Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus
Abstract
1. Hydrocephalus
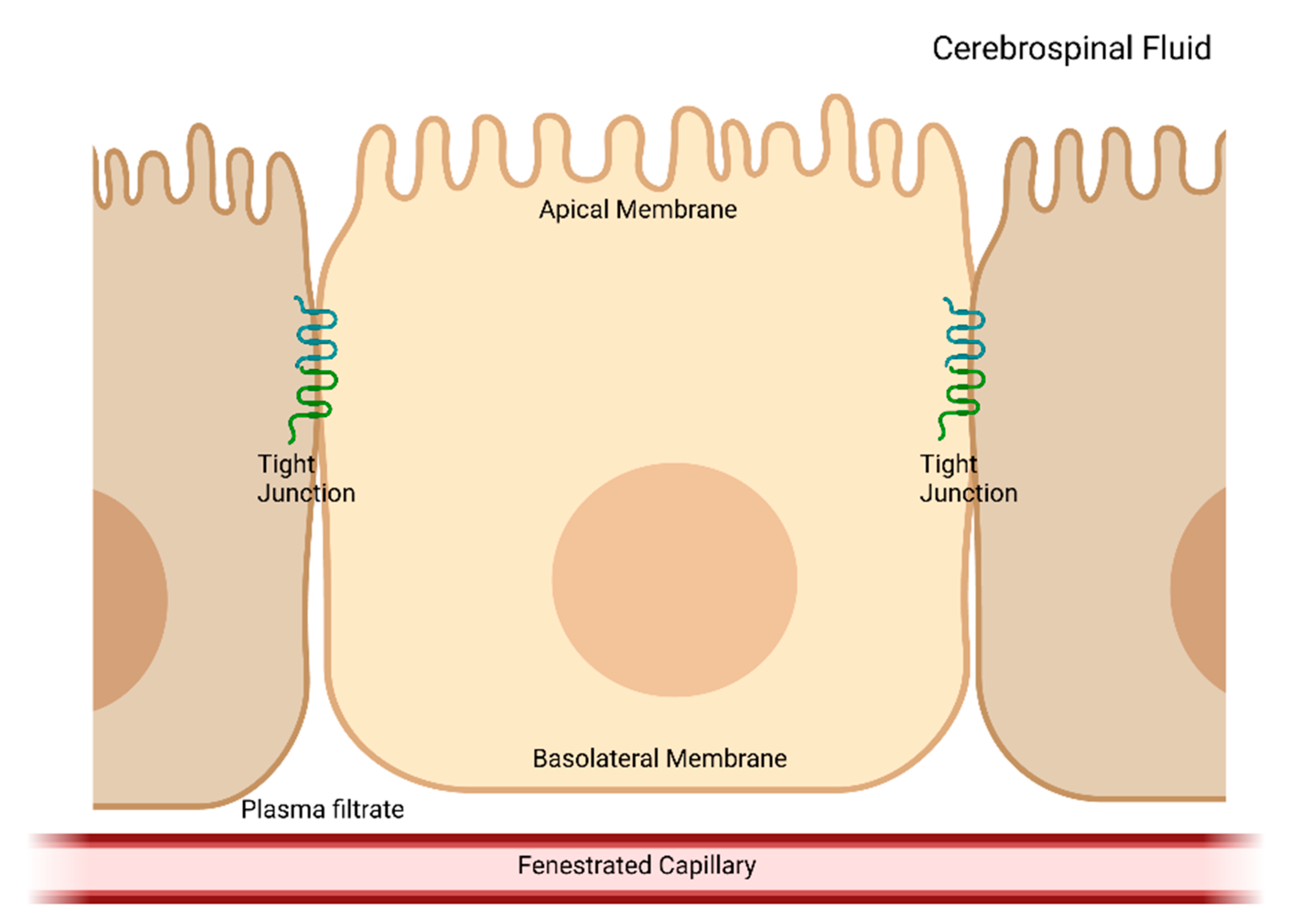
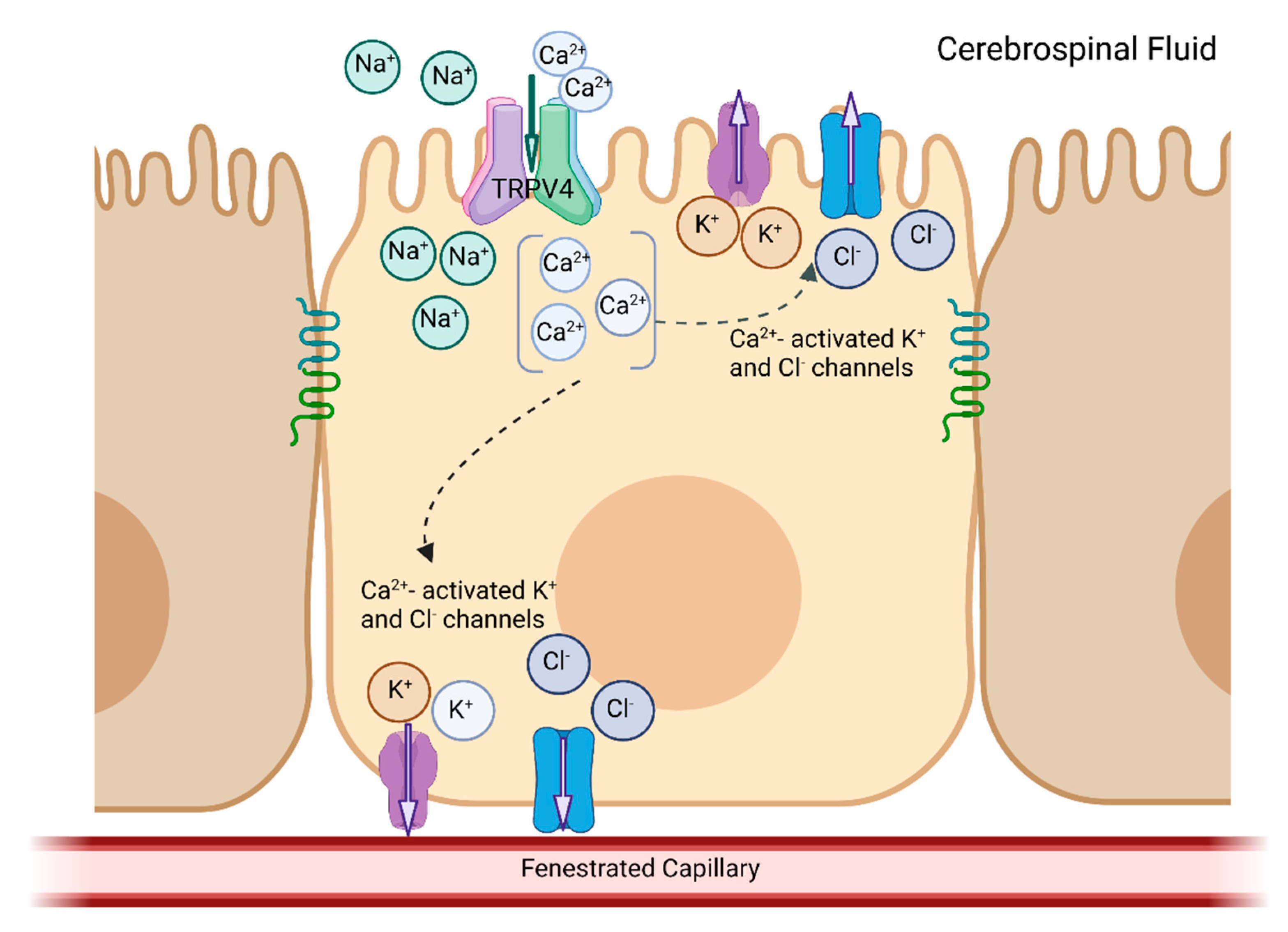
2. CSF Production
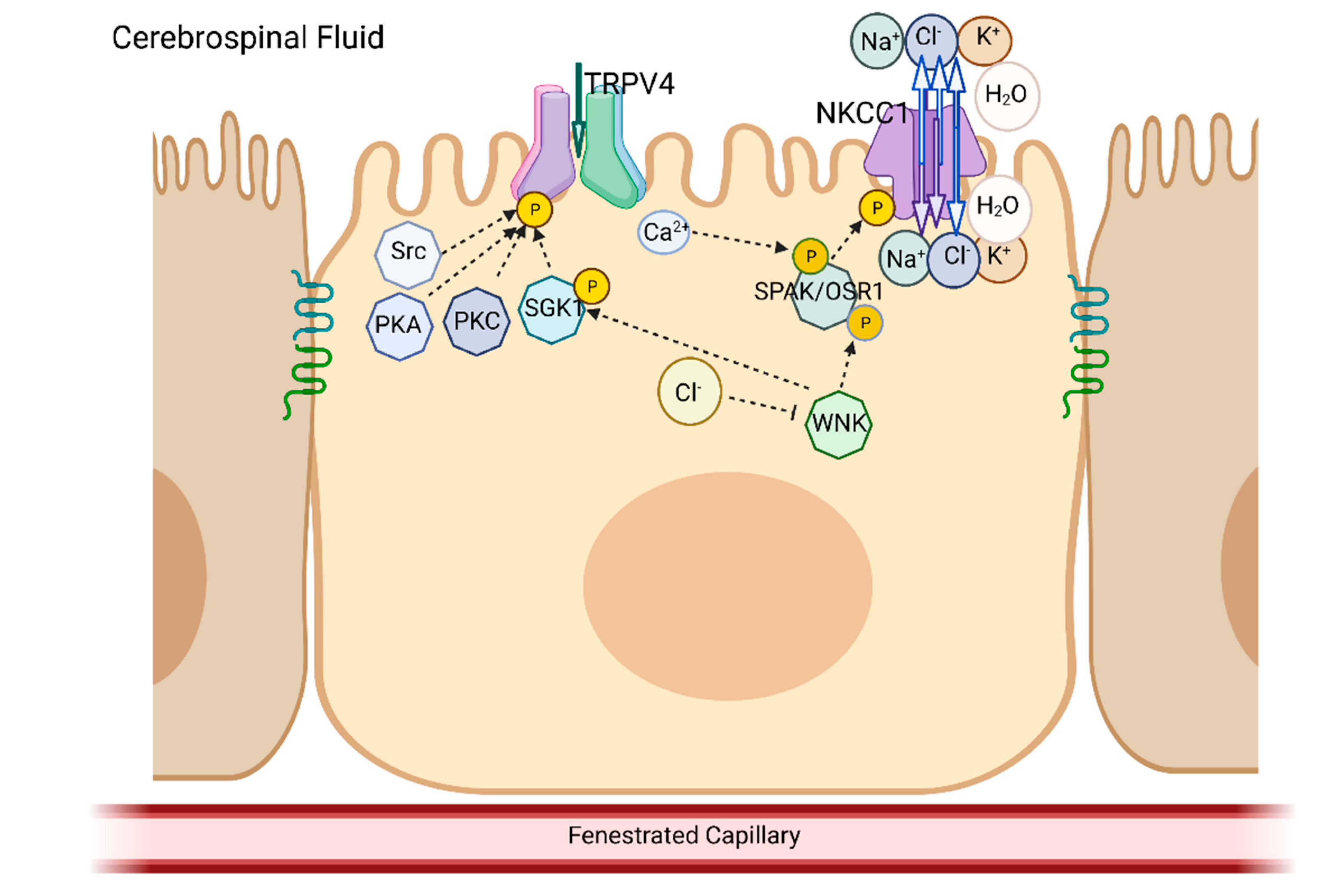
3. TRPV4
4. NKCC1
5. Overlap in the Biochemical Pathways and Kinases
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochstetler, A.; Raskin, J.; Blazer-Yost, B.L. Hydrocephalus: Historical analysis and considerations for treatment. Eur. J. Med. Res. 2022, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Sebai, M.A.; Patel, N.; Ewida, N.; Kurdi, W.; Altweijri, I.; Sogaty, S.; Almardawi, E.; Seidahmed, M.Z.; Alnemri, A.; et al. The genetic landscape of familial congenital hydrocephalus. Ann. Neurol. 2017, 81, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.E. Unlocking the genetic complexity of congenital hydrocephalus. Nat. Med. 2020, 6, 1682–1683. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.C.; Dong, W.; Kundishora, A.J.; Panchagnula, S.; Moreno-De-Luca, A.; Furey, C.G.; Allocco, A.A.; Walker, R.L.; Nelson-Williams, C.; Smith, H.; et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat. Med. 2020, 26, 1754–1765. [Google Scholar] [CrossRef]
- Hale, A.T.; Bastarache, L.; Morales, D.M.; Wellons, J.C., 3rd; Limbrick, D.D., Jr.; Gamazon, E.R. Multi-omic analysis elucidates the genetic basis of hydrocephalus. Cell Rep. 2021, 35, 109085. [Google Scholar] [CrossRef]
- Varagur, K.; Sanka, S.A.; Strahle, J.M. Syndromic hydrocephalus. Neurosurg. Clin. N. Am. 2022, 33, 67–79. [Google Scholar] [CrossRef]
- Rekate, H.L. The definition and classification of hydrocephalus: A personal recommendation to stimulate debate. Cereb. Fluid Res. 2008, 5, 287. [Google Scholar] [CrossRef]
- Simon, T.D.; Riva-Cambrin, J.; Srivastava, R.; Bratton, S.L.; Dean, J.M.; Kestle, J.R.W.; Hydrocephalus Clinical Research Network. Hospital care for children with hydrocephalus in the United States: Utilization, charges, comorbidities, and deaths. J. Neurosurg. Pediatr. 2008, 1, 131–137. [Google Scholar] [CrossRef]
- Robinson, S. Neonatal posthemorrhagic hydrocephalus from prematurity: Pathophysiology and current treatment concepts. J. Neurosurg. Pediatr. 2012, 9, 10. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Mekary, R.; Glancz, L.J.; Yunusa, I.; Baticulon, R.E.; Fieggen, G.; Wellons, J.C.; Park, K.B.; Warf, B.C. Global hydrocephalus epidemiology and incidence: Systematic review and meta-analysis. J. Neurosurg. 2018, 130, 1065–1079. [Google Scholar] [CrossRef]
- Isaacs, A.M.; Riva-Cambrin, J.; Yavin, D.; Hockley, A.; Pringsheim, T.M.; Jette, N.; Letebe, B.C.; Lowerison, M.; Dronyk, J.; Hamilton, M.G. Age-specific global epidemiology of hydrocephalus: Systemic review, metanalysis and global birth surveillance. PLoS ONE 2018, 13, e0204926. [Google Scholar] [CrossRef]
- Marmarou, A.; Abd-Elfattah Foda, M.A.; Bandoh, K.; Yoshihara, M.; Yamamoto, T.; Tsuji, O.; Zasler, N.; Ward, J.D.; Young, H.F. Posttraumatic ventriculomegaly: Hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J. Neurosurg. 1996, 85, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Guyot, L.L.; Michael, D.B. Post-traumatic hydrocephalus. Neurol. Res. 2000, 22, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Campini, R.; Angelino, E.; Rognone, F.; Pastore, I.; Oliveri, G. Posttraumatic hydrocephalus: A clinical, neuroradiodiologic and neuropsychologic assessment of long-term outcome. Arch. Phys. Med. Rehabil. 2003, 84, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Kammersgaard, L.P.; Linnemann, M.; Tibæk, M. Hydrocephalus following severe traumatic brain injury in adults. Incidence, timing, and clinical predictors during rehabilitation. NeuroRehabilitation 2013, 33, 473–480. [Google Scholar] [CrossRef]
- Oi, S.; Shimoda, M.; Shibata, M.; Honda, Y.; Togo, K.; Shinoda, M.; Tsugane, R.; Sato, O. Pathophysiology of long-standing overt ventriculomegaly in adults. J. Neurosurg. 2000, 92, 933–940. [Google Scholar] [CrossRef]
- Palandri, G.; Carretta, A.; La Corte, E.; Mazzatenta, D.; Conti, A. Longstanding overt ventriculomegaly in adults (LOVA) with patent aqueduct: Surgical outcome and etiopathogenesis of a possibly distinct form of hydrocephalus. Acta Neurochir. 2021, 163, 3343–3352. [Google Scholar] [CrossRef]
- Montemurro, N.; Indaimo, A.; Di Carlo, D.T.; Benedetto, N.; Perrini, P. Surgical treatment of long-standing overt ventriculomegaly in adults (LOVA): A comparative case series between ventriculoperitoneal shunt (VPS) and endoscopic third ventriculostomy (ETV). Int. J. Environ. Res. Public Health 2022, 19, 1926. [Google Scholar] [CrossRef]
- Williams, M.A.; Relkin, N.R. Diagnosis and management of idiopathic normal-pressure hydrocephalus. Neurol. Clin. Pract. 2013, 3, 375–385. [Google Scholar] [CrossRef]
- Jaraj, D.; Rabiei, K.; Marlow, T.; Jensen, C.; Skoog, I.; Wikkelsø, C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology 2014, 82, 1449–1454. [Google Scholar] [CrossRef]
- Jaraj, D.; Rabiei, K.; Marlow, T.; Jensen, C.; Skoog, I.; Wikkelsø, C. Mortality and dementia in untreated iNPH: A 25 year follow-up of a population-based cohort. Fluids Barriers CNS 2015, 12, 7. [Google Scholar] [CrossRef]
- Liew, B.S.; Takagi, K.; Kato, Y.; Duvuru, S.; Thanapal, S.; Mangaleswaran, B. Current updates on idiopathic normal pressure hydrocephalus. Asian J. Neurosurg. 2019, 14, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.M.; Kestle, J. Rational and methodology of the multicenter pediatric cerebrospinal fluid shunt design trial. Pediatric hydrocephalus treatment evaluation group. Child Nerv. Syst. 1996, 12, 434–447. [Google Scholar] [CrossRef]
- Kestle, J.; Milner, R.; Drake, J.M. The shunt design trial: Variation in surgical experience did not influence shunt survival. Pediatr. Neurosurg. 1999, 30, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kestle, J.; Drake, J.; Milner, R.; Sainte-Rose, C.; Cinalli, G.; Boop, F.; Piatt, J.; Haines, S.; Schiff, S.; Cochrane, D.; et al. Long-term follow-up data from the Shunt Design Trial. Pediatr. Neurosurg. 2000, 33, 230–236. [Google Scholar] [CrossRef]
- Khan, M.; Farnsworth, B.; Pope, B.R.; Sherrod, B.; Karsy, M. Impact of hospital volume on outcome after surgical treatment for hydrocephalus: A U.S. population study from the national inpatient sample. Cureus 2021, 13, e13617. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, R.V.; Nanda, A. Implanted ventricular shunts in the United States: The billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 2005, 56, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.N.; Carr, K.R.; Tomycz, L.; Wellons, J.C.; Tulipan, N. Time to first shunt failure in pediatric patients over 1 year old: A 10-year retrospective study. Pediatr. Neurosurg. 2013, 49, 353–359. [Google Scholar] [CrossRef]
- Stagno, V.; Navarrete, E.A.; Mirone, G.; Esposito, F. Management of hydrocephalus around the World. World Neurosurg. 2013, 79, S23.e17–S23.e20. [Google Scholar] [CrossRef]
- Warf, B.C. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: A prospective study in 550 African children. J. Neurosurg. 2005, 106, 475–481. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Drake, J.M.; Kestle, J.R.W.; Mallucci, C.L.; Sgouros, S.; Constantini, S.; Canadian Pediatric Neurosurgery Study Group. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J. Neurosurg. Pediatr. 2010, 6, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Leidtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Šali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-ORC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T. TRPV4 calcium entry channel: A paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 2004, 286, C195–C205. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosaatrienoic acids to activate TRPV4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, X.; Brown, R.C.; Heller, S.; O’Neil, R.G. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2007, 293, F1699–F1713. [Google Scholar] [CrossRef] [PubMed]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.; Vergnolle, N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 2011, 140, 275–285. [Google Scholar] [CrossRef]
- Henry, C.O.; Dalloneau, E.; Pérez-Berezo, M.-T.; Plata, C.; Wu, Y.; Guillon, A.; Morello, E.; Aimar, R.-F.; Potier-Cartereau, M.; Esnard, F.; et al. In vitro and in vivo evidence for an inflammatory role of the calcium channel TRPV4 in lung epithelium: Potential involvement in cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L664–L675. [Google Scholar] [CrossRef]
- Reiter, B.; Kraft, R.; Günzel, D.; Zeissig, S.; Schulzke, J.; Fromm, M.; Harteneck, C. TRPV4-mediated regulation of epithelial permeability. FASEB J. 2006, 20, 1802–1812. [Google Scholar] [CrossRef]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef]
- Narita, K.; Sasamoto, S.; Koizumi, S.; Okazaki, S.; Nakamura, H.; Inoue, T.; Takeda, S. TRPV4 regulates the integrity of the blood-cerebrospinal fluid barrier and modulates transepithelial protein transport. FASEB J. 2015, 29, 2247–2259. [Google Scholar] [CrossRef]
- Preston, D.; Simpson, S.; Halm, D.; Hochstetler, A.; Schwerk, C.; Schroten, H.; Blazer-Yost, B.L. Activation of TRPV4 stimulates transepithelial ion flux in a porcine choroid plexus cell line. Am. J. Physiol. Cell Physiol. 2018, 315, C357–C366. [Google Scholar] [CrossRef]
- Simpson, S.; Preston, D.; Schwerk, C.; Schroten, H.; Blazer-Yost, B. Cytokine and inflammatory mediator effects on TRPV4 function in choroid plexus epithelial cells. Am. J. Physiol. Cell Physiol. 2019, 317, C881–C893. [Google Scholar] [CrossRef] [PubMed]
- Hulme, L.; Hochstetler, A.; Schwerk, C.; Schroten, H.; Ishikawa, H.; Tung, C.-Y.; Perrin, B.; Blazer-Yost, B.L. Characterization of TRPV4-mediated signaling pathways in an optimized choroid plexus epithelial cell line. Am. J. Physiol. Cell Physiol. 2022, 323, C1823–C1842. [Google Scholar] [CrossRef] [PubMed]
- Darby, W.; Grace, M.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. Int. J. Biochem. Cell Biol. 2016, 78, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Willette, R.N.; Bao, W.; Nerurkar, S.; Yue, T.-L.; Doe, C.P.; Stankus, G.; Turner, G.H.; Ju, H.; Thomas, H.; Fishman, C.E.; et al. Systemic Activation of the Transient Receptor Potential Vanilloid Subtype 4 Channel Causes Endothelial Failure and Circulatory Collapse: Part 2. J. Pharmacol. Exp. Ther. 2008, 326, 443–452. [Google Scholar] [CrossRef]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22669. [Google Scholar] [CrossRef]
- Goyal, N.; Skrdla, P.; Schroyer, R.; Kumar, S.; Fernando, D.; Oughton, A.; Norton, N.; Sprecher, D.L.; Cheriyan, J. Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. Am. J. Cardiovasc. Drugs 2019, 19, 335–342. [Google Scholar] [CrossRef]
- Hochstetler, A.E.; Smith, H.M.; Preston, D.C.; Reed, M.M.; Territo, P.R.; Shim, J.W.; Fulkerson, D.; Blazer-Yost, B.L. Treatment with TRPV4 Antagonists Ameliorate Ventriculomegaly in a Rat Model of Hydrocephalus. J. Clin. Investig. Insight 2020, 5, e137646. [Google Scholar]
- Hochstetler, A.E.; Reed, M.M.; Blazer-Yost, B.L. Chapter 7, TRPV4, a Regulatory Channel in the Production of Cerebrospinal Fluid by the Choroid Plexus. In Choroid Plexus in Health and Disease; Praetorius, J., Damkier, H., Blazer-Yost, B.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lee, E.J.; Shin, S.H.; Chun, J.; Hyun, S.; Kim, Y.; Kang, S.S. The modulation of TRPV4 channel activity through its Ser 824 residue phosphorylation by SGK1. Anim. Cells Syst. 2010, 14, 99–114. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, E.J.; Hyun, S.; Chun, J.; Kim, Y.; Kang, S.S. Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area. Cell. Signal. 2012, 24, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, E.J.; Chun, J.; Hyun, S.; Kang, S.S. Phosphorylation on TRPV4 serine 824 regulates interaction with STIM1. Open Biochem. J. 2015, 9, 24–33. [Google Scholar] [CrossRef]
- Fan, H.-C.; Zhang, X.; McNaughton, P.A. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J. Biol. Chem. 2009, 284, 27884–27891. [Google Scholar] [CrossRef] [PubMed]
- Mamenko, M.; Zaika, O.L.; Boukelmoune, N.; Berrout, J.; O’Neil, R.G.; Pochynyuk, O. Discrete control of TRPV4 channel function in the distal nephron by protein kinases A. and C. J. Biol. Chem. 2013, 288, 20306–20314. [Google Scholar] [CrossRef]
- Christensen, H.L.; Păunescu, T.G.; Matchkov, V.; Barbuskaite, D.; Brown, D.; Damkier, H.H.; Praetorius, J. The V-ATPase is expressed in the choroid plexus and mediates cAMP-induced intracellular pH alterations. Physiol. Rep. 2017, 5, e13072. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Subramanya, A.; Rozansky, D.; Cohen, D.M. WNK kinases influence TRPV4 channel function and localization. Am. J. Physiol. Renal Physiol. 2006, 290, F1305–F1314. [Google Scholar] [CrossRef] [PubMed]
- Wegierski, T.; Lewandrowski, U.; Müller, B.; Sickmann, A.; Walz, G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J. Biol. Chem. 2009, 284, 2923–2933. [Google Scholar] [CrossRef]
- Ussing, H.H.; Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol. Scand. 1951, 23, 110–127. [Google Scholar] [CrossRef]
- Koefoed-Johnsen, V.; Ussing, H.H. The nature of the frog skin potential. Acta Physiol. Scand. 1958, 42, 298–308. [Google Scholar] [CrossRef]
- Geck, P.; Pietrzyk, C.; Burckhardt, B.-C.; Pfeifferl, B.; Heinz, E. Electrically silent cotransport of Na+, K+ and Cl− in ehrlich cells. Biochim. Biophys. Acta 1980, 600, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; I Rauchman, M.; Beier, D.R.; Hebert, S.C.; Gullans, S.R. Molecular cloning and chromosome localization of a putative basolateral Na(+)-K(+)-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J. Biol. Chem. 1994, 269, 25677–25683. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lytle, C.; Zhu, T.T.; Payne, J.A.; Benz, E.; Forbush, B., III. Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc. Natl. Acad. Sci. USA 1994, 91, 2201–2205. [Google Scholar] [CrossRef]
- Gamba, G.; Miyanoshita, A.; Lombardi, M.; Lytton, J.; Lee, W.S.; Hediger, M.A.; Hebert, S.C. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 1994, 269, 17713–17722. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, P.; Vanden Heuvel, G.B.; Payne, J.A.; Forbush, B., 3rd. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am. J. Physiol. Renal Physiol. 1995, 269, F405–F418. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, M.D.; Kaplan, M.R.; Peterson, L.N.; Gullans, S.R.; Hebert, S.C.; Delpire, E. Expression of the Na+K+2Cl- BSC2 in the nervous system. Am. J. Physiol. Cell Physiol. 1997, 272, C173–C183. [Google Scholar] [CrossRef]
- Zeuthen, T. Water-transporting proteins. J. Membr. Biol. 2010, 234, 57–73. [Google Scholar] [CrossRef]
- Hamann, S.; Hererra-Perez, J.J.; Zeuthen, T.; Alvarez-Leefmans, F.J. Cotransport of water by the Na+-K+2Cl- cotransporter NKCC1 in mammalian epithelial cells. J. Physiol. 2010, 588, 4089–4101. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Zhang, Y.; Liu, T.; Friedel, P.; Zhuo, W.; Somasekharan, S.; Roy, K.; Zhang, L.; Liu, Y.; et al. The structural basis of function and regulation of neuronal cotransporters NKCC1 and KCC2. Commun. Biol. 2021, 4, 226. [Google Scholar] [CrossRef]
- Javaheri, S.; Wagner, K.R. Bumetanide decreases canine cerebrospinal fluid production. In vivo evidence for NaCl cotransport in the central nervous system. J. Clin. Investig. 1993, 92, 2257–2261. [Google Scholar] [CrossRef]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Vogh, B.P.; Mangham, M.R., Jr. The effect of furosemide and bumetanide on cerebrospinal fluid formation. Brain Res. 1981, 221, 171–183. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Omileke, D.; Patabendige, A.; Spratt, N.J. CSF secretion in not altered by NKCC1 nor TRPV4 antagonism in healthy rats. Brain Sci. 2021, 11, 117. [Google Scholar] [CrossRef]
- Steffensen, A.B.; Oernbo, E.K.; Stoica, A.; Gerkau, N.J.; Barbuskaite, D.; Tritsaris, K.; Rose, C.R.; MacAulay, N. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 2018, 9, 2167. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Gagnon, K.B. Elusive role of the Na-K-2Cl cotransporter in the choroid plexus. Am. J. Physiol. Cell Physiol. 2018, 316, C522–C524. [Google Scholar] [CrossRef]
- Keep, R.F.; Xiang, J.; Betz, A.L.; Verkhratsky, A.; Nedergaard, M.; Praetorius, J.; Damkier, H.H.; Brown, P.D.; Gagnon, K.B.; Delpire, E.; et al. Potassium cotransport at the rat choroid plexus. Am. J. Physiol. Cell Physiol. 1994, 267, C1616–C1622. [Google Scholar] [CrossRef]
- Keep, R.F.; Xiang, J. Choroid plexus potassium cotransport: Modulation by osmotic stress and external potassium. J. Neurochem. 2002, 64, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Delpire, E.; Hebert, S.C.; Strange, K. Functional demonstration of Na+-K+-2Cl- cotransporter activity in isolated, polarized choroid plexus cells. Am. J. Physiol. Cell Physiol. 1998, 274, C1565–C1572. [Google Scholar] [CrossRef]
- Xu, H.; Fame, R.M.; Sadegh, C.; Sutin, J.; Narajo, C.; Syau, D.; Cui, J.; Shipley, F.B.; Vernon, A.; Gao, F.; et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat. Commun. 2021, 12, 447. [Google Scholar] [CrossRef]
- Gregoriades, J.M.C.; Madaris, A.; Alvarez, F.J.; Alvarez-Leefmans, F.J. Genetic and pharmacological inactivation of apical Na+-K+-2Cl- cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am. J. Physiol. Cell Physiol. 2018, 316, C525–C544. [Google Scholar] [CrossRef]
- Alvarez-Leefmans, F.J. CrossTalk proposal: Apical NKCC1 of choroid plexus epithelial cells works in the net inward flux mode under basal conditions, maintaining intracellular Cl− and cell volume. J. Physiol. 2020, 598, 4733–4736. [Google Scholar] [CrossRef]
- MacAulay, N.; Rose, C.R. CrossTalk opposing view: NKCC1 in the luminal membrane of choroid plexus is outwardly directed under basal conditions and contributes directly to cerebrospinal fluid secretion. J. Physiol. 2020, 598, 4737–4739. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Leefmans, F.J.; Rebuttal from Francisco, J. Alvarez-Leefmans. J. Physiol. 2020, 598, 4741–4742. [Google Scholar] [CrossRef]
- MacAulay, N.; Rose, C.R. Rebuttal from Nanna MacAulay and Christine, R. Rose. J. Physiol. 2020, 598, 4743. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Alessi, D. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J. Cell Sci. 2008, 121, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.B.; Delpire, E. Molecular physiology of SPAK and OSR1, two Ste20-related protein kinases regulating ion transport. Physiol. Rev. 2012, 92, 1577–1617. [Google Scholar] [CrossRef] [PubMed]
- Toft-Bertelsen, T.L.; Barbuskaite, D.; Heerfordt, E.K.; Lolansen, S.D.; Andreassen, S.N.; Rostgaard, N.; Olsen, M.H.; Norager, N.H.; Capion, T.; Rath, M.F.; et al. Lysophosphatidic acid as a CSF lipid in posthemorrhagic hydrocephalus that drives CSF accumulation via TRPV4-induced hyperactivation of NKCC1. Fluids Barriers CNS 2022, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Leite-Dellova, D.C.A.; Demko, J.; Sørensen, M.V.; Takagi, E.; Gleason, C.E.; Shabbir, W.; Pearce, D. WNK1 is a chloride-stimulated scaffold that regulates mTORC2 activity and ion transport. J. Cell Sci. 2022, 135, jcs260313. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16a, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of TMEM16a as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef]
- Yang, Y.D.; Cho, H.; Koo, Y.J.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Shibasaki, K.; Suzuki, Y.; Yamanaka, A.; Tominaga, M. Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J. 2014, 28, 2238–2248. [Google Scholar] [CrossRef]
- Genovese, M.; Borrelli, A.; Venturini, A.; Guidone, D.; Caci, E.; Viscido, G.; Gambardella, G.; di Bernardo, D.; Scudieri, P.; Galietta, L.J. TRPV4 and purinergic receptor signalling pathways are separately linked in airway epithelia to CFTR and TMEM16A chloride channels. J. Physiol. 2019, 597, 5859–5878. [Google Scholar] [CrossRef] [PubMed]
- Piala, A.; Moon, T.M.; Akella, R.; He, H.; Cobb, M.H.; Goldsmith, E.J. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci. Signal. 2014, 7, ra41. [Google Scholar] [CrossRef]
- Bazúa-Valenti, S.; Chávez-Canales, M.; Rojas-Vega, L.; González-Rodríguez, X.; Vázquez, N.; Rodríguez-Gama, A.; Argaiz, E.R.; Melo, Z.; Plata, C.; Ellison, D.H.; et al. The effect of WNK4 on the Na+Cl- cotransporter is modulated by intracellular chloride. J. Am. Soc. Nephrol. 2015, 26, 1781–1786. [Google Scholar] [CrossRef]
- Rodan, A.R. Intracellular chloride: A regulator of transepithelial transport in the distal nephron. Curr. Opin. Nephrol. Hypertens. 2019, 28, 360–367. [Google Scholar] [CrossRef]
- Chen, J.-C.; Lo, Y.-F.; Lin, Y.-W.; Lin, S.-H.; Huang, C.-L.; Cheng, C.-J. WNK4 kinase is a physiological intracellular chloride sensor. Proc. Natl. Acad. Sci. USA 2019, 116, 4502–4507. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ku, S.K.; Ji, H.W.; Choi, J.-H.; Shin, D.M. Ca2+ is a regulator of the WNK/OSR1/NKCC pathway in a human salivary gland cell line. Korean J. Physiol. Pharmacol. 2015, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bhuiyan, M.I.H.; Zhang, T.; Karimy, J.K.; Wu, Z.; Fiesler, V.M.; Zhang, J.; Huang, H.; Hasan, M.N.; Skrzypiec, A.E.; et al. Modulation of brain cation-Cl- cotransport via the SPAK kinase inhibitor ZT-1a. Nat. Commun. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazer-Yost, B.L. Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus. Int. J. Mol. Sci. 2023, 24, 6673. https://doi.org/10.3390/ijms24076673
Blazer-Yost BL. Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus. International Journal of Molecular Sciences. 2023; 24(7):6673. https://doi.org/10.3390/ijms24076673
Chicago/Turabian StyleBlazer-Yost, Bonnie L. 2023. "Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus" International Journal of Molecular Sciences 24, no. 7: 6673. https://doi.org/10.3390/ijms24076673
APA StyleBlazer-Yost, B. L. (2023). Consideration of Kinase Inhibitors for the Treatment of Hydrocephalus. International Journal of Molecular Sciences, 24(7), 6673. https://doi.org/10.3390/ijms24076673





