Alopecia in Patients with Collagen VI-Related Myopathies: A Novel/Unrecognized Scalp Phenotype
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
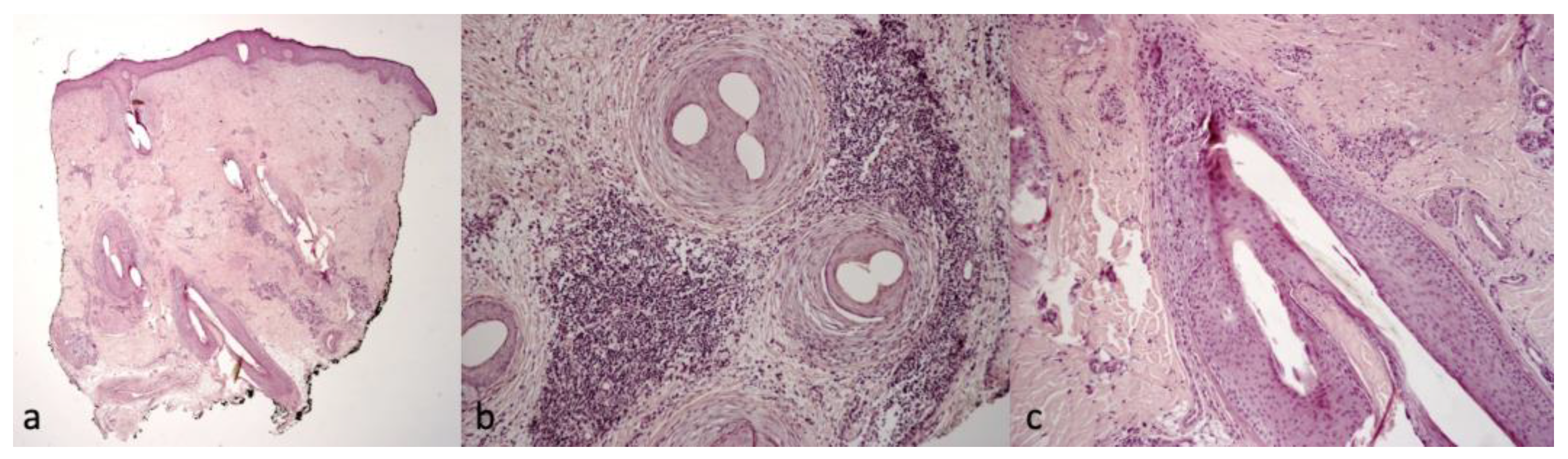
2.2. Trichoscopic and Histological Findings
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef] [Green Version]
- Maraldi, N.M.; Sabatelli, P.; Columbaro, M.; Zamparelli, A.; Manzoli, F.A.; Bernardi, P.; Bonaldo, P.; Merlini, L. Collagen VI myopathies: From the animal model to the clinical trial. Adv. Enzym. Regul. 2009, 49, 197–211. [Google Scholar] [CrossRef]
- Hessle, H.; Engvall, E. Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J. Biol. Chem. 1984, 259, 3955–3961. [Google Scholar] [CrossRef]
- Keene, D.R.; Engvall, E.; Glanville, R.W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J. Cell Biol. 1988, 107, 1995–2006. [Google Scholar] [CrossRef]
- Wiberg, C.; Hedbom, E.; Khairullina, A.; Lamandé, S.R.; Oldberg, A.; Timpl, R.; Mörgelin, M.; Heinegård, D. Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J. Biol. Chem. 2001, 276, 18947–18952. [Google Scholar] [CrossRef] [Green Version]
- Sabatelli, P.; Bonaldo, P.; Lattanzi, G.; Braghetta, P.; Bergamin, N.; Capanni, C.; Mattioli, E.; Columbaro, M.; Ognibene, A.; Pepe, G.; et al. Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol. 2001, 20, 475–486. [Google Scholar] [CrossRef]
- Allamand, V.; Merlini, L.; Bushby, K.; Consortium for Collagen VI-Related Myopathies. 166th ENMC International Workshop on Collagen type VI-related Myopathies, 22–24 May 2009, Naarden, The Netherlands. Neuromuscul. Disord. 2010, 20, 346–354. [Google Scholar] [CrossRef]
- Bönnemann, C.G. The collagen VI-related myopathies: Muscle meets its matrix. Nat. Rev. Neurol. 2011, 7, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Merlini, L.; Martoni, E.; Grumati, P.; Sabatelli, P.; Squarzoni, S.; Urciuolo, A.; Ferlini, A.; Gualandi, F.; Bonaldo, P. Autosomal recessive myosclerosis myopathy is a collagen VI disorder. Neurology 2008, 71, 1245–1253. [Google Scholar] [CrossRef]
- Echeverría, C.; Diaz, A.; Suarez, B.; Bevilacqua, J.; Bonnemann, C.; Bertini, E.; Castiglioni, C. Keloids, Spontaneous or After Minor Skin Injury: Importance of Not Missing Bethlem Myopathy. Acta Derm.-Venereol. 2017, 97, 297–298. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, A.; Muntoni, F. Skin changes in Ullrich congenital muscular dystrophy. Neuromuscul. Disord. 2008, 18, 982. [Google Scholar] [CrossRef]
- Ritter, A.M.; Lee, L.W. Keratosis pilaris in collagen type VI-related disorders. Pediatr. Dermatol. 2022, 39, 133–134. [Google Scholar] [CrossRef]
- Lee, S.S.; Hinds, B.; Sprague, J.; Barrio, V.R.; Mancuso, J.B. Atypical keratosis pilaris-like lesions in a patient with Bethlem myopathy. Pediatr. Dermatol. 2022, 39, 309–311. [Google Scholar] [CrossRef]
- Bruni, F.; Alessandrini, A.; Starace, M.; Orlando, G.; Piraccini, B. Clinical and trichoscopic features in various forms of scalp psoriasis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1830–1837. [Google Scholar] [CrossRef]
- Caputo, V.; Strafella, C.; Termine, A.; Campione, E.; Bianchi, L.; Novelli, G.; Giardina, E.; Cascella, R. RNAseq-Based Prioritization Revealed COL6A5, COL8A1, COL10A1 and MIR146A as Common and Differential Susceptibility Biomarkers for Psoriasis and Psoriatic Arthritis: Confirmation from Genotyping Analysis of 1417 Italian Subjects. Int. J. Mol. Sci. 2020, 21, 2740. [Google Scholar] [CrossRef]
- Powell, J.J.; Dawber, R.P.; Gatter, K. Folliculitis decalvans including tufted folliculitis: Clinical, histological and therapeutic findings. Br. J. Dermatol. 1999, 140, 328–333. [Google Scholar] [CrossRef]
- Fernández-Crehuet, P.; Vañó-Galván, S.; Molina-Ruiz, A.M.; Rodrigues-Barata, A.R.; Serrano-Falcón, C.; Martorell-Calatayud, A.; Arias-Santiago, S.; Barco-Nebreda, D.; Serrano, S.; Jaén, P.; et al. Trichoscopic features of folliculitis decalvans: Results in 58 Patients. Int. J. Trichol. 2017, 9, 140–141. [Google Scholar] [CrossRef] [Green Version]
- Sabatelli, P.; Gara, S.K.; Grumati, P.; Urciuolo, A.; Gualandi, F.; Curci, R.; Squarzoni, S.; Zamparelli, A.; Martoni, E.; Merlini, L.; et al. Expression of the collagen VI α5 and α6 chains in normal human skin and in skin of patients with collagen VI-related myopathies. J. Investig. Dermatol. 2011, 131, 99–107. [Google Scholar] [CrossRef]
- Hermanns-Lê, T.; Piérard, G.E.; Pierard-Franchimont, C.; Delvenne, P. Dermal Ultrastructure in Collagen VI Myopathy. Ultrastruct. Pathol. 2014, 38, 164–166. [Google Scholar] [CrossRef]
- Peltonen, J.; Hsiao, L.L.; Jaakkola, S.; Sollberg, S.; Aumailley, M.; Timpl, R.; Chu, M.L.; Uitto, J. Activation of collagen gene expression in keloids: Co-localization of type I and VI collagen and transforming growth factor-beta 1 mRNA. J. Investig. Dermatol. 1991, 97, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Doshi, B.; Khopkar, U. Trichoscopy in alopecias: Diagnosis simplified. Int. J. Trichol. 2013, 5, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss—Clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef] [PubMed]







| Age (year)/ Sex | Phenotype | Genomic Mutation | Other Cutaneous Signs | Scalp Symptoms | Scalp Clinical Findings | Trichoscopic Findings | Histological Findings | |
|---|---|---|---|---|---|---|---|---|
| 1 | 25/F | UCMD | COL6A1 c.850G>A | Keloids, follicular hyperkeratosis | Severe itching | White-yellow scales | Erythema, twisted red loop vessels, and yellow interfollicular scales | Dilated vessels in the dermis; parakeratosis, focal absence of granular layer in the epidermis, and neutrophilic infiltrate in the papillary dermis mantles (sebaceous structures) |
| 2 | 29/F | UCMD | COL6A1 c.958-2A>G | Face and neck hirsutism, keloids | Severe itching | Small cicatricial areas on vertex | Tufted hair, absence of follicular ostia in cicatricial area, and dilated elongated vessels | Dense diffuse suppurative, mixed infiltrate in the dermis. “Tufted folliculitis” and intraepidermal pustules |
| 3 | 24/F | UCMD | COL6A2 homozygous c.348dup | Keloids | Mild itching | Cicatricial patch on vertex | Severe erythema, dilated vessels, yellow scales, tufted hair, perifollicular pustules, and cicatricial areas with absence of follicular ostia | Superficial perivascular and perifollicular infiltrates of lymphocytes and neutrophils |
| 4 | 37/F | UCMD | COL6A2 c.875G>T | Keloids, follicular hyperkeratosis | Severe itching | Fine scaling | Perifollicular scales and erythema | Superficial perivascular and perifollicular infiltrates of lymphocytes and neutrophils; collection of neutrophils within infundibula |
| 5 | 24/M | UCMD | COL6A1 c.930+189C>T | Keloids, follicular hyperkeratosis | Severe itching | Hair thinning | Different hair diameters, erythema, dilated vessels, and yellow scales | Superficial perivascular and perifollicular infiltrates of lymphocytes and neutrophils |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starace, M.; Pampaloni, F.; Bruni, F.; Quadrelli, F.; Cedirian, S.; Baraldi, C.; Misciali, C.; Di Martino, A.; Sabatelli, P.; Merlini, L.; et al. Alopecia in Patients with Collagen VI-Related Myopathies: A Novel/Unrecognized Scalp Phenotype. Int. J. Mol. Sci. 2023, 24, 6678. https://doi.org/10.3390/ijms24076678
Starace M, Pampaloni F, Bruni F, Quadrelli F, Cedirian S, Baraldi C, Misciali C, Di Martino A, Sabatelli P, Merlini L, et al. Alopecia in Patients with Collagen VI-Related Myopathies: A Novel/Unrecognized Scalp Phenotype. International Journal of Molecular Sciences. 2023; 24(7):6678. https://doi.org/10.3390/ijms24076678
Chicago/Turabian StyleStarace, Michela, Francesca Pampaloni, Francesca Bruni, Federico Quadrelli, Stephano Cedirian, Carlotta Baraldi, Cosimo Misciali, Alberto Di Martino, Patrizia Sabatelli, Luciano Merlini, and et al. 2023. "Alopecia in Patients with Collagen VI-Related Myopathies: A Novel/Unrecognized Scalp Phenotype" International Journal of Molecular Sciences 24, no. 7: 6678. https://doi.org/10.3390/ijms24076678






