Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes—A Comprehensive Review
Abstract
1. Introduction
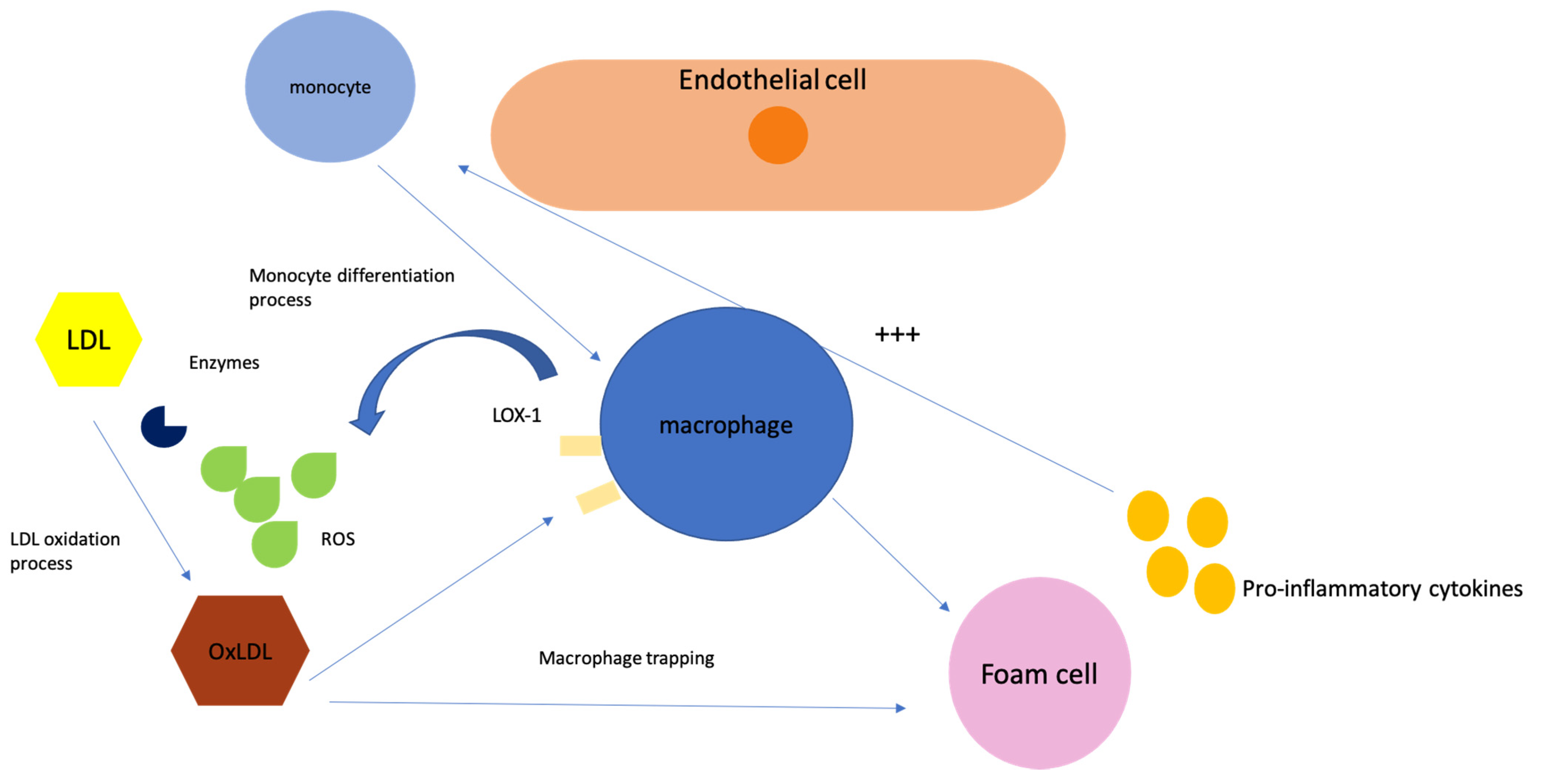
2. Role of Low-Density Lipoproteins in Cardiovascular Disease
3. What We Know So Far
4. Genetics
5. Importance of Dosing LDL Subfractions and How It Impacts Overall Cardiovascular Risk
6. Future Directions
7. Current Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Griffin, B.A. Lipoprotein atherogenicity: An overview of current mechanisms. Proc. Nutr. Soc. 1999, 58, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Hsia, J.; MacFadyen, J.G.; Monyak, J.; Ridker, P.M. Cardiovascular Event Reduction and Adverse Events Among Subjects Attaining Low-Density Lipoprotein Cholesterol <50 mg/dL with Rosuvastatin. J. Am. Coll. Cardiol. 2011, 57, 1666–1675. [Google Scholar] [CrossRef]
- McCormack, T.; Dent, R.; Blagden, M. Very low LDL-C levels may safely provide additional clinical cardiovascular benefit: The evidence to date. Int. J. Clin. Pract. 2016, 70, 886–897. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, J.C.; Grundy, S.M.; Waters, D.D.; Shear, C.; Barter, P.; Fruchart, J.-C.; Gotto, A.M.; Greten, H.; Kastelein, J.J.P.; Shepherd, J.; et al. Intensive Lipid Lowering with Atorvastatin in Patients. N. Engl. J. Med. 2005, 352, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): Characteristics of a randomized trial among 12,064 myocardial infarction survivors. Am. Heart J. 2007, 154, 815–823. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Langsted, A.; Mortensen, M.B.; Nordestgaard, B.G. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: Prospective cohort study. BMJ 2020, 371, m4266. [Google Scholar] [CrossRef]
- Rong, S.; Li, B.; Chen, L.; Sun, Y.; Du, Y.; Liu, B.; Robinson, J.G.; Bao, W. Association of Low-Density Lipoprotein Cholesterol Levels with More than 20-Year Risk of Cardiovascular and All-Cause Mortality in the General Population. J. Am. Heart Assoc. 2022, 11, e023690. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, C.; Auriel, E.; Elis, A.; Kornowski, R. The Association between Low Levels of Low Density Lipoprotein Cholesterol and Intracerebral Hemorrhage: Cause for Concern? J. Clin. Med. 2022, 11, 536. [Google Scholar] [CrossRef]
- Yen, C.; Fan, P.; Lee, C.; Chen, J.; Kuo, G.; Tu, Y.; Chu, P.; Hsu, H.; Tian, Y.; Chang, C. Association of Low-Density Lipoprotein Cholesterol Levels During Statin Treatment with Cardiovascular and Renal Outcomes in Patients with Moderate Chronic Kidney Disease. J. Am. Heart Assoc. 2022, 11, e027516. [Google Scholar] [CrossRef]
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Krauss, R.M. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 1990, 82, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A. Plasma lipoproteins: Genetic influences and clinical implications. Nat. Rev. Genet. 2009, 10, 109–121. [Google Scholar] [CrossRef]
- Dron, J.S.; Hegele, R.A. Genetics of Lipid and Lipoprotein Disorders and Traits. Curr. Genet. Med. Rep. 2016, 4, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Tichý, L.; Freiberger, T.; Blaha, V.; Satny, M.; Hubacek, J.A. Genetics of Familial Hypercholesterolemia: New Insights. Front. Genet. 2020, 11, 574474. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.A.; Hu, Y.; Zhang, H.; Xue, C.; Schmidt, E.M.; Tang, Z.-Z.; Bizon, C.; Lange, E.M.; Smith, J.D.; Turner, E.H.; et al. Whole-Exome Sequencing Identifies Rare and Low-Frequency Coding Variants Associated with LDL Cholesterol. Am. J. Hum. Genet. 2014, 94, 233–245. [Google Scholar] [CrossRef]
- Futema, M.; Plagnol, V.; Li, K.; Whittall, R.A.; Neil, H.A.W.; Seed, M.; Simon Broome Consortium; Bertolini, S.; Calandra, S.; Descamps, O.S.; et al. Whole exome sequencing of familial hypercholesterolaemia patients negative for LDLR/APOB/PCSK9 mutations. J. Med. Genet. 2014, 51, 537–544. [Google Scholar] [CrossRef]
- Futema, M.; Shah, S.; Cooper, J.A.; Li, K.; Whittall, R.A.; Sharifi, M.; Goldberg, O.; Drogari, E.; Mollaki, V.; Wiegman, A.; et al. Refinement of Variant Selection for the LDL Cholesterol Genetic Risk Score in the Diagnosis of the Polygenic Form of Clinical Familial Hypercholesterolemia and Replication in Samples from 6 Countries. Clin. Chem. 2015, 61, 231–238. [Google Scholar] [CrossRef]
- Talmud, P.J.; Shah, S.; Whittall, R.; Futema, M.; Howard, P.; Cooper, J.A.; Harrison, S.C.; Li, K.; Drenos, F.; Karpe, F.; et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: A case-control study. Lancet 2013, 381, 1293–1301. [Google Scholar] [CrossRef]
- Brahm, A.J.; Hegele, R.A. Combined hyperlipidemia: Familial but not (usually) monogenic. Curr. Opin. Lipidol. 2016, 27, 131–140. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Pencina, M.; Thanassoulis, G. ApoB: The Power of Physiology to Transform the Prevention of Cardiovascular Disease. Circ. Res. 2019, 124, 1425–1427. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Toth, P.P.; Thanassoulis, G.; Furberg, C.D. An evidence-based analysis of the National Lipid Association recommendations concerning non-HDL-C and apoB. J. Clin. Lipidol. 2016, 10, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M. All Low-Density Lipoprotein Particles Are Not Created Equal. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Rizzo Manfredi Berneis, K. Small, dense low-density-lipoproteins and the metabolic syndrome. Diabetes Metab. Res. Rev. 2007, 23, 14–20. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, A.C.; Cantin, B.; Dagenais, G.R.; Mauriège, P.; Bernard, P.-M.; Després, J.-P.; Lamarche, B. Low-Density Lipoprotein Subfractions and the Long-Term Risk of Ischemic Heart Disease in Men: 13-Year Follow-Up Data From the Québec Cardiovascular Study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 553–559. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small Dense Low-Density Lipoprotein-Cholesterol Concentrations Predict Risk for Coronary Heart Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Steffen, B.T.; Guan, W.; McClelland, R.L.; Warnick, R.; McConnell, J.; Hoefner, D.M.; Remaley, A.T. New Automated Assay of Small Dense Low-Density Lipoprotein Cholesterol Identifies Risk of Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 196–201. [Google Scholar] [CrossRef]
- Stampfer, M.J. A Prospective Study of Triglyceride Level, Low-Density Lipoprotein Particle Diameter, and Risk of Myocardial Infarction. JAMA 1996, 276, 882. [Google Scholar] [CrossRef]
- Gardner, C.D. Association of Small Low-Density Lipoprotein Particles with the Incidence of Coronary Artery Disease in Men and Women. JAMA 1996, 276, 875. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Stoian, A.P.; Janez, A.; Rizzo, M. Lipoproteins and Cardiovascular Disease: An Update on the Clinical Significance of Atherogenic Small, Dense LDL and New Therapeutical Options. Biomedicines 2021, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, P.M.; Moses, E.; Manfredi, R.; Kaspar, B.; Bruce, G.; Alberto, Z.; Vasilios, A.; de Jacqueline, G.; Winfried, M.; Klaus, G.P.; et al. “European Panel on Low Density Lipoprotein (LDL) Subclasses&”: A Statement on the Pathophysiology, Atherogenicity and Clinical Significance of LDL Subclasses. Curr. Vasc. Pharmacol. 2011, 9, 533–571. [Google Scholar] [CrossRef]
- Berneis, K.K.; Krauss, R.M. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 2002, 43, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, J.; Hak-Lemmers, H.L.; Hectors, M.P.; Demacker, P.N.; Hendriks, J.C.; Stalenhoef, A.F. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arter. Thromb. 1991, 11, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.L.; Rizzo, M.; Chait, A.; Lewis, D.M.; Blanche, P.J.; Krauss, R.M. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am. J. Med. 2001, 110, 103–110. [Google Scholar] [CrossRef]
- Macphee, C.H.; Nelson, J.J.; Zalewski, A.L. ipoprotein-associated phospholipase A2 as a target of therapy. Curr. Opin. Lipidol. 2005, 16, 442–446. [Google Scholar] [CrossRef]
- Tertov, V.V.; Kaplun, V.V.; Sobenin, I.A.; Boytsova EYu Bovin, N.V.; Orekhov, A.N. Human plasma trans-sialidase causes atherogenic modification of low density lipoprotein. Atherosclerosis 2001, 159, 103–115. [Google Scholar] [CrossRef]
- La Belle, M.; Krauss, R.M. Differences in carbohydrate content of low density lipoproteins associated with low density lipoprotein subclass patterns. J. Lipid. Res. 1990, 31, 1577–1588. [Google Scholar] [CrossRef]
- Anber, V.; Griffin, B.A.; McConnell, M.; Packard, C.J.; Shepherd, J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis 1996, 124, 261–271. [Google Scholar] [CrossRef]
- Corrado, E.; Rizzo, M.; Coppola, G.; Muratori, I.; Carella, M.; Novo, S. Endothelial dysfunction and carotid lesions are strong predictors of clinical events in patients with early stages of atherosclerosis: A 24-month follow-up study. Coron. Artery Dis. 2008, 19, 139–144. [Google Scholar] [CrossRef]
- Chaudhary, R.; Mathew, D.; Bliden, K.; Tantry, U.S.; Sharma, T.; Gesheff, M.G.; Franzese, C.J.; Pandya, S.; Toth, P.P.; Gurbel, P.A. Low-density lipoprotein 4: A novel predictor of coronary artery disease severity. Curr. Med. Res. Opin. 2017, 33, 1979–1984. [Google Scholar] [CrossRef]
- Rizzo, M.; Berneis, K. Who needs to care about small, dense low-density lipoproteins? Small, dense low-density lipoproteins. Int. J. Clin. Pract. 2007, 61, 1949–1956. [Google Scholar] [CrossRef]
- Superko, H.R. Beyond LDL Cholesterol Reduction. Circulation 1996, 94, 2351–2354. [Google Scholar] [CrossRef]
- Rizzo, M.; Pernice, V.; Frasheri, A.; Berneis, K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis 2008, 197, 237–241. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Ruotolo, G.; Brewer, H.B.; Wang, M.-D.; Liu, L.; Willey, M.B.; Deeg, M.A.; Krueger, K.A.; Nissen, S.E. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J. Clin. Lipidol. 2016, 10, 519–527. [Google Scholar] [CrossRef]
- Krauss, R.M.; Pinto, C.A.; Liu, Y.; Johnson-Levonas, A.O.; Dansky, H.M. Changes in LDL particle concentrations after treatment with the cholesteryl ester transfer protein inhibitor anacetrapib alone or in combination with atorvastatin. J. Clin. Lipidol. 2015, 9, 93–102. [Google Scholar] [CrossRef]
- Krauss, R.M.; Wojnooski, K.; Orr, J.; Geaney, J.C.; Pinto, C.A.; Liu, Y.; Wagner, J.A.; Luk, J.M.; Johnson-Levonas, A.O.; Anderson, M.S.; et al. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J. Lipid Res. 2012, 53, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kotseva, K.; Wood, D.; De Bacquer, D.; De Backer, G.; Rydén, L.; Jennings, C.; Gyberg, V.; Amouyel, P.; Bruthans, J.; Castro Conde, A.; et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur. J. Prev. Cardiol. 2016, 23, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Ip, S. Systematic Review: Association of Low-Density Lipoprotein Subfractions with Cardiovascular Outcomes. Ann. Intern. Med. 2009, 150, 474. [Google Scholar] [CrossRef]
- Sánchez-Quesada, J.L.; Pérez, A. Modified lipoproteins as biomarkers of cardiovascular risk in diabetes mellitus. Endocrinol. Nutr. (Engl. Ed.) 2013, 60, 518–528. [Google Scholar] [CrossRef] [PubMed]
| Plasmatic Lipoprotein | Density (g/mL) | Source | Composition (%) | Apolipoproteins | ||
|---|---|---|---|---|---|---|
| Lipid | Protein | Major | Others | |||
| Chylomicrons | <0.95 | intestine | 98–99 | 1–2 | ApoB-48 | ApoA-I, A-II, A-IV, A-V |
| VLDL | 0.95–1.006 | liver | 90–93 | 6–8 | ApoB-100 | ApoA-I, C-II, C-III, E, A-V |
| IDL | 1.006–1.019 | catabolism of VLDL | 89 | 11 | ApoB-100 | ApoC-II, C-III, E |
| LDL | 1.019–1.063 | catabolism of VLDL via IDL | 79 | 21 | ApoB-100 | - |
| HDL | 1.063–1.210 | liver, intestine, catabolism of CM and VLDL | 67 | 33 | ApoA-I | ApoA-II, C-III, E, M |
| Lp(a) | 1.006–1.125 | liver | 80 | 20 | Apo(a) | ApoB-100 |
| sdLDL Properties | Sequence of Processes |
|---|---|
| Reduced binding to the LDL-C receptor | Increased residence time |
| Increased penetrance of the arterial wall | Increased infiltration |
| Increased affinity for arterial proteoglycans | Increased sequestration |
| Increased susceptibility to oxidation | Increased oxidation |
| Increased total cholesterol deposits | Accelerated atherosclerosis process |
| Phenotype | Disorder | Genes Involved | Chromosome | References |
|---|---|---|---|---|
| High LDL-C | Familial hypercholesterolemia | LDLR | 19p13.3 | [14,15] |
| Familial defective apolipoprotein B | APOB | 2p24-p23 | [14,15] | |
| Autosomal dominant hypercholesterolemia type 3 (PCSK9 gain of function) | PCSK9 | 1p32.3 | [14,15] | |
| Autosomal dominant hypercholesterolemia type 4 | STAP1 | 4q13.2 | [14,15] | |
| Autosomal dominant hypercholesterolemia type 5 | APOE | 19q13 | [14,15] | |
| Autosomal recessive hypercholesterolemia | LDLRAP1 (ARH) | 1p36-p35 | [14,15] | |
| Cholesterol ester storage disease | LIPA | 10q21.31 | [14,15] | |
| Sitosterolemia | ABCG5/ABCG8 | 2p21 | [14,15] | |
| Low LDL-C | Abetalipoproteinemia (Bassen–Kornzweig syndrome) | MTTP | 4q24 | [13,14] |
| Hypobetalipoproteinemia | APOB | 2p24-p23 | [13,14] | |
| PCSK9 deficiency with low LDL-C levels (PCSK9 loss of function) | PCSK9 | 1p32.3 | [13,14] | |
| Familial combined hypolipidemia (ANGPTL3 deficiency) | ANGPTL3 | 1p31.1-p22.3 | [13,14] | |
| Chylomicron retention disease (Anderson disease) | SAR1B | 5p31.1 | [13,14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciulescu, L.A.; Scafa-Udriste, A.; Dorobantu, M. Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 6669. https://doi.org/10.3390/ijms24076669
Stanciulescu LA, Scafa-Udriste A, Dorobantu M. Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes—A Comprehensive Review. International Journal of Molecular Sciences. 2023; 24(7):6669. https://doi.org/10.3390/ijms24076669
Chicago/Turabian StyleStanciulescu, Laura Adina, Alexandru Scafa-Udriste, and Maria Dorobantu. 2023. "Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes—A Comprehensive Review" International Journal of Molecular Sciences 24, no. 7: 6669. https://doi.org/10.3390/ijms24076669
APA StyleStanciulescu, L. A., Scafa-Udriste, A., & Dorobantu, M. (2023). Exploring the Association between Low-Density Lipoprotein Subfractions and Major Adverse Cardiovascular Outcomes—A Comprehensive Review. International Journal of Molecular Sciences, 24(7), 6669. https://doi.org/10.3390/ijms24076669







