; Catania, A.; Delgado, R. Peptide Modulation of Inflammatory Processes within the Brain. Neuroimmunomodulation 1998, 5, 178–183. [Google Scholar] [CrossRef]
- Caruso, C.; Mohn, C.; Karara, A.L.; Rettori, V.; Watanobe, H.; Schiöth, H.B.; Seilicovich, A.; Lasaga, M. Alpha-Melanocyte-Stimulating Hormone through Melanocortin-4 Receptor Inhibits Nitric Oxide Synthase and Cyclooxygenase Expression in the Hypothalamus of Male Rats. Neuroendocrinology 2004, 79, 278–286. [Google Scholar] [CrossRef]
- Rajora, N.; Boccoli, G.; Burns, D.; Sharma, S.; Catania, A.P.; Lipton, J.M. α-MSH Modulates Local and Circulating Tumor Necrosis Factor-α in Experimental Brain Inflammation. J. Neurosci. 1997, 17, 2181–2186. [Google Scholar] [CrossRef] [Green Version]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Dmitrieva, V.G.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Valieva, L.V.; Sudarkina, O.Y.; Gubsky, L.V.; et al. The Peptide Drug ACTH(4-7)PGP (Semax) Suppresses mRNA Transcripts Encoding Proinflammatory Mediators Induced by Reversible Ischemia of the Rat Brain. Mol. Biol. 2021, 55, 402–411. [Google Scholar] [CrossRef]
- Yu, S.; Doycheva, D.M.; Gamdzyk, M.; Yang, Y.; Lenahan, C.; Li, G.; Li, D.; Lian, L.; Tang, J.; Lu, J.; et al. Activation of MC1R with BMS-470539 Attenuates Neuroinflammation via CAMP/PKA/Nurr1 Pathway after Neonatal Hypoxic-Ischemic Brain Injury in Rats. J. Neuroinflamm. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Carlin, A.; Airaghi, L.; Demitri, M.T.; Meda, L.; Galimberti, D.; Baron, P.; Lipton, J.M.; Catania, A. Melanocortin Peptides Inhibit Production of Proinflammatory Cytokines and Nitric Oxide by Activated Microglia. J. Leukoc. Biol. 1998, 63, 740–745. [Google Scholar] [CrossRef]
- Yue Wong, K.; Rajora, N.; Boccoli, G.; Catania, A.; Lipton, J.M. A Potential Mechanism of Local Anti-Inflammatory Action of Alpha-Melanocyte-Stimulating Hormone within the Brain: Modulation of Tumor Necrosis Factor-Alpha Production by Human Astrocytic Cells. Neuroimmunomodulation 1997, 4, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kamermans, A.; Verhoeven, T.; van het Hof, B.; Koning, J.J.; Borghuis, L.; Witte, M.; van Horssen, J.; de Vries, H.E.; Rijnsburger, M. Setmelanotide, a Novel, Selective Melanocortin Receptor-4 Agonist Exerts Anti-Inflammatory Actions in Astrocytes and Promotes an Anti-Inflammatory Macrophage Phenotype. Front. Immunol. 2019, 10, 2312. [Google Scholar] [CrossRef]
- Lipton, J.M.; Catania, A. Mechanisms of Antiinflammatory Action of the Neuroimmunomodulatory Peptide α-MSH. Ann. N. Y. Acad. Sci. 1998, 840, 373–380. [Google Scholar] [CrossRef]
- Star, R.A.; Rajora, N.; Huang, J.; Stock, R.C.; Catania, A.; Lipton, J.M. Evidence of Autocrine Modulation of Macrophage Nitric Oxide Synthase by Alpha-Melanocyte-Stimulating Hormone. Proc. Natl. Acad. Sci. USA 1995, 92, 8016–8020. [Google Scholar] [CrossRef] [Green Version]
- Neumann Andersen, G.; Nagaeva, O.; Mandrika, I.; Petrovska, R.; Muceniece, R.; Mincheva-Nilsson, L.; Wikberg, J.E.S. MC1 Receptors Are Constitutively Expressed on Leucocyte Subpopulations with Antigen Presenting and Cytotoxic Functions. Clin. Exp. Immunol. 2002, 126, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.; Rajora, N.; Capsoni, F.; Minonzio, F.; Star, R.A.; Lipton, J.M. The Neuropeptide α-MSH Has Specific Receptors on Neutrophils and Reduces Chemotaxis in Vitro. Peptides 1996, 17, 675–679. [Google Scholar] [CrossRef]
- Becher, E.; Mahnke, K.; Brzoska, T.; Kalden, D.-H.; Grabbe, S.; Luger, T.A. Human Peripheral Blood-Derived Dendritic Cells Express Functional Melanocortin Receptor MC-1R. Ann. N. Y. Acad. Sci. 2006, 885, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.W.; Hughes, T.K.; Smith, E.M. ACTH Receptor Distribution and Modulation among Murine Mononuclear Leukocyte Populations. J. Biol. Regul. Homeost. Agents 2001, 15, 156–162. [Google Scholar]
- Getting, S.J.; Gibbs, L.; Clark, A.J.; Flower, R.J.; Perretti, M. POMC Gene-Derived Peptides Activate Melanocortin Type 3 Receptor on Murine Macrophages, Suppress Cytokine Release, and Inhibit Neutrophil Migration in Acute Experimental Inflammation. J. Immunol. 1999, 162, 7446–7453. [Google Scholar] [CrossRef]
- BUGGY, J.J. Binding of α-Melanocyte-Stimulating Hormone to Its G-Protein-Coupled Receptor on B-Lymphocytes Activates the Jak/STAT Pathway. Biochem. J. 1998, 331, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, M.; Nagaev, I.; Meyer, M.K.; Nagaeva, O.; Wikberg, J.; Mincheva-Nilsson, L.; Andersen, G.N. Melanocortin 2, 3 and 4 Receptor Gene Expressions Are Downregulated in CD8+ T Cytotoxic Lymphocytes and CD19 + B Lymphocytes in Rheumatoid Arthritis Responding to TNF- α Inhibition. Scand. J. Immunol. 2017, 86, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muceniece, R.; Dambrova, M. Melanocortins in Brain Inflammation: The Role of Melanocortin Receptor Subtypes. In Melanocortins: Multiple Actions and Therapeutic Potential; Springer: New York, NY, USA, 2010; Volume 681, pp. 61–70. [Google Scholar]
- Kishi, T.; Aschkenasi, C.J.; Lee, C.E.; Mountjoy, K.G.; Saper, C.B.; Elmquist, J.K. Expression of Melanocortin 4 Receptor MRNA in the Central Nervous System of the Rat. J. Comp. Neurol. 2003, 457, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Roselli-Rehfuss, L.; Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Low, M.J.; Tatro, J.B.; Entwistle, M.L.; Simerly, R.B.; Cone, R.D. Identification of a Receptor for Gamma Melanotropin and Other Proopiomelanocortin Peptides in the Hypothalamus and Limbic System. Proc. Natl. Acad. Sci. USA 1993, 90, 8856–8860. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.; Getting, S. Melanocortin Receptor Type 3 as a Potential Target for Anti-Inflammatory Therapy. Curr. Drug Target -Inflamm. Allergy 2004, 3, 311–315. [Google Scholar] [CrossRef]
- Lasaga, M.; Debeljuk, L.; Durand, D.; Scimonelli, T.N.; Caruso, C. Role of α-Melanocyte Stimulating Hormone and Melanocortin 4 Receptor in Brain Inflammation. Peptides 2008, 29, 1825–1835. [Google Scholar] [CrossRef]
- Caruso, C.; Durand, D.; Schiöth, H.B.; Rey, R.; Seilicovich, A.; Lasaga, M. Activation of Melanocortin 4 Receptors Reduces the Inflammatory Response and Prevents Apoptosis Induced by Lipopolysaccharide and Interferon-γ in Astrocytes. Endocrinology 2007, 148, 4918–4926. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, K.W.; Reyes, T.M. Central Blockade of Melanocortin Receptors Attenuates the Metabolic and Locomotor Responses to Peripheral Interleukin-1β Administration. Neuropharmacology 2008, 54, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cernackova, A.; Durackova, Z.; Trebaticka, J.; Mravec, B. Neuroinflammation and Depressive Disorder: The Role of the Hypothalamus. J. Clin. Neurosci. 2020, 75, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, A.; Martínez-Cengotitabengoa, M.; Peters-Corbett, A.; Diaz-Gutierrez, M.J.; Martínez-Cengotitabengoa, M. Alterations of the HPA Axis Observed in Patients with Major Depressive Disorder and Their Relation to Early Life Stress: A Systematic Review. Neuropsychobiology 2020, 79, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Priftis, K.N. Regulation of the Hypothalamic-Pituitary-Adrenal Axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Ostrander, M.M.; Mueller, N.K.; Figueiredo, H. Limbic System Mechanisms of Stress Regulation: Hypothalamo-Pituitary-Adrenocortical Axis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 1201–1213. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Glucocorticoid-Sensitive Hippocampal Neurons Are Involved in Terminating the Adrenocortical Stress Response. Proc. Natl. Acad. Sci. USA 1984, 81, 6174–6177. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, L.; Sapolsky, R. The Role of the Hippocampus in Feedback Regulation of the Hypothalamic-Pituitary-Adrenocortical Axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef]
- Cole, A.B.; Montgomery, K.; Bale, T.L.; Thompson, S.M. What the Hippocampus Tells the HPA Axis: Hippocampal Output Attenuates Acute Stress Responses via Disynaptic Inhibition of CRF+ PVN Neurons. Neurobiol. Stress 2022, 20, 100473. [Google Scholar] [CrossRef]
- Herman, J.; Schafer, M.; Young, E.; Thompson, R.; Douglass, J.; Akil, H.; Watson, S. Evidence for Hippocampal Regulation of Neuroendocrine Neurons of the Hypothalamo-Pituitary-Adrenocortical Axis. J. Neurosci. 1989, 9, 3072–3082. [Google Scholar] [CrossRef] [Green Version]
- Diorio, D.; Viau, V.; Meaney, M. The Role of the Medial Prefrontal Cortex (Cingulate Gyrus) in the Regulation of Hypothalamic-Pituitary-Adrenal Responses to Stress. J. Neurosci. 1993, 13, 3839–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, S.; Conforti, N.; Itzik, A.; Weidenfeld, J. Differential Effect of Amygdaloid Lesions on CRF-41, ACTH and Corticosterone Responses Following Neural Stimuli. Brain Res. 1994, 658, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.T.; Phillips, J.J.; McCracken, J.T.; Sadow, T.F. Adrenal Gland Volume in Major Depression: Relationship to Basal and Stimulated Pituitary-Adrenal Cortical Axis Function. Biol. Psychiatry 1996, 40, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Cassidy, F.; Naftolowitz, D.; Tatham, N.E.; Wilson, W.H.; Iranmanesh, A.; Liu, P.Y.; Veldhuis, J.D. Pathophysiology of Hypercortisolism in Depression. Acta Psychiatr. Scand. 2007, 115, 90–103. [Google Scholar] [CrossRef]
- Vreeburg, S.A.; Hoogendijk, W.J.G.; van Pelt, J.; DeRijk, R.H.; Verhagen, J.C.M.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W.J.H. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity. Arch. Gen. Psychiatry 2009, 66, 617. [Google Scholar] [CrossRef] [Green Version]
- Dienes, K.A.; Hazel, N.A.; Hammen, C.L. Cortisol Secretion in Depressed, and at-Risk Adults. Psychoneuroendocrinology 2013, 38, 927–940. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, K.R.R.; Doraiswamy, P.M.; Lurie, S.N.; Figiel, G.S.; Husain, M.M.; Boyko, O.B.; ELLINWOOD, E.H.; Nemeroff, C.B. Pituitary Size in Depression*. J. Clin. Endocrinol. Metab. 1991, 72, 256–259. [Google Scholar] [CrossRef]
- Delvecchio, G.; Altamura, A.C.; Soares, J.C.; Brambilla, P. Pituitary Gland in Bipolar Disorder and Major Depression: Evidence from Structural MRI Studies. J. Affect. Disord. 2017, 218, 446–450. [Google Scholar] [CrossRef]
- Wu, T.-C.; Chen, H.-T.; Chang, H.-Y.; Yang, C.-Y.; Hsiao, M.-C.; Cheng, M.-L.; Chen, J.-C. Mineralocorticoid Receptor Antagonist Spironolactone Prevents Chronic Corticosterone Induced Depression-like Behavior. Psychoneuroendocrinology 2013, 38, 871–883. [Google Scholar] [CrossRef]
- Kvarta, M.D.; Bradbrook, K.E.; Dantrassy, H.M.; Bailey, A.M.; Thompson, S.M. Corticosterone Mediates the Synaptic and Behavioral Effects of Chronic Stress at Rat Hippocampal Temporoammonic Synapses. J. Neurophysiol. 2015, 114, 1713–1724. [Google Scholar] [CrossRef] [Green Version]
- Sterner, E.Y.; Kalynchuk, L.E. Behavioral and Neurobiological Consequences of Prolonged Glucocorticoid Exposure in Rats: Relevance to Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cui, X.-Y.; Cui, S.-Y.; Ye, H.; Hu, X.; Zhao, H.-L.; Liu, Y.-T.; Zhang, Y.-H. Depression-like Behaviors Induced by Chronic Corticosterone Exposure via Drinking Water: Time-Course Analysis. Neurosci. Lett. 2018, 687, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Gallagher, P.; Del-Estal, D.; Hearn, A.; Ferrier, I.N.; Young, A.H. Hypothalamic–Pituitary–Adrenal Axis Function in Patients with Chronic Depression. Psychol. Med. 2002, 32, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.M.; Davis, M.C.; Otte, C.; Mohr, D.C. Depression and Cortisol Responses to Psychological Stress: A Meta-Analysis. Psychoneuroendocrinology 2005, 30, 846–856. [Google Scholar] [CrossRef]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary Cortisol in Depressed Patients versus Control Persons: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef]
- Carroll, B.J.; Iranmanesh, A.; Keenan, D.M.; Cassidy, F.; Wilson, W.H.; Veldhuis, J.D. Pathophysiology of Hypercortisolism in Depression: Pituitary and Adrenal Responses to Low Glucocorticoid Feedback. Acta Psychiatr. Scand. 2012, 125, 478–491. [Google Scholar] [CrossRef] [Green Version]
- Stokes, P.E. Pretreatment DST and Hypothalamic-Pituitary-Adrenocortical Function in Depressed Patients and Comparison Groups. Arch. Gen. Psychiatry 1984, 41, 257. [Google Scholar] [CrossRef]
- Herbert, J. Cortisol and Depression: Three Questions for Psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef]
- Carpenter, W.T.; Bunney, W.E. Adrenal Cortical Activity in Depressive Illness. Am. J. Psychiatry 1971, 128, 31–40. [Google Scholar] [CrossRef]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder—Translating Findings from Humans to Animal Models and Back. Front. Psychiatry 2020, 10, 974. [Google Scholar] [CrossRef]
- Pariante, C.M.; Miller, A.H. Glucocorticoid Receptors in Major Depression: Relevance to Pathophysiology and Treatment. Biol. Psychiatry 2001, 49, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.W.; Hu, F.; Miller, A.H. Cytokine-Effects on Glucocorticoid Receptor Function: Relevance to Glucocorticoid Resistance and the Pathophysiology and Treatment of Major Depression. Brain. Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Ising, M.; Künzel, H.E.; Binder, E.B.; Nickel, T.; Modell, S.; Holsboer, F. The Combined Dexamethasone/CRH Test as a Potential Surrogate Marker in Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Gallagher, P.; Smith, M.S.; Ferrier, I.N.; Young, A.H. The Dex/CRH Test—Is It Better than the DST? Psychoneuroendocrinology 2006, 31, 889–894. [Google Scholar] [CrossRef]
- Mokhtari, M.; Arfken, C.; Boutros, N. The DEX/CRH Test for Major Depression: A Potentially Useful Diagnostic Test. Psychiatry Res. 2013, 208, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F.; Barden, N. Antidepressants and Hypothalamic-Pituitary-Adrenocortical Regulation. Endocr. Rev. 1996, 17, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Pariante, C.M. The Effects of Antidepressants on the Hypothalamic-Pituitary-Adrenal Axis. Drug News Perspect. 2006, 19, 603. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, S.; Dose, T.; Lucae, S.; Kloiber, S.; Menke, A.; Hennings, J.; Spieler, D.; Uhr, M.; Holsboer, F.; Ising, M. Suppressive Effect of Mirtazapine on the HPA System in Acutely Depressed Women Seems to Be Transient and Not Related to Antidepressant Action. Psychoneuroendocrinology 2009, 34, 238–248. [Google Scholar] [CrossRef]
- Menke, A. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front. Psychiatry 2019, 10, 101. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, Z.; Yan, H.; Guo, W. Efficacy of Treatments Targeting Hypothalamic-Pituitary-Adrenal Systems for Major Depressive Disorder: A Meta-Analysis. Front. Pharmacol. 2021, 12, 732157. [Google Scholar] [CrossRef]
- Blasey, C.M.; DeBattista, C.; Roe, R.; Block, T.; Belanoff, J.K. A Multisite Trial of Mifepristone for the Treatment of Psychotic Depression: A Site-by-Treatment Interaction. Contemp. Clin. Trials 2009, 30, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Kling, M.A.; Coleman, V.H.; Schulkin, J. Glucocorticoid Inhibition in the Treatment of Depression: Can We Think Outside the Endocrine Hypothalamus? Depress. Anxiety 2009, 26, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, G.; Enache, D.; Gianotti, L.; Schatzberg, A.F.; Young, A.H.; Pariante, C.M.; Mondelli, V. Baseline Cortisol and the Efficacy of Antiglucocorticoid Treatment in Mood Disorders: A Meta-Analysis. Psychoneuroendocrinology 2019, 110, 104420. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Gallucci, W.T.; Gold, P.W.; Chrousos, G.P. Multiple Feedback Regulatory Loops upon Rat Hypothalamic Corticotropin-Releasing Hormone Secretion. Potential Clinical Implications. J. Clin. Investig. 1988, 82, 767–774. [Google Scholar] [CrossRef]
- Motta, M.; Mangili, G.; Martini, L. A “Short” Feedback Loop in the Control of ACTH Secretion. Endocrinology 1965, 77, 392–395. [Google Scholar] [CrossRef]
- Sawchenko, P.E.; Arias, C. Evidence for Short-Loop Feedback Effects of ACTH on CRF and Vasopressin Expression in Parvocellular Neurosecretory Neurons. J. Neuroendocrinol. 1995, 7, 721–731. [Google Scholar] [CrossRef]
- Seiden, G.; Brodish, A. Physiological Evidence for ‘Short-Loop’ Feedback Effects of ACTH on Hypothalamic CRF. Neuroendocrinology 1971, 8, 154–164. [Google Scholar] [CrossRef]
- Suda, T.; Yajima, F.; Tomori, N.; Sumitomo, T.; Nakagami, Y.; Ushiyama, T.; Demura, H.; Shizume, K. Inhibitory effect of adrenocorticotropin on corticotropin-releasing factor release from rat hypothalamus in vitro. Endocrinology 1986, 118, 459–461. [Google Scholar] [CrossRef]
- Tozawa, F.; Suda, T.; Dobashi, I.; Ohmori, N.; Kasagi, Y.; Demura, H. Central Administration of α-Melanocyte-Stimulating Hormone Inhibits Corticotropin-Releasing Factor Release in Adrenalectomized Rats. Neurosci. Lett. 1994, 174, 117–119. [Google Scholar] [CrossRef]
- Schioth, H.; Muceniece, R.; Larsson, M.; Wikberg, J. The Melanocortin 1, 3, 4 or 5 Receptors Do Not Have a Binding Epitope for ACTH beyond the Sequence of Alpha-MSH. J. Endocrinol. 1997, 155, 73–78. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Barsh, G.S.; Akil, H.; Watson, S.J. Interaction between α-Melanocyte-Stimulating Hormone and Corticotropin-Releasing Hormone in the Regulation of Feeding and Hypothalamo-Pituitary-Adrenal Responses. J. Neurosci. 2003, 23, 7863–7872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegant, V.M.; Jolles, J.; Colbern, D.L.; Zimmermann, E.; Hendrik Gispen, W. Intracerebroventricular Acth Activates the Pituitary-Adrenal System:Dissociation from a Behavioral Response. Life Sci. 1979, 25, 1791–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Frijtag, J.C.; Croiset, G.; Hendrik Gispen, W.; Adan, R.A.H.; Wiegant, V.M. The Role of Central Melanocortin Receptors in the Activation of the Hypothalamus-Pituitary-Adrenal-Axis and the Induction of Excessive Grooming. Br. J. Pharmacol. 1998, 123, 1503–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, K. Brain Melanocortin Receptors Are Involved In CRH-Mediated HPA Axis Activity and Thermogenesis. Open Neuroendocrinol. J. 2011, 4, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.K.; Mul, J.D.; Clemmensen, C.; Egan, A.E.; Begg, D.P.; Halcomb, K.; Seeley, R.J.; Herman, J.P.; Ulrich-Lai, Y.M. Loss of Melanocortin-4 Receptor Function Attenuates HPA Responses to Psychological Stress. Psychoneuroendocrinology 2014, 42, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Serova, L.I.; Laukova, M.; Alaluf, L.G.; Sabban, E.L. Blockage of Melanocortin-4 Receptors by Intranasal HS014 Attenuates Single Prolonged Stress-Triggered Changes in Several Brain Regions. J. Neurochem. 2014, 131, 825–835. [Google Scholar] [CrossRef]
- Vecsernyés, M.; Biró, É.; Gardi, J.; Julesz, J.; Telegdy, G. Involvement of Endogenous Corticotropin-Releasing Factor in Mediation of Neuroendocrine and Behavioral Effects to Alpha-Melanocyte-Stimulating Hormone. Endocr. Res. 2000, 26, 347–356. [Google Scholar] [CrossRef]
- Weiss, J.M.; Sundar, S.K.; Cierpial, M.A.; Ritchie, J.C. Effects of Interleukin-1 Infused into Brain Are Antagonized by α-MSH in a Dose-Dependent Manner. Eur. J. Pharmacol. 1991, 192, 177–179. [Google Scholar] [CrossRef]
- Shalts, E.; Feng, Y.J.; Ferin, M.; Wardlaw, S.L. Alpha-Melanocyte-Stimulating Hormone Antagonizes the Neuroendocrine Effects of Corticotropin-Releasing Factor and Interleukin-1 Alpha in the Primate. Endocrinology 1992, 131, 132–138. [Google Scholar] [CrossRef]
- Rivier, C.; Chizzonite, R.; Vale, W. In the Mouse, the Activation of the Hypothalamic Pituitary-Adrenal Axis by a Lipopolysaccharide (Endotoxin) Is Mediated through Interleukin-1. Endocrinology 1989, 125, 2800–2805. [Google Scholar] [CrossRef]
- Daynes, R.A.; Robertson, B.A.; Cho, B.H.; Burnham, D.K.; Newton, R. Alpha-Melanocyte-Stimulating Hormone Exhibits Target Cell Selectivity in Its Capacity to Affect Interleukin 1-Inducible Responses in Vivo and in Vitro. J. Immunol. 1987, 139, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lyson, K.; McCann, S.M. Alpha-Melanocyte-Stimulating Hormone Abolishes IL-1- and IL-6-Lnduced Corticotropin-Releasing Factor Release from the Hypothalamus in Vitro. Neuroendocrinology 1993, 58, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Cragnolini, A.B.; Perelló, M.; Schiöth, H.B.; Scimonelli, T.N. α-MSH and γ-MSH Inhibit IL-1β Induced Activation of the Hypothalamic–Pituitary–Adrenal Axis through Central Melanocortin Receptors. Regul. Pept. 2004, 122, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Vulliémoz, N.R.; Xiao, E.; Xia-Zhang, L.; Ferin, M.; Wardlaw, S.L. Melanocortin Modulation of Inflammatory Cytokine and Neuroendocrine Responses to Endotoxin in the Monkey. Endocrinology 2006, 147, 1878–1883. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, F.; Qin, D.; Chen, H.; Wang, J.; Wang, J.; Song, S.; Wang, C.; Wang, Y.; Liu, S.; et al. The Role of Brain Derived Neurotrophic Factor in Central Nervous System. Front. Aging Neurosci. 2022, 14, 986443. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF Signaling in Context: From Synaptic Regulation to Psychiatric Disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Xue, Y.; Liang, H.; Yang, R.; Deng, K.; Tang, M.; Zhang, M. The Role of Pro- and Mature Neurotrophins in the Depression. Behav. Brain Res. 2021, 404, 113162. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.-M. Decreased Serum Brain-Derived Neurotrophic Factor Levels in Major Depressed Patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Bus, B.A.A.; Spinhoven, P.; Penninx, B.W.J.H.; Kenis, G.; Prickaerts, J.; Voshaar, R.O.; Elzinga, B.M. Serum Levels of Brain-Derived Neurotrophic Factor in Major Depressive Disorder: State–Trait Issues, Clinical Features and Pharmacological Treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.; et al. Alterations of Serum Levels of Brain-Derived Neurotrophic Factor (BDNF) in Depressed Patients with or without Antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Deveci, A.; Taneli, F. The Effect of Chronic Antidepressant Treatment on Serum Brain-Derived Neurotrophic Factor Levels in Depressed Patients: A Preliminary Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265. [Google Scholar] [CrossRef]
- Gervasoni, N.; Aubry, J.-M.; Bondolfi, G.; Osiek, C.; Schwald, M.; Bertschy, G.; Karege, F. Partial Normalization of Serum Brain-Derived Neurotrophic Factor in Remitted Patients after a Major Depressive Episode. Neuropsychobiology 2005, 51, 234–238. [Google Scholar] [CrossRef]
- Tadić, A.; Wagner, S.; Schlicht, K.F.; Peetz, D.; Borysenko, L.; Dreimüller, N.; Hiemke, C.; Lieb, K. The Early Non-Increase of Serum BDNF Predicts Failure of Antidepressant Treatment in Patients with Major Depression: A Pilot Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Duman, R.; Sanacora, G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.-J.; Kim, J.-M.; Lee, J.-Y.; Kim, S.-Y.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Kim, H.-R.; Shin, M.-G.; Yoon, J.-S. BDNF Promoter Methylation and Suicidal Behavior in Depressive Patients. J. Affect. Disord. 2013, 151, 679–685. [Google Scholar] [CrossRef]
- Dunham, J.S.; Deakin, J.F.W.; Miyajima, F.; Payton, A.; Toro, C.T. Expression of Hippocampal Brain-Derived Neurotrophic Factor and Its Receptors in Stanley Consortium Brains. J. Psychiatr. Res. 2009, 43, 1175–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, M.T.; Shannon Weickert, C.; Webster, M.J. Decreased BDNF and TrkB MRNA Expression in Multiple Cortical Areas of Patients with Schizophrenia and Mood Disorders. Transl. Psychiatry 2014, 4, e389. [Google Scholar] [CrossRef] [Green Version]
- Karege, F.; Vaudan, G.; Schwald, M.; Perroud, N.; La Harpe, R. Neurotrophin Levels in Postmortem Brains of Suicide Victims and the Effects of Antemortem Diagnosis and Psychotropic Drugs. Mol. Brain Res. 2005, 136, 29–37. [Google Scholar] [CrossRef]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.-F.; Young, L.T. Increased Hippocampal Bdnf Immunoreactivity in Subjects Treated with Antidepressant Medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hamani, C.; Machado, D.C.; Hipólide, D.C.; Dubiela, F.P.; Suchecki, D.; Macedo, C.E.; Tescarollo, F.; Martins, U.; Covolan, L.; Nobrega, J.N. Deep Brain Stimulation Reverses Anhedonic-Like Behavior in a Chronic Model of Depression: Role of Serotonin and Brain Derived Neurotrophic Factor. Biol. Psychiatry 2012, 71, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Zhu, J. Effects of Sleep Deprivation on Behaviors and Abnormal Hippocampal BDNF/MiR-10B Expression in Rats with Chronic Stress Depression. Int. J. Clin. Exp. Pathol. 2015, 8, 586–593. [Google Scholar] [PubMed]
- Taliaz, D.; Loya, A.; Gersner, R.; Haramati, S.; Chen, A.; Zangen, A. Resilience to Chronic Stress Is Mediated by Hippocampal Brain-Derived Neurotrophic Factor. J. Neurosci. 2011, 31, 4475–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, E.; Gersner, R.; Wilf-Yarkoni, A.; Raizel, H.; Dar, D.E.; Richter-Levin, G.; Levit, O.; Zangen, A. Age-Dependent Effects of Chronic Stress on Brain Plasticity and Depressive Behavior. J. Neurochem. 2008, 107, 522–532. [Google Scholar] [CrossRef]
- Schnydrig, S.; Korner, L.; Landweer, S.; Ernst, B.; Walker, G.; Otten, U.; Kunz, D. Peripheral Lipopolysaccharide Administration Transiently Affects Expression of Brain-Derived Neurotrophic Factor, Corticotropin and Proopiomelanocortin in Mouse Brain. Neurosci. Lett. 2007, 429, 69–73. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Araujo, D.M.; Hefti, F. Systemic Interleukin-1β Decreases Brain-Derived Neurotrophic Factor Messenger RNA Expression in the Rat Hippocampal Formation. Neuroscience 1993, 53, 297–301. [Google Scholar] [CrossRef]
- Guan, Z.; Fang, J. Peripheral Immune Activation by Lipopolysaccharide Decreases Neurotrophins in the Cortex and Hippocampus in Rats. Brain. Behav. Immun. 2006, 20, 64–71. [Google Scholar] [CrossRef]
- Taliaz, D.; Stall, N.; Dar, D.E.; Zangen, A. Knockdown of Brain-Derived Neurotrophic Factor in Specific Brain Sites Precipitates Behaviors Associated with Depression and Reduces Neurogenesis. Mol. Psychiatry 2010, 15, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Hoshaw, B.A.; Malberg, J.E.; Lucki, I. Central Administration of IGF-I and BDNF Leads to Long-Lasting Antidepressant-like Effects. Brain Res. 2005, 1037, 204–208. [Google Scholar] [CrossRef]
- Siuciak, J.A.; Lewis, D.R.; Wiegand, S.J.; Lindsay, R.M. Antidepressant-Like Effect of Brain-Derived Neurotrophic Factor (BDNF). Pharmacol. Biochem. Behav. 1997, 56, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, Y.; Chen, A.C.-H.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-Derived Neurotrophic Factor Produces Antidepressant Effects in Behavioral Models of Depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nibuya, M.; Morinobu, S.; Duman, R. Regulation of BDNF and TrkB MRNA in Rat Brain by Chronic Electroconvulsive Seizure and Antidepressant Drug Treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef] [PubMed]
- Coppell, A.; Pei, Q.; Zetterström, T.S. Bi-Phasic Change in BDNF Gene Expression Following Antidepressant Drug Treatment. Neuropharmacology 2003, 44, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.P.R.; Mørk, A. The Effect of Escitalopram, Desipramine, Electroconvulsive Seizures and Lithium on Brain-Derived Neurotrophic Factor MRNA and Protein Expression in the Rat Brain and the Correlation to 5-HT and 5-HIAA Levels. Brain Res. 2004, 1024, 183–192. [Google Scholar] [CrossRef]
- Calabrese, F.; Molteni, R.; Maj, P.F.; Cattaneo, A.; Gennarelli, M.; Racagni, G.; Riva, M.A. Chronic Duloxetine Treatment Induces Specific Changes in the Expression of BDNF Transcripts and in the Subcellular Localization of the Neurotrophin Protein. Neuropsychopharmacology 2007, 32, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant Drugs Act by Directly Binding to TRKB Neurotrophin Receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Casarotto, P.; Umemori, J.; Castrén, E. BDNF Receptor TrkB as the Mediator of the Antidepressant Drug Action. Front. Mol. Neurosci. 2022, 15, 1032224. [Google Scholar] [CrossRef]
- Alboni, S.; Tascedda, F.; Corsini, D.; Benatti, C.; Caggia, F.; Capone, G.; Barden, N.; Blom, J.M.C.; Brunello, N. Stress Induces Altered CRE/CREB Pathway Activity and BDNF Expression in the Hippocampus of Glucocorticoid Receptor-Impaired Mice. Neuropharmacology 2011, 60, 1337–1346. [Google Scholar] [CrossRef]
- Ridder, S.; Chourbaji, S.; Hellweg, R.; Urani, A.; Zacher, C.; Schmid, W.; Zink, M.; Hörtnagl, H.; Flor, H.; Henn, F.A.; et al. Mice with Genetically Altered Glucocorticoid Receptor Expression Show Altered Sensitivity for Stress-Induced Depressive Reactions. J. Neurosci. 2005, 25, 6243–6250. [Google Scholar] [CrossRef] [Green Version]
- Numakawa, T.; Adachi, N.; Richards, M.; Chiba, S.; Kunugi, H. Brain-Derived Neurotrophic Factor and Glucocorticoids: Reciprocal Influence on the Central Nervous System. Neuroscience 2013, 239, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.; Hoetelmans, R.W.; de Kloet, E.R.; Vreugdenhil, E. Corticosterone Regulates Expression of BDNF and TrkB but Not NT-3 and TrkC MRNA in the Rat Hippocampus. J. Neurosci. Res. 1997, 48, 334–341. [Google Scholar] [CrossRef]
- Chao, H.M.; Sakai, R.R.; Ma, L.Y.; McEwen, B.S. Adrenal Steroid Regulation of Neurotrophic Factor Expression in the Rat Hippocampus. Endocrinology 1998, 139, 311. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Cintra, A.; Belluardo, N.; Sommer, W.; Bhatnagar, M.; Bader, M.; Ganten, D.; Fuxe, K. Gluco- and Mineralocorticoid Receptor-Mediated Regulation of Neurotrophic Factor Gene Expression in the Dorsal Hippocampus and the Neocortex of the Rat. Eur. J. Neurosci. 2000, 12, 2918–2934. [Google Scholar] [CrossRef] [PubMed]
- Lambert, W.M.; Xu, C.-F.; Neubert, T.A.; Chao, M.V.; Garabedian, M.J.; Jeanneteau, F.D. Brain-Derived Neurotrophic Factor Signaling Rewrites the Glucocorticoid Transcriptome via Glucocorticoid Receptor Phosphorylation. Mol. Cell Biol. 2013, 33, 3700–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanneteau, F.; Garabedian, M.J.; Chao, M.V. Activation of Trk Neurotrophin Receptors by Glucocorticoids Provides a Neuroprotective Effect. Proc. Natl. Acad. Sci. USA 2008, 105, 4862–4867. [Google Scholar] [CrossRef] [Green Version]
- Dolotov, O.V.; Karpenko, E.A.; Inozemtseva, L.S.; Seredenina, T.S.; Levitskaya, N.G.; Rozyczka, J.; Dubynina, E.V.; Novosadova, E.V.; Andreeva, L.A.; Alfeeva, L.Y.; et al. Semax, an Analog of ACTH(4–10) with Cognitive Effects, Regulates BDNF and TrkB Expression in the Rat Hippocampus. Brain Res. 2006, 1117, 54–60. [Google Scholar] [CrossRef]
- Shadrina, M.; Kolomin, T.; Agapova, T.; Agniullin, Y.; Shram, S.; Slominsky, P.; Lymborska, S.; Myasoedov, N. Comparison of the Temporary Dynamics of NGF and BDNF Gene Expression in Rat Hippocampus, Frontal Cortex, and Retina Under Semax Action. J. Mol. Neurosci. 2010, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.R.; Peter, J.-C.; Lecourt, A.-C.; Barde, Y.-A.; Hofbauer, K.G. Melanocortin-4 Receptor Activation Stimulates Hypothalamic Brain-Derived Neurotrophic Factor Release to Regulate Food Intake, Body Temperature and Cardiovascular Function. J. Neuroendocrinol. 2007, 19, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-Derived Neurotrophic Factor Regulates Energy Balance Downstream of Melanocortin-4 Receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenadel, M.G.; Thearle, M.S.; Grice, B.A.; Huang, H.; Dai, M.-H.; Tao, Y.-X.; Hunter, L.A.; Palaguachi, G.I.; Mou, Z.; Kim, R.C.; et al. Brain-Derived Neurotrophic Factor in Human Subjects with Function-Altering Melanocortin-4 Receptor Variants. Int. J. Obes. 2014, 38, 1068–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bariohay, B.; Roux, J.; Tardivel, C.; Trouslard, J.; Jean, A.; Lebrun, B. Brain-Derived Neurotrophic Factor/Tropomyosin-Related Kinase Receptor Type B Signaling Is a Downstream Effector of the Brainstem Melanocortin System in Food Intake Control. Endocrinology 2009, 150, 2646–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saba, J.; Carniglia, L.; Ramírez, D.; Turati, J.; Imsen, M.; Durand, D.; Lasaga, M.; Caruso, C. Melanocortin 4 Receptor Activation Protects Striatal Neurons and Glial Cells from 3-Nitropropionic Acid Toxicity. Mol. Cell Neurosci. 2019, 94, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Dolotov, O.V.; Karpenko, E.A.; Seredenina, T.S.; Inozemtseva, L.S.; Levitskaya, N.G.; Zolotarev, Y.A.; Kamensky, A.A.; Grivennikov, I.A.; Engele, J.; Myasoedov, N.F. Semax, an Analogue of Adrenocorticotropin (4–10), Binds Specifically and Increases Levels of Brain-Derived Neurotrophic Factor Protein in Rat Basal Forebrain. J. Neurochem. 2006, 97, 82–86. [Google Scholar] [CrossRef]
- Liao, Y.; Xing, Q.; Li, Q.; Zhang, J.; Pan, R.; Yuan, Z. Astrocytes in Depression and Alzheimer’s Disease. Front. Med. 2021, 15, 829–841. [Google Scholar] [CrossRef]
- Dolotov, O.V.; Inozemtseva, L.S.; Myasoedov, N.F.; Grivennikov, I.A. Stress-Induced Depression and Alzheimer’s Disease: Focus on Astrocytes. Int. J. Mol. Sci. 2022, 23, 4999. [Google Scholar] [CrossRef]
- Caruso, C.; Carniglia, L.; Durand, D.; Gonzalez, P.V.; Scimonelli, T.N.; Lasaga, M. Melanocortin 4 Receptor Activation Induces Brain-Derived Neurotrophic Factor Expression in Rat Astrocytes through Cyclic AMP–Protein Kinase A Pathway. Mol. Cell Endocrinol. 2012, 348, 47–54. [Google Scholar] [CrossRef]
- Ramírez, D.; Saba, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Melanocortin 4 Receptor Activates ERK-CFos Pathway to Increase Brain-Derived Neurotrophic Factor Expression in Rat Astrocytes and Hypothalamus. Mol. Cell Endocrinol. 2015, 411, 28–37. [Google Scholar] [CrossRef]
- Shadrina, M.I.; Dolotov, O.V.; Grivennikov, I.A.; Slominsky, P.A.; Andreeva, L.A.; Inozemtseva, L.S.; Limborska, S.A.; Myasoedov, N.F. Rapid Induction of Neurotrophin MRNAs in Rat Glial Cell Cultures by Semax, an Adrenocorticotropic Hormone Analog. Neurosci. Lett. 2001, 308, 115–118. [Google Scholar] [CrossRef]
- Dubynina, E.V.; Inozemtseva, L.S.; Markov, D.D.; Yatsenko, K.A.; Dolotov, O.V.; Grivennikov, I.A. Alpha-Melanocyte-Stimulating Hormone Increases the Expression of Vascular Endothelial Growth Factor in Rat Hippocampal Astrocytes in Vitro. Neurochem. J. 2009, 3, 267–271. [Google Scholar] [CrossRef]
- Deyama, S.; Bang, E.; Kato, T.; Li, X.-Y.; Duman, R.S. Neurotrophic and Antidepressant Actions of Brain-Derived Neurotrophic Factor Require Vascular Endothelial Growth Factor. Biol. Psychiatry 2019, 86, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y. Mitogen- and Stress-Activated Protein Kinase-1 Activation Is Involved in Melanocortin-Induced BDNF Expression in Neuro2a Neuronal Cells. Neuroreport 2020, 31, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.A.; Hen, R. Neurogenesis and Affective Disorders. Eur. J. Neurosci. 2011, 33, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Marriott, M.; Nahmias, C.; MacQueen, G.M. Lower Hippocampal Volume in Patients Suffering from Depression: A Meta-Analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Videbech, P. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, M.C.; Yucel, K.; Nazarov, A.; MacQueen, G.M. A Meta-Analysis Examining Clinical Predictors of Hippocampal Volume in Patients with Major Depressive Disorder. J. Psychiatry Neurosci. 2009, 34, 41–54. [Google Scholar]
- Cole, J.; Costafreda, S.G.; McGuffin, P.; Fu, C.H.Y. Hippocampal Atrophy in First Episode Depression: A Meta-Analysis of Magnetic Resonance Imaging Studies. J. Affect. Disord. 2011, 134, 483–487. [Google Scholar] [CrossRef]
- Nolan, M.; Roman, E.; Nasa, A.; Levins, K.J.; O’Hanlon, E.; O’Keane, V.; Willian Roddy, D. Hippocampal and Amygdalar Volume Changes in Major Depressive Disorder: A Targeted Review and Focus on Stress. Chronic Stress 2020, 4, 247054702094455. [Google Scholar] [CrossRef]
- Koolschijn, P.C.M.P.; van Haren, N.E.M.; Lensvelt-Mulders, G.J.L.M.; Hulshoff Pol, H.E.; Kahn, R.S. Brain Volume Abnormalities in Major Depressive Disorder: A Meta-Analysis of Magnetic Resonance Imaging Studies. Hum. Brain Mapp. 2009, 30, 3719–3735. [Google Scholar] [CrossRef]
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Hen, R.; Belzung, C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol. Psychiatry 2008, 64, 293–301. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Magarinos, A.M. Stress Effects on Morphology and Function of the Hippocampus. Ann. N. Y. Acad. Sci. 1997, 821, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M. Role of Corticosteroid Hormones in the Dentate Gyrus. Prog. Brain Res. 2007, 163, 355–370. [Google Scholar] [PubMed]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, D.; Zaffe, D.; Ottani, A.; Spaccapelo, L.; Galantucci, M.; Minutoli, L.; Bitto, A.; Irrera, N.; Contri, M.; Altavilla, D.; et al. Treatment of Cerebral Ischemia with Melanocortins Acting at MC4 Receptors Induces Marked Neurogenesis and Long-Lasting Functional Recovery. Acta Neuropathol. 2011, 122, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Spaccapelo, L.; Galantucci, M.; Neri, L.; Contri, M.; Pizzala, R.; D’Amico, R.; Ottani, A.; Sandrini, M.; Zaffe, D.; Giuliani, D.; et al. Up-Regulation of the Canonical Wnt-3A and Sonic Hedgehog Signaling Underlies Melanocortin-Induced Neurogenesis after Cerebral Ischemia. Eur. J. Pharmacol. 2013, 707, 78–86. [Google Scholar] [CrossRef]
- Giuliani, D.; Neri, L.; Canalini, F.; Calevro, A.; Ottani, A.; Vandini, E.; Sena, P.; Zaffe, D.; Guarini, S. NDP-α-MSH Induces Intense Neurogenesis and Cognitive Recovery in Alzheimer Transgenic Mice through Activation of Melanocortin MC4 Receptors. Mol. Cell Neurosci. 2015, 67, 13–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhang, D.; Lu, Z.; Man, J. Effects of RO27-3225 on Neurogenesis, PDGFRβ+ Cells and Neuroinflammation after Cerebral Infarction. Int. Immunopharmacol. 2020, 81, 106281. [Google Scholar] [CrossRef]
- Trullas, R.; Skolnick, P. Functional Antagonists at the NMDA Receptor Complex Exhibit Antidepressant Actions. Eur. J. Pharmacol. 1990, 185, 1–10. [Google Scholar] [CrossRef]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging Evidence for the Widespread Role of Glutamatergic Dysfunction in Neuropsychiatric Diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hara, H.; Kimura, H. Role of the AMPA Receptor in Antidepressant Effects of Ketamine and Potential of AMPA Receptor Potentiators as a Novel Antidepressant. Neuropharmacology 2023, 222, 109308. [Google Scholar] [CrossRef] [PubMed]
- Trifiletti, R.R.; Pranzatelli, M.R. ACTH Binds to [3H]MK-801-Labelled Rat Hippocampal NMDA Receptors. Eur. J. Pharmacol. 1992, 226, 377–379. [Google Scholar] [CrossRef] [PubMed]
- de Barioglio, S.R.; Brito, M.I. Effect of Alpha-MSH upon Cyclic AMP Levels Induced by the Glutamatergic Agonists NMDA, Quisqualic Acid, and Kainic Acid. Peptides 1996, 17, 1303–1306. [Google Scholar] [CrossRef]
- Vasileva, E.V.; Kondrakhin, E.A.; Abdullina, A.A.; Salimov, R.M.; Kovalev, G.I. Predominance of Nootropic or Anxiolytic Effects of Selank, Semax, and Noopept Peptides Depending on the Route of Administration to BALB/c and C57BL/6 Mice. Neurochem. J. 2020, 14, 268–278. [Google Scholar] [CrossRef]
- Grigoriev, V.V.; Andreeva, L.A.; Zamoyski, V.L.; Shevchenko, V.P.; Bachurin, S.O.; Myasoedov, N.F. The Action of the Peptide Drug Semax on the Currents of AMPA Receptors of Rat Cerebellar Purkinje Cells. Dokl. Biochem. Biophys. 2015, 460, 47–48. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, W.-Y.; Cheng, E.Y.L.; Fu, A.K.Y.; Ip, N.Y. Melanocortin-4 Receptor Regulates Hippocampal Synaptic Plasticity through a Protein Kinase A-Dependent Mechanism. J. Neurosci. 2013, 33, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.K.; Huang, K.W.; Grueter, B.A.; Rothwell, P.E.; Malenka, R.C. Anhedonia Requires MC4R-Mediated Synaptic Adaptations in Nucleus Accumbens. Nature 2012, 487, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Skiteva, O.; Zhang, X.; Svenningsson, P.; Chergui, K. Ketamine and Its Metabolite (2R,6R)-Hydroxynorketamine Induce Lasting Alterations in Glutamatergic Synaptic Plasticity in the Mesolimbic Circuit. Mol. Psychiatry 2018, 23, 2066–2077. [Google Scholar] [CrossRef] [Green Version]
- Bright, U.; Akirav, I. Modulation of Endocannabinoid System Components in Depression: Pre-Clinical and Clinical Evidence. Int. J. Mol. Sci. 2022, 23, 5526. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Mehta, V.; Singh, S.; Kumar, R.; Bungau, S. Integrating Endocannabinoid Signalling In Depression. J. Mol. Neurosci. 2021, 71, 2022–2034. [Google Scholar] [CrossRef]
- Yong, Y.; Cakir, I.; Lining Pan, P.; Biddinger, J.E.; Bluett, R.J.; Mackie, K.; Bingham, N.; Patel, S.; Ghamari-Langroudi, M. Endogenous Cannabinoids Are Required for MC4R-Mediated Control of Energy Homeostasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2015990118. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Drago, F. Endocannabinoid System, Stress and HPA Axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Vergoni, A.V.; Petrosino, S.; Ottani, A.; Pocai, A.; Bertolini, A.; Di Marzo, V. Regulation of Hypothalamic Endocannabinoid Levels by Neuropeptides and Hormones Involved in Food Intake and Metabolism: Insulin and Melanocortins. Neuropharmacology 2008, 54, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; DeJonckheere, C.; Vandervorst, C.; Schotte, C.; Cosyns, P.; Raus, J.; Suy, E. Abnormal Pituitary Function during Melancholia: Reduced α-Melanocyte-Stimulating Hormone Secretion and Increased Intact ACTH Non-Suppression. J. Affect. Disord. 1991, 22, 149–157. [Google Scholar] [CrossRef]
- Hidese, S.; Yoshida, F.; Ishida, I.; Matsuo, J.; Hattori, K.; Kunugi, H. Plasma Neuropeptide Levels in Patients with Schizophrenia, Bipolar Disorder, or Major Depressive Disorder and Healthy Controls: A Multiplex Immunoassay Study. Neuropsychopharmacol. Rep. 2022, 43, 57–68. [Google Scholar] [CrossRef]
- Berrettini, W.H.; Nurnberger, J.I.; Chan, J.S.D.; Chrousos, G.P.; Gaspar, L.; Gold, P.W.; Seidah, N.G.; Simmons-Alling, S.; Goldin, L.R.; Chrétien, M.; et al. Pro-Opiomelanocortin-Related Peptides in Cerebrospinal Fluid: A Study of Manic-Depressive Disorder. Psychiatry Res. 1985, 16, 287–302. [Google Scholar] [CrossRef]
- Sandman, C. Enhancement of Attention in Man with ACTH/MSH 4–10. Physiol. Behav. 1975, 15, 427–431. [Google Scholar] [CrossRef]
- Wu, G.-S.; Luo, H.-R.; Dong, C.; Mastronardi, C.; Licinio, J.; Wong, M.-L. Sequence Polymorphisms of MC1R Gene and Their Association with Depression and Antidepressant Response. Psychiatr. Genet. 2011, 21, 14–18. [Google Scholar] [CrossRef]
- Amin, M.; Ott, J.; Wu, R.; Postolache, T.T.; Gragnoli, C. Implication of Melanocortin Receptor Genes in the Familial Comorbidity of Type 2 Diabetes and Depression. Int. J. Mol. Sci. 2022, 23, 8350. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and Obesity: Evidence of Shared Biological Mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Fulton, S.; Décarie-Spain, L.; Fioramonti, X.; Guiard, B.; Nakajima, S. The Menace of Obesity to Depression and Anxiety Prevalence. Trends Endocrinol. Metab. 2022, 33, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Baldini, G.; Phelan, K.D. The Melanocortin Pathway and Control of Appetite-Progress and Therapeutic Implications. J. Endocrinol. 2019, 241, R1–R33. [Google Scholar] [CrossRef] [PubMed]
- Kühnen, P.; Krude, H.; Biebermann, H. Melanocortin-4 Receptor Signalling: Importance for Weight Regulation and Obesity Treatment. Trends Mol. Med. 2019, 25, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Y. The Central Melanocortin System and Human Obesity. J. Mol. Cell Biol. 2020, 12, 785–797. [Google Scholar] [CrossRef]
- Yeo, G.S.H.; Chao, D.H.M.; Siegert, A.-M.; Koerperich, Z.M.; Ericson, M.D.; Simonds, S.E.; Larson, C.M.; Luquet, S.; Clarke, I.; Sharma, S.; et al. The Melanocortin Pathway and Energy Homeostasis: From Discovery to Obesity Therapy. Mol. Metab. 2021, 48, 101206. [Google Scholar] [CrossRef]
- Kokare, D.M.; Dandekar, M.P.; Singru, P.S.; Gupta, G.L.; Subhedar, N.K. Involvement of α-MSH in the Social Isolation Induced Anxiety- and Depression-like Behaviors in Rat. Neuropharmacology 2010, 58, 1009–1018. [Google Scholar] [CrossRef]
- Chaki, S.; Hirota, S.; Funakoshi, T.; Suzuki, Y.; Suetake, S.; Okubo, T.; Ishii, T.; Nakazato, A.; Okuyama, S. Anxiolytic-Like and Antidepressant-Like Activities of MCL0129 (1-[( S )-2-(4-Fluorophenyl)-2-(4-Isopropylpiperadin-1-Yl)Ethyl]-4-[4-(2-Methoxynaphthalen-1-Yl)Butyl]Piperazine), a Novel and Potent Nonpeptide Antagonist of the Melanocortin-4 Receptor. J. Pharmacol. Exp. Ther. 2003, 304, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Chaki, S.; Oshida, Y.; Ogawa, S.; Funakoshi, T.; Shimazaki, T.; Okubo, T.; Nakazato, A.; Okuyama, S. MCL0042: A Nonpeptidic MC4 Receptor Antagonist and Serotonin Reuptake Inhibitor with Anxiolytic- and Antidepressant-like Activity. Pharmacol. Biochem. Behav. 2005, 82, 621–626. [Google Scholar] [CrossRef]
- Liu, J.; Garza, J.C.; Truong, H.V.; Henschel, J.; Zhang, W.; Lu, X.-Y. The Melanocortinergic Pathway Is Rapidly Recruited by Emotional Stress and Contributes to Stress-Induced Anorexia and Anxiety-Like Behavior. Endocrinology 2007, 148, 5531–5540. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Garza, J.C.; Li, W.; Lu, X.-Y. Melanocortin-4 Receptor in the Medial Amygdala Regulates Emotional Stress-Induced Anxiety-like Behaviour, Anorexia and Corticosterone Secretion. Int. J. Neuropsychopharmacol. 2013, 16, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serova, L.I.; Laukova, M.; Alaluf, L.G.; Sabban, E.L. Intranasal Infusion of Melanocortin Receptor Four (MC4R) Antagonist to Rats Ameliorates Development of Depression and Anxiety Related Symptoms Induced by Single Prolonged Stress. Behav. Brain Res. 2013, 250, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sabban, E.L.; Serova, L.I.; Alaluf, L.G.; Laukova, M.; Peddu, C. Comparative Effects of Intranasal Neuropeptide Y and HS014 in Preventing Anxiety and Depressive-like Behavior Elicited by Single Prolonged Stress. Behav. Brain Res. 2015, 295, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaki, S.; Okuyama, S. Involvement of Melanocortin-4 Receptor in Anxiety and Depression. Peptides 2005, 26, 1952–1964. [Google Scholar] [CrossRef]
- Chaki, S.; Ogawa, S.; Toda, Y.; Funakoshi, T.; Okuyama, S. Involvement of the Melanocortin MC4 Receptor in Stress-Related Behavior in Rodents. Eur. J. Pharmacol. 2003, 474, 95–101. [Google Scholar] [CrossRef]
- Kokare, D.M.; Chopde, C.T.; Subhedar, N.K. Participation of α-Melanocyte Stimulating Hormone in Ethanol-Induced Anxiolysis and Withdrawal Anxiety in Rats. Neuropharmacology 2006, 51, 536–545. [Google Scholar] [CrossRef]
- Corda, M.G.; Orlandi, M.; Fratta, W. Proconflict Effect of ACTH1−24: Interaction with Benzodiazepines. Pharmacol. Biochem. Behav. 1990, 36, 631–634. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Vaziri, S.; Wilson, C.A. Behavioral Effects of α-MSH and MCH after Central Administration in the Female Rat. Peptides 1996, 17, 171–177. [Google Scholar] [CrossRef]
- Bruschetta, G.; Jin, S.; Liu, Z.-W.; Kim, J.D.; Diano, S. MC4R Signaling in Dorsal Raphe Nucleus Controls Feeding, Anxiety, and Depression. Cell Rep. 2020, 33, 108267. [Google Scholar] [CrossRef]
- Cragnolini, A.B.; Schiöth, H.B.; Scimonelli, T.N. Anxiety-like Behavior Induced by IL-1β Is Modulated by α-MSH through Central Melanocortin-4 Receptors. Peptides 2006, 27, 1451–1456. [Google Scholar] [CrossRef]
- Goyal, S.; Kokare, D.; Chopde, C.; Subhedar, N. Alpha-Melanocyte Stimulating Hormone Antagonizes Antidepressant-like Effect of Neuropeptide Y in Porsolt’s Test in Rats. Pharmacol. Biochem. Behav. 2006, 85, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Deak, T.; Bellamy, C.; D’Agostino, L.G.; Rosanoff, M.; McElderry, N.K.; Bordner, K.A. Behavioral Responses during the Forced Swim Test Are Not Affected by Anti-Inflammatory Agents or Acute Illness Induced by Lipopolysaccharide. Behav. Brain Res. 2005, 160, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Scollan, E.L.; Ehrensing, R.H.; Schally, A.V.; Coy, D.H. Enkephalin and Other Peptides Reduce Passiveness. Pharmacol. Biochem. Behav. 1978, 9, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Vorvul, A.O.; Bobyntsev, I.I.; Medvedeva, O.A.; Mukhina, A.Y.; Svishcheva, M.V.; Azarova, I.E.; Andreeva, L.A.; Myasoedov, N.F. ACTH(6-9)-Pro-Gly-Pro Ameliorates Anxiety-like and Depressive-like Behaviour and Gut Mucosal Microbiota Composition in Rats under Conditions of Chronic Restraint Stress. Neuropeptides 2022, 93, 102247. [Google Scholar] [CrossRef] [PubMed]
- Markov, D.D.; Yatsenko, K.A.; Inozemtseva, L.S.; Grivennikov, I.A.; Myasoedov, N.F.; Dolotov, O.V. Systemic N-Terminal Fragments of Adrenocorticotropin Reduce Inflammation- and Stress-Induced Anhedonia in Rats. Psychoneuroendocrinology 2017, 82, 173–186. [Google Scholar] [CrossRef]
- Kitamura, Y.; Araki, H.; Gomita, Y. Influence of ACTH on the Effects of Imipramine, Desipramine and Lithium on Duration of Immobility of Rats in the Forced Swim Test. Pharmacol. Biochem. Behav. 2002, 71, 63–69. [Google Scholar] [CrossRef]
- Kitamura, Y.; Akiyama, K.; Kitagawa, K.; Shibata, K.; Kawasaki, H.; Suemaru, K.; Araki, H.; Sendo, T.; Gomita, Y. Chronic Coadministration of Carbamazepine Together with Imipramine Produces Antidepressant-like Effects in an ACTH-Induced Animal Model of Treatment–Resistant Depression: Involvement of 5-HT2A Receptors? Pharmacol. Biochem. Behav. 2008, 89, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.J.; Burnett, S.A.; Hasebe, K.; McGillivray, J.A.; Gray, L.J.; McGee, S.L.; Walder, K.; Berk, M.; Tye, S.J. Chronic Adrenocorticotrophic Hormone Treatment Alters Tricyclic Antidepressant Efficacy and Prefrontal Monoamine Tissue Levels. Behav. Brain Res. 2013, 242, 76–83. [Google Scholar] [CrossRef]
- Churruca, I.; Portillo, M.P.; Casis, L.; Gutiérrez, A.; Macarulla, M.T.; Echevarría, E. Effects of Fluoxetine Administration on Hypothalamic Melanocortin System in Obese Zucker Rats. Neuropeptides 2008, 42, 293–299. [Google Scholar] [CrossRef]
- Baker, R.A.; Herkenham, M.; Brady, L.S. Effects of Long-Term Treatment with Antidepressant Drugs on Proopiomelanocortin and Neuropeptide Y MRNA Expression in the Hypothalamic Arcuate Nucleus of Rats. J. Neuroendocrinol. 1996, 8, 337–343. [Google Scholar] [CrossRef]
- Ortuño, M.J.; Schneeberger, M.; Ilanges, A.; Marchildon, F.; Pellegrino, K.; Friedman, J.M.; Ducy, P. Melanocortin 4 Receptor Stimulation Prevents Antidepressant-Associated Weight Gain in Mice Caused by Long-Term Fluoxetine Exposure. J. Clin. Investig. 2021, 131, e151976. [Google Scholar] [CrossRef] [PubMed]
- van der Klaauw, A.A.; Keogh, J.M.; Henning, E.; Stephenson, C.; Kelway, S.; Trowse, V.M.; Subramanian, N.; O’Rahilly, S.; Fletcher, P.C.; Farooqi, I.S. Divergent Effects of Central Melanocortin Signalling on Fat and Sucrose Preference in Humans. Nat. Commun. 2016, 7, 13055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panaro, B.L.; Cone, R.D. Melanocortin-4 Receptor Mutations Paradoxically Reduce Preference for Palatable Foods. Proc. Natl. Acad. Sci. USA 2013, 110, 7050–7055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippert, R.N.; Ellacott, K.L.J.; Cone, R.D. Gender-Specific Roles for the Melanocortin-3 Receptor in the Regulation of the Mesolimbic Dopamine System in Mice. Endocrinology 2014, 155, 1718–1727. [Google Scholar] [CrossRef] [Green Version]
- Eliason, N.L.; Martin, L.; Low, M.J.; Sharpe, A.L. Melanocortin Receptor Agonist Melanotan-II Microinjected in the Nucleus Accumbens Decreases Appetitive and Consumptive Responding for Food. Neuropeptides 2022, 96, 102289. [Google Scholar] [CrossRef]
- Shanmugarajah, L.; Dunigan, A.I.; Frantz, K.J.; Roseberry, A.G. Altered Sucrose Self-Administration Following Injection of Melanocortin Receptor Agonists and Antagonists into the Ventral Tegmental Area. Psychopharmacology 2017, 234, 1683–1692. [Google Scholar] [CrossRef]
- Yen, H.-H.; Roseberry, A.G. Decreased Consumption of Rewarding Sucrose Solutions after Injection of Melanocortins into the Ventral Tegmental Area of Rats. Psychopharmacology 2015, 232, 285–294. [Google Scholar] [CrossRef]
- Pandit, R.; Omrani, A.; Luijendijk, M.C.M.; de Vrind, V.A.J.; Van Rozen, A.J.; Ophuis, R.J.A.O.; Garner, K.; Kallo, I.; Ghanem, A.; Liposits, Z.; et al. Melanocortin 3 Receptor Signaling in Midbrain Dopamine Neurons Increases the Motivation for Food Reward. Neuropsychopharmacology 2016, 41, 2241–2251. [Google Scholar] [CrossRef]
- Allen, A.T.; Heaton, E.C.; Shapiro, L.P.; Butkovich, L.M.; Yount, S.T.; Davies, R.A.; Li, D.C.; Swanson, A.M.; Gourley, S.L. Inter-Individual Variability Amplified through Breeding Reveals Control of Reward-Related Action Strategies by Melanocortin-4 Receptor in the Dorsomedial Striatum. Commun. Biol. 2022, 5, 116. [Google Scholar] [CrossRef]
- Pandit, R.; van der Zwaal, E.M.; Luijendijk, M.C.M.; Brans, M.A.D.; van Rozen, A.J.; Oude Ophuis, R.J.A.; Vanderschuren, L.J.M.J.; Adan, R.A.H.; la Fleur, S.E. Central Melanocortins Regulate the Motivation for Sucrose Reward. PLoS ONE 2015, 10, e0121768. [Google Scholar] [CrossRef]
- Figlewicz, D.P.; Jay, J.L.; Acheson, M.A.; Magrisso, I.J.; West, C.H.; Zavosh, A.; Benoit, S.C.; Davis, J.F. Moderate High Fat Diet Increases Sucrose Self-Administration in Young Rats. Appetite 2013, 61, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabeza de Vaca, S.; Hao, J.; Afroz, T.; Krahne, L.L.; Carr, K.D. Feeding, Body Weight, and Sensitivity to Non-Ingestive Reward Stimuli during and after 12-Day Continuous Central Infusions of Melanocortin Receptor Ligands. Peptides 2005, 26, 2314–2321. [Google Scholar] [CrossRef]
- Grieb, Z.A.; Cross, E.A.; Albers, H.E. Alpha-Melanocyte-Stimulating Hormone (AMSH) Modulates the Rewarding Properties of Social Interactions in an Oxytocin Receptor-Dependent Manner in Syrian Hamsters (Mesocricetus Auratus). Physiol. Behav. 2022, 252, 113828. [Google Scholar] [CrossRef] [PubMed]
- Klawonn, A.M.; Fritz, M.; Nilsson, A.; Bonaventura, J.; Shionoya, K.; Mirrasekhian, E.; Karlsson, U.; Jaarola, M.; Granseth, B.; Blomqvist, A.; et al. Motivational Valence Is Determined by Striatal Melanocortin 4 Receptors. J. Clin. Investig. 2018, 128, 3160–3170. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; He, Y.; Wang, C.; Xu, P.; Yang, Y.; Cai, X.; Liu, H.; Yu, K.; Pei, Z.; Hyseni, I.; et al. A POMC-Originated Circuit Regulates Stress-Induced Hypophagia, Depression, and Anhedonia. Mol. Psychiatry 2020, 25, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Legrand, R.; Lucas, N.; Breton, J.; Déchelotte, P.; Fetissov, S.O. Dopamine Release in the Lateral Hypothalamus Is Stimulated by α-MSH in Both the Anticipatory and Consummatory Phases of Feeding. Psychoneuroendocrinology 2015, 56, 79–87. [Google Scholar] [CrossRef]
- Rudman, D.; Hollins, B.M.; Kutner, M.H.; Moffitt, S.D.; Lynn, M.J. Three Types of Alpha-Melanocyte-Stimulating Hormone: Bioactivities and Half-Lives. Am. J. Physiol. Metab. 1983, 245, E47–E54. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Lamers, C. Overcoming the Shortcomings of Peptide-Based Therapeutics. Futur. Drug Discov. 2022, 4. [Google Scholar] [CrossRef]
- Wilson, J. Low Permeability of the Blood-Brain Barrier to Nanomolar Concentrations of Immunoreactive Alpha-Melanotropin. Psychopharmacology 1988, 96, 262–266. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Mezey, E.; Cassell, M.D.; Williams, T.H.; Mueller, G.P.; O’Donohue, T.L.; Palkovits, M. Topographical Distribution of Pro-Opiomelanocortin-Derived Peptides (ACTH/β-END/α-MSH) in the Rat Median Eminence. Brain Res. 1985, 329, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, M.; Khan, M.N.; Walsh, R.J.; Baquiran, G.B.; Renaud, L.P.; Bourque, C.; Sgro, S.; Gauthier, S.; Chretien, M.; Posner, B.I. NH2-Terminal Specificity and Axonal Localization of Adrenocorticotropin Binding Sites in Rat Median Eminence. Proc. Natl. Acad. Sci. USA 1985, 82, 1271–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatro, J.B. Melanotropin Receptors in the Brain Are Differentially Distributed and Recognize Both Corticotropin and α-Melanocyte Stimulating Hormone. Brain Res. 1990, 536, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tatro, J.B. Brain Receptors for Central and Peripheral Melanotropins. Ann. N. Y. Acad. Sci. 1993, 680, 621–625. [Google Scholar] [CrossRef]
- Potaman, V.N.; Antonova, L.V.; Dubynin, V.A.; Zaitzev, D.A.; Kamensky, A.A.; Myasoedov, N.F.; Nezavibatko, V.N. Entry of the Synthetic ACTH(4–10) Analogue into the Rat Brain Following Intravenous Injection. Neurosci. Lett. 1991, 127, 133–136. [Google Scholar] [CrossRef]
- Shevchenko, K.V.; Nagaev, I.Y.; Alfeeva, L.Y.; Andreeva, L.A.; Kamenskii, A.A.; Levitskaya, N.G.; Shevchenko, V.P.; Grivennikova, I.A.; Myasoedov, N.F. Kinetics of Semax Penetration into the Brain and Blood of Rats after Its Intranasal Administration. Russ. J. Bioorganic Chem. 2006, 32, 57–62. [Google Scholar] [CrossRef]
- Trivedi, P.; Jiang, M.; Tamvakopoulos, C.C.; Shen, X.; Yu, H.; Mock, S.; Fenyk-Melody, J.; Van der Ploeg, L.H.; Guan, X.-M. Exploring the Site of Anorectic Action of Peripherally Administered Synthetic Melanocortin Peptide MT-II in Rats. Brain Res. 2003, 977, 221–230. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular Pathways of Major Depressive Disorder Converge on the Synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]

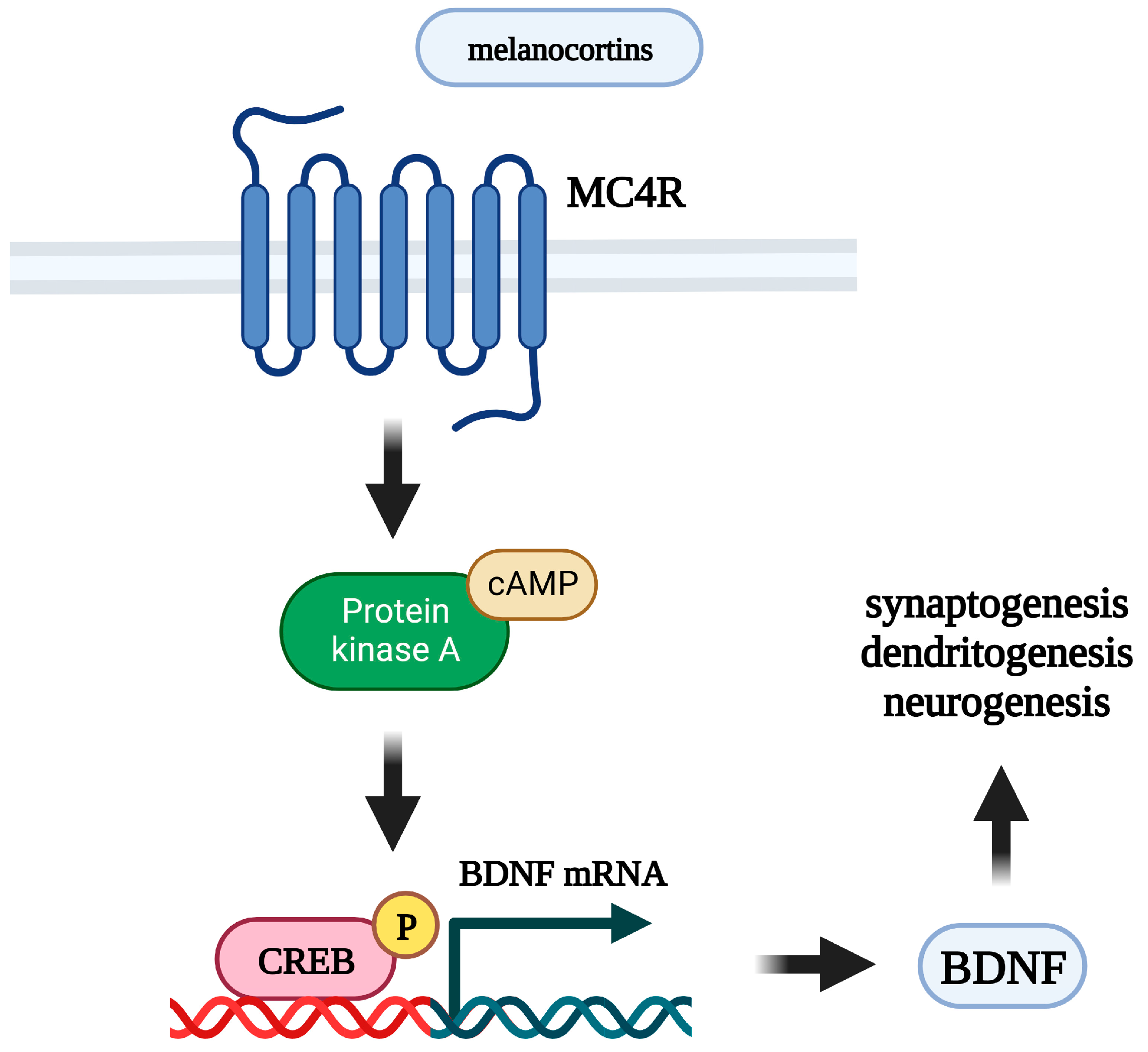
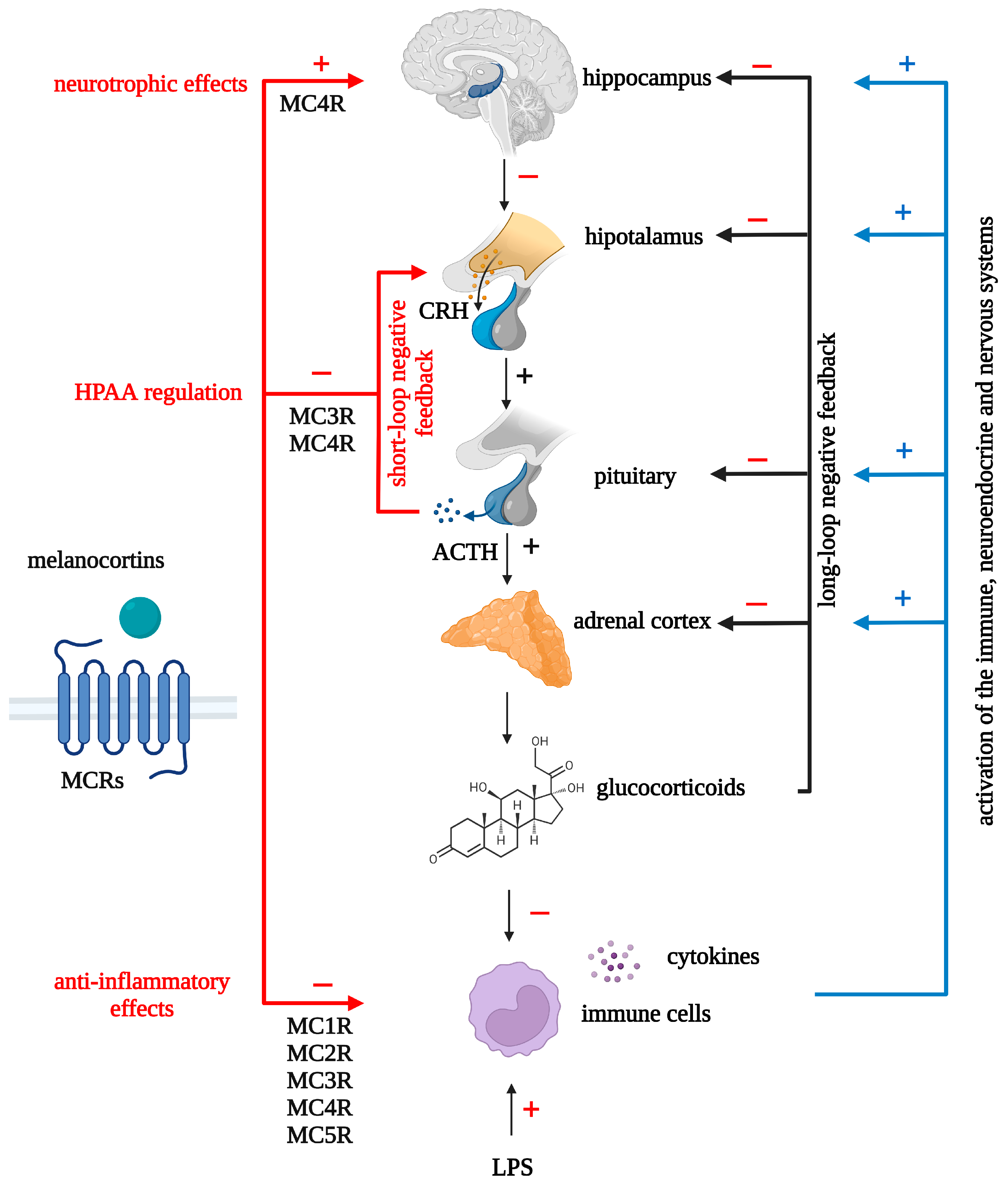
| Peptide | Amino Acid Sequence |
|---|---|
| ACTH | Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-Arg-Pro-Val-Lys-Val-Tyr-Pro-Asn-Gly-Ala-Glu-Asp-Glu-Ser-Ala-Glu-Ala-Phe-Pro-Leu-Glu-Phe |
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 |
| β-MSH | H-Ala-Glu-Lys-Lys-Asp-Glu-Gly-Pro-Tyr-Arg-Met-Glu-His-Phe-Arg-Trp-Gly-Ser-Pro-Pro-Lys-Asp-OH |
| γ1-MSH | H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-NH2 |
| γ2-MSH | H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-OH |
| γ3-MSH | H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-Asp-Arg-Phe-Gly-Arg-Arg-Asn-Ser-Ser-Ser-Ser-Gly-Ser-Ser-Gly-Ala-Gly-Gln-OH |
| Subjects | Experimental Model | Agonist | Dose | Site of Injection | Outcome | Agonist Effects Inhibitor | Dose | Site of Injection | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Studies performed in vivo | |||||||||
| Male albino rats | Conscious and freely moving rats | α-MSH | 1 μg | VTA | Dopamine ↓ and DOPAC ↑ in the caudate putamen and nucleus accumbens | - | - | - | [61] |
| Long–Evans rats | Conscious and freely moving rats | AgRP (inverse agonist) | 1 nmol | ICV | Neuronal activation within midbrain dopamine neurons and dopamine turnover in the medial prefrontal cortex ↑ | - | - | - | [62] |
| Male SD rats | Anaesthetized rats | α-MSH | 10 nmol | VTA | Dopamine and DOPAC levels in the nucleus accumbens ↑ | HS131 | 1 nmol | VTA | [64] |
| Male Wistar rats | Conscious and freely moving rats | MT-II | 0.0625, 0.625 µg | ICV | Changes in dopamine D1-like and D2-like receptor binding in several brain regions | - | - | - | [65] |
| Male Wistar rats | Anaesthetized rats | MT-II | 3 nmol | ICV | Firing rate of locus coeruleus noradrenergic neurons ↓ and firing rate of dorsal raphe nucleus serotonergic neurons ↑ | - | - | - | [67] |
| AgRP (inverse agonist) | 1 nmol | ICV | Firing rate of locus coeruleus noradrenergic neurons ↑ | ||||||
| Male C57BL/10 Bg mice | Conscious and freely moving mice | ACTH | 20 IU/kg | IP | Tyrosine hydroxylase activity in the locus coeruleus ↑ | - | - | - | [68] |
| ACTH1-24 | 20 μg/kg | ||||||||
| ACTH4-10 | 20 μg/kg | ||||||||
| Male SD and Wistar rats | Conscious and freely moving mice | ACTH4-10 | 25, 50 µg | SC | Catecholamine synthesis in the whole brain and brain stem ↑ | - | - | - | [69] |
| Male C57/bl mice and male SD rats | Conscious and freely moving rats and mice | Semax | 0.15 mg/kg | IP | Extracellular striatal level of 5-HIAA ↑ | - | - | - | [71] |
| Studies performed in vitro | |||||||||
| Male Wistar rats | Striatal tissue | ACTH1-24 | 10 μM | - | Inhibition of association and dissociation of dopamine D2 agonist to the dopamine D2 receptor | - | - | - | [66] |
| Male SD rats (synaptosomal preparation from cortical areas) | FST | α-MSH | 1, 4 µg | SC | Inhibition of FST-activated [3H]-5-HT re-uptake | - | - | - | [70] |
| Subjects | Experimental Model | Agonist | Dose | Site of Injection | Outcome | Agonist Effects Inhibitor | Dose | Site of Injection | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Studies performed in vivo | |||||||||
| White rabbits | LPS-induced fever | α-MSH | 2.5 µg | IV | Antipyretic effect | - | - | - | [116] |
| 200 ng | ICV | ||||||||
| White rabbits | IL-6 and TNF-α-induced fever | α-MSH | 200 ng | ICV | Antipyretic effect | - | - | - | [117] |
| White rabbits | Leukocytic pyrogen-induced fever | α-MSH | 200 ng | ICV | Antipyretic effect | - | - | - | [118] |
| NDP-α-MSH | 10, 20 ng | ||||||||
| Male SD rats | LPS-induced fever | α-MSH | 300 ng/rat | ICV | Antipyretic effect | SHU 9119 | 200 ng | ICV | [119] |
| Male SD rats | LPS-induced fever | α-MSH | 25–100 µg/kg | IP | Antipyretic effect; IL-6 ↓ | SHU 9119 | 200 ng | ICV | [120] |
| Male SD rats | LPS-induced fever | α-MSH | 1 µg | ICV | Antipyretic effect | HS014 | 1 µg | ICV | [121] |
| Male Wistar rats | LPS-induced endotoxemia | α-MSH | 3 nmol/rat | ICV | iNOS, COX-2 ↓ | HS024 | 1 nmol/rat | ICV | [123] |
| Male BALB/c mice | LPS challenge | α-MSH | 10 µg | ICV | TNF-α ↓ | - | - | - | [124] |
| 50 µg | IP | ||||||||
| Male Wistar rats | Transient cerebral ischaemia | Semax | 10 µg/100 g | IP | Expression of genes related to inflammatory processes ↓ | - | - | - | [125] |
| Male Wistar rats | Transient focal cerebral ischaemia | Semax | 10 µg/100 g | IP | IL-1α, IL-1β, IL-6, Ccl3, Cxcl2 ↓ | - | - | - | [126] |
| Unsexed SD rat pups | Neonatal hypoxia-ischemia brain injury | BMS-470539 | 50, 160, 500 μg/kg | Intranasal | IL-1β, TNF-α, IL-6 ↓ | - | - | - | [127] |
| Male Swiss Albino mice | Experimental gouty arthritis | ACTH4–10 | 100 µg | SC | KC, IL-1β ↓ | SHU 9119 | 10 µg | IP | [137] |
| α-MSH | 10 µg | ||||||||
| β-MSH | 10 µg | ||||||||
| Studies performed in vitro | |||||||||
| Rat hypothalamic explants | LPS + IFN-γ | α-MSH | 5 µM | - | iNOS ↓ | - | - | - | [123] |
| Mice brain tissue | LPS challenge | α-MSH | 10−15–10−8 M | - | TNF-α ↓ | - | - | - | [124] |
| Murine microglial cell line (N9) | LPS + IFN-γ | α-MSH | 1, 10, 25, 50, 100 µM | - | NO, TNF-α, IL-6 ↓ | - | - | - | [128] |
| α-MSH11–13 | |||||||||
| ACTH1–24 | |||||||||
| Anaplastic astrocytoma cell line A-172 | LPS + phorbol 12-myristate 13-acetate | α-MSH | 10−17–10−10 M | - | TNF-α ↓ | - | - | - | [129] |
| Human astrocytoma cells (U373) | TNF-α + IFN-γ | Setmelanotide | 0.001–10 µM | - | CCL2, CXCL10 ↓IL-6, IL-11 ↑ | SHU 9119 | 10 µM | - | [130] |
| RAW 264.7 cells | LPS + IFN-γ | α-MSH | 5 × 10−13–5 × 10−5 M | - | Nitrite accumulation, iNOS ↓ | - | - | - | [132] |
| Human monocyte-derived dendritic cells | - | α-MSH | 10−12 M | - | CD86, CD40 ↓ | - | - | - | [135] |
| Mice peritoneal macrophages | Monosodium urate crystals | ACTH | ACTH (100 ng/mL) | - | KC, phagocytosis ↓ | - | - | - | [137] |
| ACTH4–10 | 100 µg/mL | ||||||||
| α-MSH | 10 µg/mL | ||||||||
| β-MSH | 10–30 µg/mL | ||||||||
| Cultured rat astrocytes | LPS + IFN-γ | α-MSH | 5 µM | - | NO, PGE2, iNOS, COX-2 ↓ | HS024 | 0.5 µM | - | [145] |
| Subjects | Experimental Model | Agonist | Dose | Site of Injection | Outcome | Agonist Effects Inhibitor | Dose | Site of Injection | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Studies performed in vivo | |||||||||
| Male SD rats | ACTH implants into the median eminence | ACTH | 5 U | Median eminence | Corticosterone ↓ | - | - | - | [190] |
| Male SD rats | Adrenalectomized and hypophysectomized animals | ACTH | 0.5, 2 mg/mL (1 μL/h) | SC | Number of AVP- and CRF-positive PVN neurons ↓ | - | - | - | [191] |
| Male SD rats | Adrenalectomized and hypophysectomized animals | ACTH | 2 U | SC | CRH ↓ | - | - | - | [192] |
| Male Wistar rats | Adrenalectomized animals | α-MSH | 166, 1663 ng | ICV | Plasma ACTH levels ↓ CRF levels in the median eminence ↓ | - | - | - | [194] |
| Male SD rats | Conscious and freely moving rats | MT-II | 0.5, 1 nmol | ICV | Plasma corticosterone and CRH mRNA ↑ | HS014 | 0.25, 0.5, 1.0 nmol | ICV | [196] |
| Male Wistar rats | Conscious and freely moving rats | [D-Phe7]ACTH4-10 | 5 µg | ICV | Plasma corticosterone ↑ | - | - | - | [197] |
| ACTH1-16 | 1 µg | ||||||||
| ACTH1-24 | 1 µg | ||||||||
| Male Wistar rats | Conscious and freely moving rats | ACTH1-24 | 0.3–3 µg | ICV | Plasma ACTH and corticosterone ↑ | SHU 9119 | 1, 3 µg | ICV | [198] |
| [D-Arg8]ACTH4-10 | 5, 10 µg | ICV | |||||||
| Male Wistar rats | Acute central CRH administration | - | - | - | Plasma ACTH levels ↓ | SHU 9119 (solo effect) | 0.5 nmol/day over 14 days | ICV | [199] |
| Male rats | Homozygous or heterozygous male rats with loss of function mutation in MC4R | - | - | - | Stress-induced increase in circulating ACTH and corticosterone was reduced by loss of MC4R function | - | - | - | [200] |
| Male SD rats | Single prolonged stress | - | - | - | Plasma corticosterone ↓ | HS014 (solo effect) | 100 µg | Intranasal | [201] |
| Male Wistar rats | Conscious and freely moving rats | α-MSH | 50, 500 µg | SC | Plasma corticosterone ↑ | - | - | - | [202] |
| Male SD rats | FST | α-MSH | 1, 4 µg | SC | Plasma corticosterone ↓ | - | - | - | [70] |
| Male SD rats | IL-1β infusion | α-MSH | 0.1, 1.0, 10 ng | ICV | Plasma ACTH and corticosterone ↓ | - | - | - | [203] |
| Female rhesus monkeys | IL-1α infusion | α-MSH | 120 µg/h for 2 h | ICV | Plasma cortisol ↓ | - | - | - | [204] |
| Male BALB/c mice | LPS and IL-1β-induced HPAA activation | α-MSH | 10 and 30 µg | IP and SC | Plasma ACTH ↓ | - | - | - | [205] |
| CR:SW mice | IL-1β-induced HPAA activation | α-MSH | 30 µg | IV | Plasma corticosterone ↓ | - | - | - | [206] |
| NDP-α-MSH | 15 µg | ||||||||
| Male Wistar rats | IL-1β-induced HPAA activation | γ-MSH | 1 µg | ICV | Plasma corticosterone ↓ | SHU 9119 | 1 µg | ICV | [208] |
| α-MSH | 0.1 µg | HS014 | 1 µg | ICV | |||||
| Female rhesus monkeys | LPS-induced HPAA activation | NDP-α-MSH | 20 µg/h for 7 h | IV | Plasma ACTH and cortisol ↓ | SHU 9119 | 20 µg/h for 7 h | IV | [209] |
| Male SD rats | LPS-induced fever | α-MSH | 100 µg/kg | IP | Plasma ACTH and corticosterone ↓ | SHU 9119 | 200 ng | ICV | [120] |
| Studies performed in vitro | |||||||||
| Rat hypothalamic explants | 5HT-, ACh-, and NE-stimulated CRH secretion | ACTH | 10−10–10−7 M | - | CRH ↓ | - | - | - | [189] |
| α-MSH | |||||||||
| Rat hypothalamic explants | Rat hypothalamic perifusion system | ACTH | 0.22–22 nM | - | CRH ↓ | - | - | - | [193] |
| ACTH1-24 | 2.2 nM | ||||||||
| ACTH1-17 | 2.2 nM | ||||||||
| α-MSH | 2.2 nM | ||||||||
| Rat hypothalamic explants | IL-6-stimulated CRH release | ACTH1-24 | 10−15–10−13 M | - | CRH ↓ | - | - | - | [207] |
| α-MSH | 10−13–10−11 M | ||||||||
| Subjects | Experimental Model | Agonist | Dose | Site of Injection | Outcome | Agonist Effects Inhibitor | Dose | Site of Injection | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Studies performed in vivo | |||||||||
| Male rats | Conscious and freely moving rats | Semax | 50, 500 μg/kg | Intranasal | Hippocampal BDNF protein levels ↑ | - | - | - | [252] |
| Male Wistar rats | Conscious and freely moving rats | Semax | 50 μg/kg | Intranasal | Hippocampal BDNF mRNA levels ↑ | - | - | - | [253] |
| MC4R−/− null mice | MC4R−/− null mice | - | - | - | Hypothalamic BDNF mRNA levels ↓ | - | - | - | [255] |
| Male Wistar Han rats | Conscious and freely moving rats | MT-II | 1 nmol/rat | ICV | BDNF protein levels within the dorsal vagal complex ↑ | SHU 9119 | 0.5 nmol/rat | ICV | [257] |
| Male Wistar rats | Conscious and freely moving rats | α-MSH | 0.5 mg/kg | IP | Striatal BDNF mRNA ↑ | - | - | - | [258] |
| Male Wistar rats | Conscious and freely moving rats | Semax | 50, 250 μg/kg | Intranasal | BDNF protein levels within the basal forebrain ↑ | - | - | - | [259] |
| Male Wistar rats | Conscious and freely moving rats | α-MSH | 0.5 mg/kg | IP | Hypothalamic BDNF mRNA levels ↑ | - | - | - | [263] |
| Studies performed in vitro | |||||||||
| Rat hypothalamic explants | Isolated rat hypothalami | MK1 | 1, 10 µM | - | BDNF release ↑ | SHU 9119 | 3 µM | - | [254] |
| Rat astrocytes | Rat cultured astrocytes | NDP-α-MSH | 0.1, 1, 10 μM | - | BDNF mRNA and protein levels ↑ | - | - | - | [262] |
| Rat astrocytes | Rat cultured astrocytes | NDP-α-MSH | 1 μM | - | BDNF mRNA ↑ | - | - | - | [263] |
| Rat glial cells | Glial cell cultures from rat basal forebrain | Semax | 10 μM | - | BDNF mRNA ↑ | - | - | - | [264] |
| Murine neuronal cells | Murine neuroblastoma Neuro2a cells | NDP-α-MSH | 10–10,000 nM | - | BDNF mRNA ↑ | - | - | - | [267] |
| Subjects | Experimental Model | Tests | Agonist | Dose | Site of Injection | Outcome | Agonist Effects Inhibitor | Dose | Site of Injection | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy normal, male subjects | - | Tasks designed to assess emotionality or arousal | ACTH/MSH4-10 | 15 mg | IV | Anxiety ↓ | - | - | - | [303] |
| Male SD rats | Social isolation induced anxiety- and depression-like behaviors | FST, EPM | - | - | - | Anxiety and depression-like behavior ↓ | HS014 (solo effect) | 1–10 nmol/rat | ICV | [312] |
| Male ICR mice, male Wistar rats, male SD rats | Conscious and freely moving mice and rats | EPM, light/dark exploration test, marble-burying behavior, learned helplessness test, FST | - | - | - | Anxiety and depression-like behavior ↓ | MCL0129 (solo effect) | 1–30 mg/kg | PO and SC | [313] |
| Male SD rats | Conscious and freely moving rats | Vogel test, EPM | - | - | - | Anxiety ↓ | MCL0042 (solo effect) | 1–10 mg/kg | SC | [314] |
| Male SD rats | Acute restraint and FST | EPM | - | - | - | Anxiety ↓ | SHU 9119 (solo effect) | 0.05, 0.5 nmol | ICV | [315] |
| Male SD rats | Conscious and freely moving rats and acute restraint stress | EPM | Cyclo (β-Ala-His-D-Phe-Arg-Trp-Glu)-NH2 | 0.1, 1 nmol | Medial amygdala | Anxiogenic-like effects of agonist and anxiolytic-like effect of antagonist | SHU 9119 | 0.5, 1.0 nmol | Medial amygdala | [316] |
| Male SD rats | Single prolonged stress | FST, EPM | - | - | - | Anxiety and depression-like behavior ↓ | HS014 (solo effect) | 3.5 ng, 100 μg | Intranasal | [317] |
| Male SD rats | Single prolonged stress | FST, EPM | - | - | - | Anxiety and depression-like behavior ↓ | HS014 (solo effect) | 3.5 ng, 100 μg | Intranasal | [318] |
| Male ICR mice, male SD rats | Stress-induced anxiogenic-like behavior | Vogel test, light/dark exploration test | α-MSH | 3, 10 μg | ICV | Anxiogenic-like effects of agonists and anxiolytic-like effect of antagonist | MCL0020 | 0.01–0.1 nmol | ICV | [320] |
| MT-II | 0.1, 0.3, 1 μg | |||||||||
| Male SD rats | Ethanol-induced anxiolysis | EPM | α-MSH | 0.5–5 μg/rat | ICV | Anxiogenic-like effects of agonist and anxiolytic-like effect of antagonist | HS014 | 1–10 nM/rat | ICV | [321] |
| antiserum against α-MSH | ICV | |||||||||
| Male SD rats | Conscious and freely moving rats | Conflict test | ACTH1-24 | 0.1–10 μg | ICV | Anxiety ↑ | - | - | - | [322] |
| α-MSH | 0.25–5 μg | |||||||||
| Female Wistar rats | Conscious and freely moving rats | EPM | α-MSH | 100 ng | Medial preoptic area | Anxiety ↑ | - | - | - | [323] |
| Male Wistar rats | IL-1β-induced anxiety-like behavior | EPM | α-MSH | 0.2 μg | ICV | Anxiety ↓ | HS014 | 2 μg | ICV | [325] |
| γ-MSH | 2 μg | |||||||||
| Male SD rats | Conscious and freely moving rats | FST | α-MSH | 100–400 ng/rat | ICV | Antidepressant-like effect of antagonist and prodepressant effect of agonist | HS014 | 0.01–0.07 ng/rat | ICV | [326] |
| Male albino rats | Conscious and freely moving rats | FST | α-MSH | 1 mg/kg | IP | Antidepressant-like effect | - | - | - | [328] |
| Male Wistar rats | Chronic immobilization stress-induced anxiety-like and depressive-like behaviour | FST, EPM | ACTH6-9-Pro-Gly-Pro | 5, 50, 500 μg/kg | IP | Antidepressant and anxiolytic effects | - | - | - | [329] |
| Male SD rats | CUMS and LPS-induced depressive-like behaviour | Sucrose preference test | α-MSH | 100 μg/kg | IP | Antidepressant effects | SHU 9119 | 4.3 μg/kg | IP | [330] |
| ACTH4-10 | 58 μg/kg | |||||||||
| Male Wistar rats | Immobility-decreasing effect of imipramine and desipramine | FST | ACTH1-24 | 100 μg/day for 1, 3, 7, 14 days | SC | Prodepressant effect | - | - | - | [331] |
| Male Wistar rats | Immobility-decreasing effect of imipramine | FST | ACTH1-24 | 100 μg/day for 14 days | SC | Prodepressant effect | - | - | - | [332] |
| Male SD rats | Immobility-decreasing effect of imipramine | FST | ACTH1-24 | 100 μg/day for 14 days | IP | Prodepressant effect | - | - | - | [333] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markov, D.D.; Dolotov, O.V.; Grivennikov, I.A. The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs. Int. J. Mol. Sci. 2023, 24, 6664. https://doi.org/10.3390/ijms24076664
Markov DD, Dolotov OV, Grivennikov IA. The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs. International Journal of Molecular Sciences. 2023; 24(7):6664. https://doi.org/10.3390/ijms24076664
Chicago/Turabian StyleMarkov, Dmitrii D., Oleg V. Dolotov, and Igor A. Grivennikov. 2023. "The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs" International Journal of Molecular Sciences 24, no. 7: 6664. https://doi.org/10.3390/ijms24076664
APA StyleMarkov, D. D., Dolotov, O. V., & Grivennikov, I. A. (2023). The Melanocortin System: A Promising Target for the Development of New Antidepressant Drugs. International Journal of Molecular Sciences, 24(7), 6664. https://doi.org/10.3390/ijms24076664






