Literature Review: The sFlt1/PlGF Ratio and Pregestational Maternal Comorbidities: New Risk Factors to Predict Pre-Eclampsia
Abstract
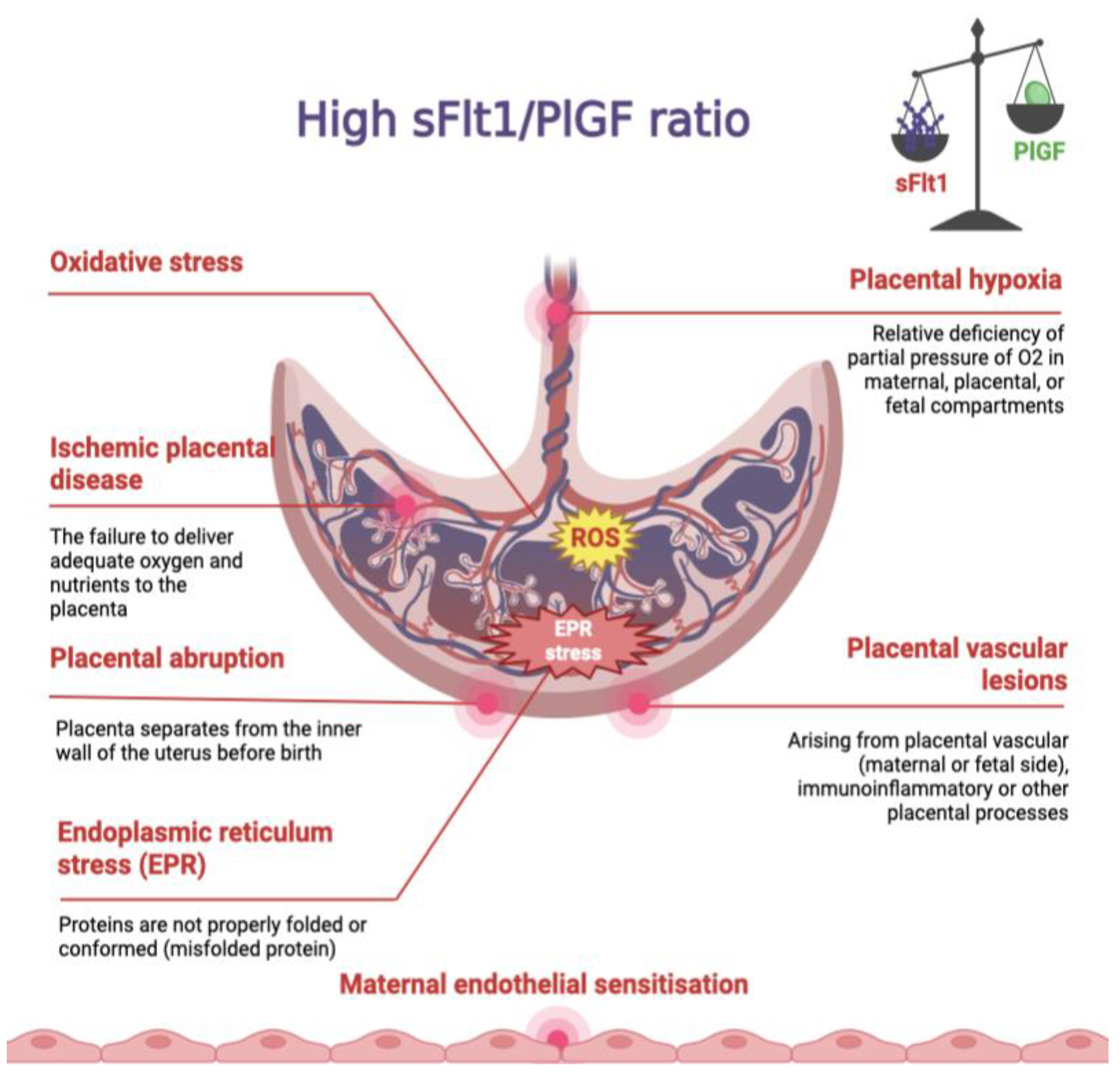
:1. Pre-Eclampsia
2. sFlt-1 and PlGF
3. sFlt1/PlGF Ratio
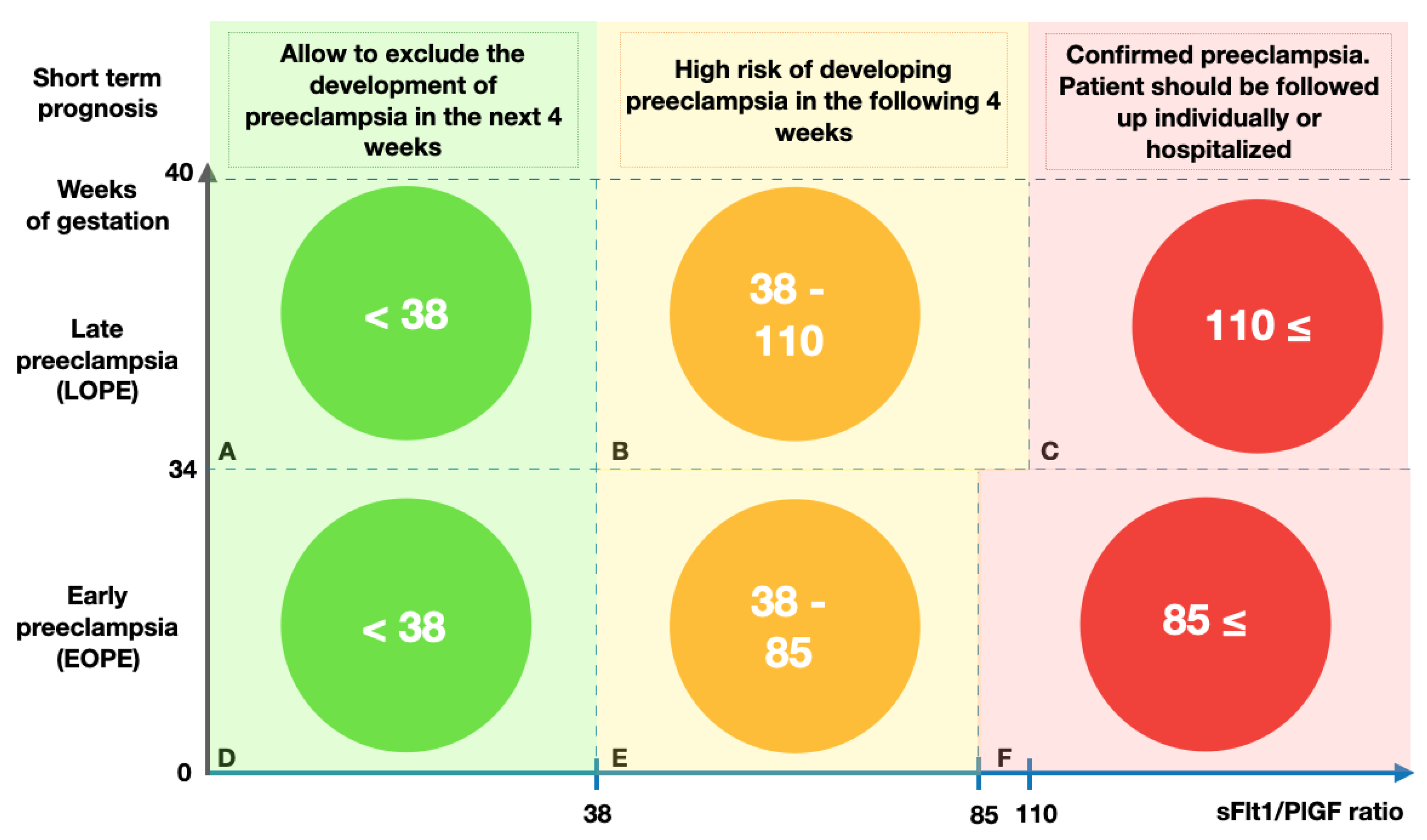
4. Short-Term Pre-Eclampsia Predictions Using the sFlt1/PlGF Ratio
5. sFlt1/PlGF Ratio and HELLP
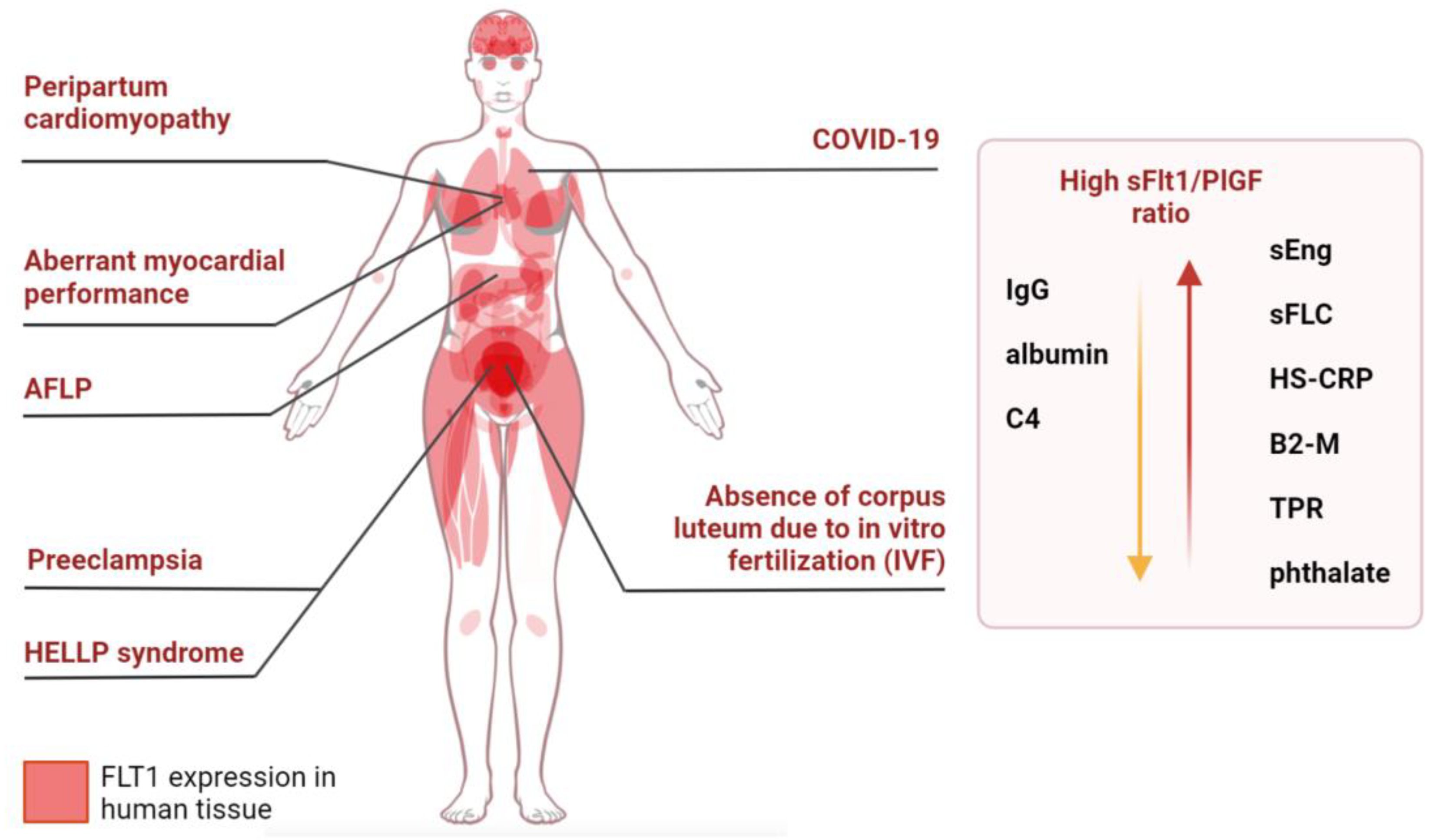
6. sFlt1/PlGF and Maternal Pregestational Comorbidities
7. sFlt1/PlGF Ratio and Chronic Hypertension
8. sFlt1/PlGF Ratio and Cardiovascular Disease
9. sFlt1/PlGF Ratio and Rheumatoid Arthritis
10. sFlt1/PlGF Ratio and Chronic Kidney Disease
11. sFlt1/PlGF Ratio and Acute Fatty Liver of Pregnancy
12. sFlt1/PlGF Ratio and Obesity in Pregnant Women
13. sFlt1/PlGF Ratio and Diabetes
14. sFlt1/PlGF Ratio and Serum and Urine Biomarkers of Pregnant Women
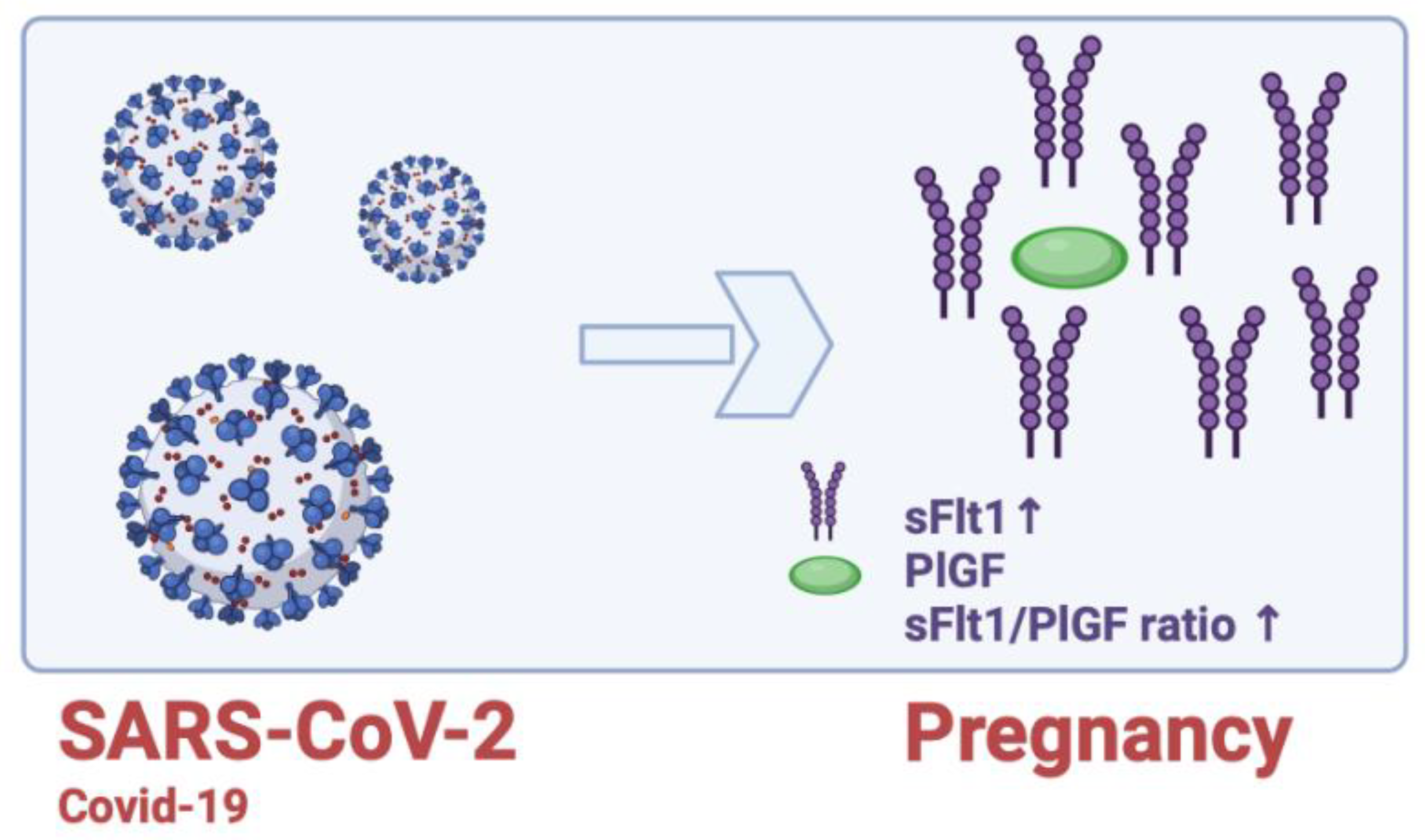
15. sFlt1/PlGF Ratio and COVID-19
16. sFlt1/PlGF Ratio and HIV Infection
17. sFlt-1, PlGF and Thyroid Hormones
18. sFlt1/PlGF Ratio and In Vitro Fertilization (IVF)
19. sFlt1/PlGF Ratio and Cigarette Smoking
20. sFlt1/PlGF Ratio and Breast Cancer in Pregnant Women
21. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Khalil, A.; O’Brien, P.; Townsend, R. Current best practice in the management of hypertensive disorders in pregnancy. Integr. Blood Press. Control 2016, 9, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, E323–E333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluivers, A.C.M.; Biesbroek, A.; Visser, W.; Saleh, L.; Russcher, H.; Danser, J.A.H.; Neuman, R.I. Angiogenic imbalance in pre-eclampsia and fetal growth restriction: Enhanced soluble Fms-like tyrosine kinase-1 binding or diminished production of placental growth factor? Ultrasound Obstet. Gynecol. 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.; Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165961. [Google Scholar] [CrossRef]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef]
- Caillon, H.; Tardif, C.; Dumontet, E.; Winer, N.; Masson, D. Evaluation of sFlt-1/PlGF Ratio for Predicting and Improving Clinical Management of Pre-eclampsia: Experience in a Specialized Perinatal Care Center. Ann. Lab. Med. 2018, 38, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Rowson, S.; Reddy, M.; De Guingand, D.; Langston-Cox, A.; Marshall, S.; Costa, F.D.S.; Palmer, K. Comparison of circulating total sFLT-1 to placental-specific sFLT-1 e15a in women with suspected preeclampsia. Placenta 2022, 120, 73–78. [Google Scholar] [CrossRef]
- Hauser, S.; Weich, H.A. A heparin-binding form of placenta growth factor (PLGF-2) is expressed in human umbilical vein endothelial cells and in placenta. Growth Factors 1993, 9, 259–268. [Google Scholar] [CrossRef]
- Cao, Y.; Ji, W.-R.; Qi, P.; Rosin, A.; Cao, Y. Placenta growth factor: Identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem. Biophys. Res. Commun. 1997, 235, 493–498. [Google Scholar] [CrossRef]
- Yang, W.; Ahn, H.; Hinrichs, M.; Torry, R.J.; Torry, D.S. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J. Reprod. Immunol. 2003, 60, 53–60. [Google Scholar] [CrossRef]
- Dewerchin, M.; Carmeliet, P. PlGF: A Multitasking Cytokine with Disease-Restricted Activity. Cold Spring Harb. Perspect. Med. 2012, 2, a011056. [Google Scholar] [CrossRef]
- Chau, K.; Hennessy, A.; Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 2017, 31, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [Green Version]
- De Vivo, A.; Baviera, G.; Giordano, D.; Todarello, G.; Corrado, F.; D’Anna, R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet. Gynecol. Scand. 2008, 87, 837–842. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Zhang, W.; Trudinger, B.J.; Lee, V.W. Discordant clinical presentations of preeclampsia and intrauterine fetal growth restriction with similar pro- and anti-angiogenic profiles. J. Matern. Neonatal Med. 2014, 27, 1854–1859. [Google Scholar] [CrossRef]
- Nanjo, S.; Minami, S.; Mizoguchi, M.; Yamamoto, M.; Yahata, T.; Toujima, S.; Shiro, M.; Kobayashi, A.; Muragaki, Y.; Ino, K. Levels of serum-circulating angiogenic factors within 1 week prior to delivery are closely related to conditions of pregnant women with pre-eclampsia, gestational hypertension, and/or fetal growth restriction. J. Obstet. Gynaecol. Res. 2017, 43, 1805–1814. [Google Scholar] [CrossRef]
- Schrey-Petersen, S.; Stepan, H. Anti-angiogenesis and Preeclampsia in 2016. Curr. Hypertens. Rep. 2017, 19, 6. [Google Scholar] [CrossRef]
- Leaños-Miranda, A.; Méndez-Aguilar, F.; Ramírez-Valenzuela, K.L.; Serrano-Rodríguez, M.; Berumen-Lechuga, G.; Molina-Pérez, C.J.; Isordia-Salas, I.; Campos-Galicia, I. Circulating angiogenic factors are related to the severity of gestational hypertension and preeclampsia, and their adverse outcomes. Medicine 2017, 96, e6005. [Google Scholar] [CrossRef]
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80. [Google Scholar] [CrossRef]
- Dathan-Stumpf, A.; Rieger, A.; Verlohren, S.; Wolf, C.; Stepan, H. sFlt-1/PlGF ratio for prediction of preeclampsia in clinical routine: A pragmatic real-world analysis of healthcare resource utilisation. PLoS ONE 2022, 17, e0263443. [Google Scholar] [CrossRef] [PubMed]
- Signore, C.; Mills, J.L.; Qian, C.; Yu, K.; Lam, C.; Epstein, F.H.; Karumanchi, S.A.; Levine, R.J. Circulating angiogenic factors and placental abruption. Obstet. Gynecol. 2006, 108, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Charnock-Jones, D.S. Placental hypoxia, endoplasmic reticulum stress and maternal endothelial sensitisation by sFLT1 in pre-eclampsia. J. Reprod. Immunol. 2016, 114, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Smith, L.; Modest, A.M.; Salahuddin, S.; Karumanchi, S.; Rana, S.; Young, B.C. Angiogenic factors and prediction for ischemic placental disease in future pregnancies. Pregnancy Hypertens. 2021, 25, 12–17. [Google Scholar] [CrossRef]
- Baltajian, K.; Hecht, J.L.; Wenger, J.B.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Zsengeller, Z.K.; Thadhani, R.; Karumanchi, S.A.; Rana, S. Placental lesions of vascular insufficiency are associated with anti-angiogenic state in women with preeclampsia. Hypertens. Pregnancy 2014, 33, 427–439. [Google Scholar] [CrossRef]
- Anto, E.O.; Coall, D.A.; Addai-Mensah, O.; Wiafe, Y.A.; Owiredu, W.K.B.A.; Obirikorang, C.; Annani-Akollor, M.E.; Adua, E.; Tawiah, A.; Acheampong, E.; et al. Early gestational profiling of oxidative stress and angiogenic growth mediators as predictive, preventive and personalised (3P) medical approach to identify suboptimal health pregnant mothers likely to develop preeclampsia. EPMA J. 2021, 12, 517–534. [Google Scholar] [CrossRef]
- Li, H.; Gu, B.; Zhang, Y.; Lewis, D.; Wang, Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 2005, 26, 210–217. [Google Scholar] [CrossRef]
- Castro, K.R.; Prado, K.M.; Lorenzon, A.R.; Hoshida, M.S.; Alves, E.A.; Francisco, R.P.V.; Zugaib, M.; Marques, A.L.X.; Silva, E.C.O.; Fonseca, E.J.S.; et al. Serum From Preeclamptic Women Triggers Endoplasmic Reticulum Stress Pathway and Expression of Angiogenic Factors in Trophoblast Cells. Front. Physiol. 2022, 12, 799653. [Google Scholar] [CrossRef]
- Daponte, A.; Pournaras, S.; Polyzos, N.P.; Tsezou, A.; Skentou, H.; Anastasiadou, F.; Lialios, G.; Messinis, I.E. Soluble FMS-like tyrosine kinase-1 (sFlt-1) and serum placental growth factor (PLGF) as biomarkers for ectopic pregnancy and missed abortion. J. Clin. Endocrinol. Metab. 2011, 96, E1444–E1451. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.; Stenman, U.-H.; Nuutila, M.; Paavonen, J.; Hiilesmaa, V.; Ylikorkala, O. Failure of second-trimester measurement of soluble endoglin and other angiogenic factors to predict placental abruption. Prenat. Diagn. 2007, 27, 1143–1146. [Google Scholar] [CrossRef]
- Herraiz, I.; Dröge, L.A.; Gómez-Montes, E.; Henrich, W.; Galindo, A.; Verlohren, S. Characterization of the Soluble FMS-like tyrosine kinase-1 to placental growth factor ratio in pregnancies complicated by fetal growth restriction. Obstet. Gynecol. 2014, 124 Pt 1, 265–273. [Google Scholar] [CrossRef]
- Zhang, K.; Zen, M.; Popovic, N.L.; Lee, V.; Alahakoon, T.I. Urinary placental growth factor in preeclampsia and fetal growth restriction: An alternative to circulating biomarkers? J. Obstet. Gynaecol. Res. 2019, 45, 1828–1836. [Google Scholar] [CrossRef]
- Stepan, H.; Hund, M.; Andraczek, T. Combining Biomarkers to Predict Pregnancy Complications and Redefine Preeclampsia: The Angiogenic-Placental Syndrome. Hypertension 2020, 75, 918–926. [Google Scholar] [CrossRef]
- Huhn, E.A.; Kreienbühl, A.; Hoffmann, I.; Schoetzau, A.; Lange, S.; De Tejada, B.M.; Hund, M.; Hoesli, I.; Lapaire, O. Diagnostic Accuracy of Different Soluble fms-Like Tyrosine Kinase 1 and Placental Growth Factor Cut-Off Values in the Assessment of Preterm and Term Preeclampsia: A Gestational Age Matched Case-Control Study. Front. Med. 2018, 5, 325. [Google Scholar] [CrossRef] [Green Version]
- Tang, P.; Xu, J.; Xie, B.-J.; Wang, Q.-M. Use of serum and urinary soluble sFlt-1 and PLGF in the diagnosis of preeclampsia. Hypertens. Pregnancy 2017, 36, 48–52. [Google Scholar] [CrossRef]
- Cui, L.; Shu, C.; Liu, Z.; Tong, W.; Cui, M.; Wei, C.; Tang, J.J.; Liu, X.; Hu, J.; Jiang, J.; et al. The expression of serum sEGFR, sFlt-1, sEndoglin and PLGF in preeclampsia. Pregnancy Hypertens. 2018, 13, 127–132. [Google Scholar] [CrossRef]
- Sroka, D.; Verlohren, S. Short Term Prediction of Preeclampsia. Matern. Med. 2021, 3, 107–115. [Google Scholar] [CrossRef]
- Stepan, H.; Galindo, A.; Hund, M.; Schlembach, D.; Sillman, J.; Surbek, D.; Vatish, M. Clinical utility of sFlt -1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet. Gynecol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Bolla, D.M.; Papadia, A.; Raio, L. The sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 1785–1786. [Google Scholar] [CrossRef] [Green Version]
- Sabrià, E.; Lequerica-Fernández, P.; Ganuza, P.L.; Ángeles, E.E.; Escudero, A.I.; Martínez-Morillo, E.; Alvárez, F.V. Use of the sFlt-1/PlGF ratio to rule out preeclampsia requiring delivery in women with suspected disease. Is the evidence reproducible? Clin. Chem. Lab. Med. 2018, 56, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Stepan, H.; Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. 2012, 122, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New Gestational Phase–Specific Cutoff Values for the Use of the Soluble Fms-Like Tyrosine Kinase-1/Placental Growth Factor Ratio as a Diagnostic Test for Preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, K.; Grittner, U.; Engels, T.; Pape, J.; Denk, B.; Henrich, W.; Verlohren, S. The importance of repeated measurements of the sFlt-1/PlGF ratio for the prediction of preeclampsia and intrauterine growth restriction. J. Périnat. Med. 2014, 42, 61–68. [Google Scholar] [CrossRef]
- Leaños-Miranda, A.; Nolasco-Leaños, A.G.; Carrillo-Juárez, R.I.; Molina-Pérez, C.J.; Sillas-Pardo, L.J.; Jiménez-Trejo, L.M.; Isordia-Salas, I.; Ramírez-Valenzuela, K.L. Usefulness of the sFlt-1/PlGF (Soluble fms-Like Tyrosine Kinase-1/Placental Growth Factor) Ratio in Diagnosis or Misdiagnosis in Women With Clinical Diagnosis of Preeclampsia. Hypertension 2020, 76, 892–900. [Google Scholar] [CrossRef]
- Jayasena, C.; Abbara, A.; Comninos, A.; Narayanaswamy, S.; Maffe, J.G.; Izzi-Engbeaya, C.; Oldham, J.; Lee, T.; Sarang, Z.; Malik, Z.; et al. Novel circulating placental markers prokineticin-1, soluble Fms-like tyrosine kinase-1, soluble endoglin and placental growth factor and association with late miscarriage. Hum. Reprod. 2016, 31, 2681–2688. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.L.; Cavalli, R.D.C.; Korkes, H.A.; Filho, E.V.D.C.; Peraçoli, J.C. Diagnosis and Management of Preeclampsia: Suggested Guidance on the Use of Biomarkers. Diagnóstico e tratamento da pré-eclâmpsia: Sugestão para o uso adequado dos biomarcadores. Rev. Bras. Ginecol. Obstet. 2022, 44, 878–883. [Google Scholar] [CrossRef]
- Trottmann, F.; Baumann, M.; Amylidi-Mohr, S.; Surbek, D.; Risch, L.; Mosimann, B.; Raio, L. Angiogenic profiling in HELLP syndrome cases with or without hypertension and proteinuria. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 93–96. [Google Scholar] [CrossRef]
- Tranquilli, A.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.; Steyn, W.; Zeeman, G.; Brown, M. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013, 3, 44–47. [Google Scholar] [CrossRef]
- Schnabel, A.; Blois, S.M.; Meint, P.; Freitag, N.; Ernst, W.; Barrientos, G.; Conrad, M.L.; Rose, M.; Seelbach-Göbel, B. Elevated systemic galectin-1 levels characterize HELLP syndrome. J. Reprod. Immunol. 2016, 114, 38–43. [Google Scholar] [CrossRef]
- Suzuki, H.; Nagayama, S.; Hirashima, C.; Takahashi, K.; Takahashi, H.; Ogoyama, M.; Nagayama, M.; Shirasuna, K.; Matsubara, S.; Ohkuchi, A. Markedly higher sFlt-1/PlGF ratio in a woman with acute fatty liver of pregnancy compared with HELLP syndrome. J. Obstet. Gynaecol. Res. 2019, 45, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single–cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Duhig, K.E.; Shennan, A.H. Recent advances in the diagnosis and management of pre-eclampsia. F1000Prime Rep. 2015, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Nzelu, D.; Dumitrascu-Biris, D.; Nicolaides, K.H.; Kametas, N.A. Chronic hypertension: First-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age. Am. J. Obstet. Gynecol. 2018, 218, 337.e1–337.e7. [Google Scholar] [CrossRef] [Green Version]
- Panaitescu, A.M.; Syngelaki, A.; Prodan, N.; Akolekar, R.; Nicolaides, K.H. Chronic hypertension and adverse pregnancy outcome: A cohort study. Ultrasound Obstet. Gynecol. 2017, 50, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Nzelu, D.; Biris, D.; Karampitsakos, T.; Nicolaides, K.K.; Kametas, N.A. First trimester serum angiogenic and anti-angiogenic factors in women with chronic hypertension for the prediction of preeclampsia. Am. J. Obstet. Gynecol. 2020, 222, 374.e1–374.e9. [Google Scholar] [CrossRef]
- Perni, U.; Sison, C.; Sharma, V.; Helseth, G.; Hawfield, A.; Suthanthiran, M.; August, P. Angiogenic factors in superimposed preeclampsia: A longitudinal study of women with chronic. Hypertension 2012, 59, 740–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, R.A.; Hoshida, M.S.; Alves, E.A.; Zugaib, M.; Francisco, R.P.V. Preeclampsia and superimposed preeclampsia: The same disease? The role of angiogenic biomarkers. Hypertens. Pregnancy 2016, 35, 139–149. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 24 January 2023).
- Iwama, H.; Uemura, S.; Naya, N.; Imagawa, K.-I.; Takemoto, Y.; Asai, O.; Onoue, K.; Okayama, S.; Somekawa, S.; Kida, Y.; et al. Cardiac expression of placental growth factor predicts the improvement of chronic phase left ventricular function in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2006, 47, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochholzer, W.; Reichlin, T.; Stelzig, C.; Hochholzer, K.; Meissner, J.; Breidthardt, T.; Reiter, M.; Duehsler, B.; Freidank, H.; Winkler, K.; et al. Impact of soluble fms-like tyrosine kinase-1 and placental growth factor serum levels for risk stratification and early diagnosis in patients with suspected acute myocardial infarction. Eur. Heart J. 2011, 32, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Hammadah, M.; Georgiopoulou, V.V.; Kalogeropoulos, A.P.; Weber, M.; Wang, X.; Samara, M.A.; Wu, Y.; Butler, J.; Tang, W.W. Elevated Soluble Fms-Like Tyrosine Kinase-1 and Placental-Like Growth Factor Levels Are Associated With Development and Mortality Risk in Heart Failure. Circ. Heart Fail. 2016, 9, e002115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draker, N.; Torry, D.S.; Torry, R. Placenta growth factor and sFlt-1 as biomarkers in ischemic heart disease and heart failure: A review. Biomark. Med. 2019, 13, 785–799. [Google Scholar] [CrossRef]
- Matsumoto, T.; Uemura, S.; Takeda, Y.; Matsui, M.; Okada, S.; Nishida, T.; Soeda, T.; Okayama, S.; Somekawa, S.; Ishigami, K.-I.; et al. An elevated ratio of placental growth factor to soluble FMS-like tyrosine kinase-1 predicts adverse outcomes in patients with stable coronary artery disease. Intern. Med. 2013, 52, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Sinning, C.; Schnabel, R.B.; Zeller, T.; Seiffert, M.; Rupprecht, H.J.; Lackner, K.J.; Blankenberg, S.; Bickel, C.; Westermann, D. Prognostic use of soluble fms-like tyrosine kinase-1 and placental growth factor in patients with coronary artery disease. Biomark. Med. 2016, 10, 95–106. [Google Scholar] [CrossRef]
- Poldervaart, J.M.; Röttger, E.; Dekker, M.S.; Zuithoff, N.P.A.; Verheggen, P.W.H.M.; De Vrey, E.A.; Wildbergh, T.X.; Hof, A.W.J.V.; Mosterd, A.; Hoes, A.W. No Added Value of Novel Biomarkers in the Diagnostic Assessment of Patients Suspected of Acute Coronary Syndrome. PLoS ONE 2015, 10, e0132000. [Google Scholar] [CrossRef]
- Klingenberg, R.; Aghlmandi, S.; Räber, L.; Gencer, B.; Nanchen, D.; Heg, D.; Carballo, S.; Rodondi, N.; Mach, F.; Windecker, S.; et al. Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT-proBNP and hsCRP with the GRACE score. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 129–138. [Google Scholar] [CrossRef]
- Tsiaras, S.; Poppas, A. Cardiac disease in pregnancy: Value of Echocardiography. Curr. Cardiol. Rep. 2010, 12, 250–256. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Baltabaeva, A.; Liberati, M.; Thilaganathan, B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011, 57, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Persico, M.G.; Vincenti, V.; DiPalma, T. Structure, expression and receptor-binding properties of placenta growth factor (PlGF). Poxviruses 1999, 237, 31–40. [Google Scholar] [CrossRef]
- Chen, C.W.; Jaffe, I.Z.; Karumanchi, S.A. Pre-eclampsia and cardiovascular disease. Cardiovasc. Res. 2014, 101, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Gimenez, C.; Mendoza, M.; Cruz-Lemini, M.; Galian-Gay, L.; Sanchez-Garcia, O.; Granato, C.; Rodriguez-Sureda, V.; Rodriguez-Palomares, J.; Carreras-Moratonas, E.; Cabero-Roura, L.; et al. Angiogenic Factors and Long-Term Cardiovascular Risk in Women That Developed Preeclampsia During Pregnancy. Hypertension 2020, 76, 1808–1816. [Google Scholar] [CrossRef]
- Akhter, T.; Wikström, A.-K.; Larsson, M.; Wikström, G.; Naessen, T. Association between angiogenic factors and signs of arterial aging in women with pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Gibbone, E.; Wright, A.; Campos, R.V.; Sierra, A.S.; Nicolaides, K.H.; Charakida, M. Maternal cardiac function at 19–23 weeks’ gestation in prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2021, 57, 739–747. [Google Scholar] [CrossRef]
- Behrens, I.; Basit, S.; Lykke, J.A.; Ranthe, M.F.; Wohlfahrt, J.; Bundgaard, H.; Melbye, M.; Boyd, H.A. Hypertensive disorders of pregnancy and peripartum cardiomyopathy: A nationwide cohort study. PLoS ONE 2019, 14, e0211857. [Google Scholar] [CrossRef] [Green Version]
- Mebazaa, A.; Seronde, M.-F.; Gayat, E.; Tibazarwa, K.; Anumba, D.O.; Akrout, N.; Sadoune, M.; Sarb, J.; Arrigo, M.; Motiejunaite, J.; et al. Imbalanced Angiogenesis in Peripartum Cardiomyopathy―Diagnostic Value of Placenta Growth Factor. Circ. J. 2017, 81, 1654–1661. [Google Scholar] [CrossRef] [Green Version]
- Ullmo, J.; Cruz-Lemini, M.; Sánchez-García, O.; Bos-Real, L.; De La Llama, P.F.; Calero, F.; Domínguez-Gallardo, C.; Garrido-Gimenez, C.; Trilla, C.; Carreras-Costa, F.; et al. Cardiac dysfunction and remodeling regulated by anti-angiogenic environment in patients with preeclampsia: The ANGIOCOR prospective cohort study protocol. BMC Pregnancy Childbirth 2021, 21, 816. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, E.A. Pregnancy and rheumatoid arthritis. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 64, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kong, J.-S.; Lee, S.; Yoo, S.-A.; Koh, J.H.; Jin, J.; Kim, W.-U. Angiogenic cytokines can reflect the synovitis severity and treatment response to biologics in rheumatoid arthritis. Exp. Mol. Med. 2020, 52, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Therneau, T.M.; Gabriel, S.E. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010, 62, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wu, T.; Jin, T.; Zhang, Y.; Wang, J.; Qi, J.; Li, Y.; Jiang, H.; Zhang, J.; Jiang, Z.; et al. Maternal and fetal outcomes in pregnant women with rheumatoid arthritis: A systematic review and meta-analysis. Clin. Rheumatol. 2022, 42, 855–870. [Google Scholar] [CrossRef]
- Neuman, R.I.; Smeele, H.T.W.; Danser, A.H.J.; Dolhain, R.J.E.M.; Visser, W. The sFlt-1 to PlGF ratio in pregnant women with rheumatoid arthritis: Impact of disease activity and sulfasalazine use. Rheumatology 2021, 61, 628–635. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Hladunewich, M.A. Chronic Kidney Disease and Pregnancy. Semin. Nephrol. 2017, 37, 337–346. [Google Scholar] [CrossRef]
- Suarez, M.L.G.; Kattah, A.; Grande, J.P.; Garovic, V. Renal Disorders in Pregnancy: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 119–130. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cabiddu, G.; Attini, R.; Vigotti, F.N.; Maxia, S.; Lepori, N.; Tuveri, M.; Massidda, M.; Marchi, C.; Mura, S.; et al. Risk of Adverse Pregnancy Outcomes in Women with CKD. J. Am. Soc. Nephrol. 2015, 26, 2011–2022. [Google Scholar] [CrossRef] [Green Version]
- Piccoli, G.B.; Gaglioti, P.; Attini, R.; Parisi, S.; Bossotti, C.; Olearo, E.; Oberto, M.; Ferraresi, M.; Rolfo, A.; Versino, E.; et al. Pre-eclampsia or chronic kidney disease? The flow hypothesis. Nephrol. Dial. Transplant. 2013, 28, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Molina-Pérez, C.J.; Nolasco-Leaños, A.G.; Carrillo-Juárez, R.I.; Leaños-Miranda, A. Clinical usefulness of angiogenic factors in women with chronic kidney disease and suspected superimposed preeclampsia. J. Nephrol. 2022, 35, 1699–1708. [Google Scholar] [CrossRef]
- Karge, A.; Beckert, L.; Moog, P.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Abel, K.; Flechsenhar, S.; Kuschel, B.; Graupner, O. Role of sFlt-1/PIGF ratio and uterine Doppler in pregnancies with chronic kidney disease suspected with Pre-eclampsia or HELLP syndrome. Pregnancy Hypertens. 2020, 22, 160–166. [Google Scholar] [CrossRef]
- Rolfo, A.; Attini, R.; Nuzzo, A.M.; Piazzese, A.; Parisi, S.; Ferraresi, M.; Todros, T.; Piccoli, G.B. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013, 83, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Rolfo, A.; Attini, R.; Tavassoli, E.; Neve, F.V.; Nigra, M.; Cicilano, M.; Nuzzo, A.M.; Giuffrida, D.; Biolcati, M.; Nichelatti, M.; et al. Is It Possible to Differentiate Chronic Kidney Disease and Preeclampsia by means of New and Old Biomarkers? A Prospective Study. Dis. Markers 2015, 2015, 127083. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.B.; Byrne, J.J.; Cunningham, F.G. Acute Fatty Liver of Pregnancy. Clin. Obstet. Gynecol. 2020, 63, 152–164. [Google Scholar] [CrossRef]
- Rath, W.; Tsikouras, P.; Stelzl, P. HELLP Syndrome or Acute Fatty Liver of Pregnancy: A Differential Diagnostic Challenge: Common Features and Differences. Geburtshilfe Frauenheilkd. 2020, 80, 499–507. [Google Scholar] [CrossRef]
- Trottmann, F.; Raio, L.; Amylidi-Mohr, S.; Mosimann, B.; Campos, A.J.; Messerli, F.H.; Risch, L.; Baumann, M.U. Soluble fms-like tyrosine kinase 1 (sFlt-1): A novel biochemical marker for acute fatty liver of pregnancy. Acta Obstet. Gynecol. Scand. 2021, 100, 1876–1884. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 24 January 2023).
- Schummers, L.; Hutcheon, J.A.; Bodnar, L.M.; Lieberman, E.; Himes, K.P. Risk of adverse pregnancy outcomes by prepregnancy body mass index: A population-based study to inform prepregnancy weight loss counseling. Obstet. Gynecol. 2015, 125, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Suwaki, N.; Masuyama, H.; Nakatsukasa, H.; Masumoto, A.; Sumida, Y.; Takamoto, N.; Hiramatrsu, Y. Hypoadiponectinemia and circulating angiogenic factors in overweight patients complicated with pre-eclampsia. Am. J. Obstet. Gynecol. 2006, 195, 1687–1692. [Google Scholar] [CrossRef]
- Zera, C.A.; Seely, E.W.; Wilkins-Haug, L.E.; Lim, K.-H.; Parry, S.I.; McElrath, T.F. The association of body mass index with serum angiogenic markers in normal and abnormal pregnancies. Am. J. Obstet. Gynecol. 2014, 211, 247.e1–247.e7. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, T.; Finnpec, F.T.; Heinonen, S.; Hämäläinen, E.; Pulkki, K.; Romppanen, J.; Laivuori, H. Impact of obesity on angiogenic and inflammatory markers in the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) cohort. Int. J. Obes. 2019, 43, 1070–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimberger, S.; Mueller, A.; Ratnaparkhi, R.; Perdigao, J.L.; Rana, S. Angiogenic factor abnormalities and risk of peripartum complications and prematurity among urban predominantly obese parturients with chronic hypertension. Pregnancy Hypertens. 2020, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Allshouse, A.; Silver, R.M.; Grobman, W.A.; Simhan, H.; Haas, D.; Reddy, U.M.; Blue, N.R. High early pregnancy body mass index is associated with alterations in first and second trimester angiogenic biomarkers. Am. J. Obstet. Gynecol. MFM 2022, 4, 100614. [Google Scholar] [CrossRef] [PubMed]
- Karge, A.; Desing, L.; Haller, B.; Ortiz, J.U.; Lobmaier, S.M.; Kuschel, B.; Graupner, O. Performance of sFlt-1/PIGF Ratio for the Prediction of Perinatal Outcome in Obese Pre-Eclamptic Women. J. Clin. Med. 2022, 11, 3023. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 January 2023).
- Salzer, L.; Tenenbaum-Gavish, K.; Hod, M. Metabolic disorder of pregnancy (understanding pathophysiology of diabetes and preeclampsia). Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 328–338. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, N. Gestational Diabetes Mellitus and Preeclampsia: Correlation and Influencing Factors. Front. Cardiovasc. Med. 2022, 9, 831297. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diabetes Rep. 2015, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Hanson, U.; Persson, B. Outcome of Pregnancies complicated by type 1 insulin-dependent diabetes in Sweden: Acute pregnancy complications, neonatal mortality and morbidity. Am. J. Perinatol. 1993, 10, 330–333. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Lee, V.W.; Mclean, M.; Athayde, N.; Lanzarone, V.; Khoshnow, Q.; Peek, M.J.; Cheung, N.W. The Association of Falling Insulin Requirements With Maternal Biomarkers and Placental Dysfunction: A Prospective Study of Women With Preexisting Diabetes in Pregnancy. Diabetes Care 2017, 40, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.L.; Smilen, K.E.; Bianco, A.T.; Moshier, E.L.; Ferrara, L.A.; Stone, J.L. Predictive value of combined serum biomarkers for adverse pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 89–94. [Google Scholar] [CrossRef]
- Zen, M.; Padmanabhan, S.; Zhang, K.; Kirby, A.; Cheung, N.W.; Lee, V.W.; Alahakoon, T.I. Urinary and Serum Angiogenic Markers in Women With Preexisting Diabetes During Pregnancy and Their Role in Preeclampsia Prediction. Diabetes Care 2020, 43, 67–73. [Google Scholar] [CrossRef]
- Yu, Y.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Scholz, H.; Henriksen, T.; Lorentzen, B.; Clausen, T.; Garg, S.K.; Menard, M.K.; et al. Anti-angiogenic factors and pre-eclampsia in type 1 diabetic women. Diabetologia 2009, 52, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Holmes, V.A.; Young, I.S.; Patterson, C.C.; Maresh, M.J.; Pearson, D.W.; Walker, J.D.; McCance, D.R.; Diabetes and Preeclampsia Intervention Trial (DAPIT) Study Group. The role of angiogenic and antiangiogenic factors in the second trimester in the prediction of preeclampsia in pregnant women with type 1 Diabetes. Diabetes Care 2013, 36, 3671–3677. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, A.M.; Giuffrida, D.; Moretti, L.; Re, P.; Grassi, G.; Menato, G.; Rolfo, A. Placental and maternal sFlt1/PlGF expression in gestational diabetes mellitus. Sci. Rep. 2021, 11, 2312. [Google Scholar] [CrossRef]
- Philips, E.M.; Trasande, L.; Kahn, L.G.; Gaillard, R.; Steegers, E.A.P.; Jaddoe, V.W.V. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum. Reprod. 2019, 34, 365–373. [Google Scholar] [CrossRef]
- Portelli, M.; Baron, B. Clinical Presentation of Preeclampsia and the Diagnostic Value of Proteins and Their Methylation Products as Biomarkers in Pregnant Women with Preeclampsia and Their Newborns. J. Pregnancy 2018, 2018, 2632637. [Google Scholar] [CrossRef] [Green Version]
- Ohkuchi, A.; Hirashima, C.; Matsubara, S.; Suzuki, H.; Takahashi, K.; Usui, R.; Suzuki, M. Serum sFlt1:PlGF ratio, PlGF, and soluble endoglin levels in gestational proteinuria. Hypertens. Pregnancy 2009, 28, 95–108. [Google Scholar] [CrossRef]
- Abascal-Saiz, A.; Duque-Alcorta, M.; Fioravantti, V.; Antolín, E.; Fuente-Luelmo, E.; Haro, M.; Ramos-Álvarez, M.P.; Perdomo, G.; Bartha, J.L. The Relationship between Angiogenic Factors and Energy Metabolism in Preeclampsia. Nutrients 2022, 14, 2172. [Google Scholar] [CrossRef]
- Anton, L.; Merrill, D.C.; Neves, L.A.; Gruver, C.; Moorefield, C.; Brosnihan, K.B. Angiotensin II and angiotensin-(1-7) decrease sFlt1 release in normal but not preeclamptic chorionic villi: An in vitro study. Reprod. Biol. Endocrinol. 2010, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Cerdeira, A.S.; Wenger, J.; Salahuddin, S.; Lim, K.-H.; Ralston, S.J.; Thadhani, R.I.; Karumanchi, S.A. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS ONE 2012, 7, e48259. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, A.; Reisch, B.; Mavarani, L.; Oppong, M.D.; Kimmig, R.; Mach, P.; Schmidt, B.; Köeninger, A.; Gellhaus, A. Soluble endoglin versus sFlt-1/PlGF ratio: Detection of preeclampsia, HELLP syndrome, and FGR in a high-risk cohort. Hypertens. Pregnancy 2022, 41, 159–172. [Google Scholar] [CrossRef]
- Sarween, N.; Drayson, M.T.; Hodson, J.; Knox, E.M.; Plant, T.; Day, C.J.; Lipkin, G.W. Humoral immunity in late-onset Pre-eclampsia and linkage with angiogenic and inflammatory markers. Am. J. Reprod. Immunol. 2018, 80, e13041. [Google Scholar] [CrossRef]
- Doherty, A.; Carvalho, J.C.; Drewlo, S.; El-Khuffash, A.; Downey, K.; Dodds, M.; Kingdom, J. Altered hemodynamics and hyperuricemia accompany an elevated sFlt-1/PLGF ratio before the onset of early severe preeclampsia. J. Obstet. Gynaecol. Can. 2014, 36, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Sahay, A.S.; Patil, V.V.; Sundrani, D.P.; Joshi, A.A.; Wagh, G.N.; Gupte, S.A.; Joshi, S.R. A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertens. Res. 2014, 37, 753–758. [Google Scholar] [CrossRef]
- Sathiya, R.; Rajendran, J.; Sumathi, S. COVID-19 and Preeclampsia: Overlapping Features in Pregnancy. Rambam Maimonides Med. J. 2022, 13, e0007. [Google Scholar] [CrossRef]
- Giardini, V.; Ornaghi, S.; Gambacorti-Passerini, C.; Casati, M.; Carrer, A.; Acampora, E.; Vasarri, M.V.; Arienti, F.; Vergani, P. Imbalanced Angiogenesis in Pregnancies Complicated by SARS-CoV-2 Infection. Viruses 2022, 14, 2207. [Google Scholar] [CrossRef]
- Soldavini, C.M.; Di Martino, D.; Sabattini, E.; Ornaghi, S.; Sterpi, V.; Erra, R.; Invernizzi, F.; Tine’, G.; Giardini, V.; Vergani, P.; et al. sFlt-1/PlGF ratio in hypertensive disorders of pregnancy in patients affected by COVID-19. Pregnancy Hypertens. 2022, 27, 103–109. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Espino-Y-Sosa, S.; Poon, L.C.; Solis-Paredes, J.M.; Estrada-Gutierrez, G.; Espejel-Nuñez, A.; Juarez-Reyes, A.; Etchegaray-Solana, A.; Alfonso-Guillen, Y.; Aguilar-Andrade, L.; et al. Increased levels of soluble fms-like tyrosine kinase-1 are associated with adverse outcome in pregnant women with COVID-19. Ultrasound Obstet. Gynecol. 2022, 59, 202–208. [Google Scholar] [CrossRef]
- Govender, R.; Moodley, J.; Naicker, T. The COVID-19 Pandemic: An Appraisal of its Impact on Human Immunodeficiency Virus Infection and Pre-Eclampsia. Curr. Hypertens. Rep. 2021, 23, 9. [Google Scholar] [CrossRef]
- Masiá, M.; Padilla, S.; Fernández, M.; Rodríguez, C.; Moreno, A.; Oteo, J.A.; Antela, A.; Moreno, S.; del Amo, J.; Gutiérrez, F.; et al. Oxidative Stress Predicts All-Cause Mortality in HIV-Infected Patients. PLoS ONE 2016, 11, e0153456. [Google Scholar] [CrossRef] [Green Version]
- Baliga, R.S.; Chaves, A.A.; Jing, L.; Ayers, L.W.; Bauer, J.A. AIDS-related vasculopathy: Evidence for oxidative and inflammatory pathways in murine and human AIDS. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1373–H1380. [Google Scholar] [CrossRef]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- Govender, N.; Naicker, T.; Rajakumar, A.; Moodley, J. Soluble fms-like tyrosine kinase-1 and soluble endoglin in HIV-associated preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 100–105. [Google Scholar] [CrossRef]
- Sansone, M.; Sarno, L.; Saccone, G.; Berghella, V.; Maruotti, G.; Migliucci, A.; Capone, A.; Martinelli, P. Risk of Preeclampsia in Human Immunodeficiency Virus–Infected Pregnant Women. Obstet. Gynecol. 2016, 127, 1027–1032. [Google Scholar] [CrossRef]
- Kalumba, V.; Moodley, J.; Naidoo, T.D. Is the prevalence of pre-eclampsia affected by HIV/AIDS? A retrospective case-control study: Cardiovascular topics. Cardiovasc. J. Afr. 2013, 24, 24–27. [Google Scholar] [CrossRef]
- Padayachee, S.; Govender, N.; Naicker, T. Does HAART dysregulate angiogenesis in HIV infected preeclampsia? Niger. J. Physiol. Sci. 2022, 37, 29–34. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Derakhshan, A.; Männistö, T.; Bliddal, S.; Popova, P.V.; Carty, D.M.; Chen, L.; Taylor, P.; Mosso, L.; Oken, E.; et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: A systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2022, 10, 243–252. [Google Scholar] [CrossRef]
- Levine, R.J.; Vatten, L.J.; Horowitz, G.L.; An, H.; Romundstad, P.R.; Yu, K.F.; Hollenberg, A.N.; Hellevik, A.I.; Asvold, B.O.; Karumanchi, S.A. Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of reduced thyroid function: Nested case-control and population based study. BMJ 2009, 339, b4336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korevaar, T.I.M.; Steegers, E.A.P.; de Rijke, Y.B.; Visser, W.E.; Jaddoe, V.W.V.; Visser, T.J.; Medici, M.; Peeters, R.P. Placental Angiogenic Factors Are Associated With Maternal Thyroid Function and Modify hCG-Mediated FT4 Stimulation. J. Clin. Endocrinol. Metab. 2015, 100, E1328–E1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasi-Hashiani, A.; Omani-Samani, R.; Mohammadi, M.; Amini, P.; Navid, B.; Alizadeh, A.; Morasae, E.K.; Maroufizadeh, S. Assisted reproductive technology and the risk of preeclampsia: An updated systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- von Versen-Höynck, F.; Schaub, A.M.; Chi, Y.-Y.; Chiu, K.-H.; Liu, J.; Lingis, M.; Williams, R.S.; Rhoton-Vlasak, A.; Nichols, W.W.; Fleischmann, R.R.; et al. Increased Preeclampsia Risk and Reduced Aortic Compliance With In Vitro Fertilization Cycles in the Absence of a Corpus Luteum. Hypertension 2019, 73, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Berisha, B. Regulation of corpus luteum function in cattle—An overview. Reprod. Domest. Anim. 2004, 39, 241–251. [Google Scholar] [CrossRef]
- Conrad, K.P.; Baker, V.L.; Marshall, S.A.; Leo, C.H.; Senadheera, S.N.; Girling, J.E.; Tare, M.; Parry, L.J.; Davison, J.M. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R69–R72. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Cantonwine, D.; Little, S.E.; McElrath, T.F.; Parry, S.I.; Lim, K.-H.; Wilkins-Haug, L.E. Angiogenic markers in pregnancies conceived through in vitro fertilization. Am. J. Obstet. Gynecol. 2015, 213, 212.e1–212.e8. [Google Scholar] [CrossRef]
- Biasoni, V.; Patriarca, A.; Dalmasso, P.; Bertagna, A.; Manieri, C.; Benedetto, C.; Revelli, A. Ovarian sensitivity index is strongly related to circulating AMH and may be used to predict ovarian response to exogenous gonadotropins in IVF. Reprod. Biol. Endocrinol. 2011, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Li, H.W.R.; Lee, V.C.Y.; Ho, P.C.; Ng, E. Ovarian sensitivity index is a better measure of ovarian responsiveness to gonadotrophin stimulation than the number of oocytes during in-vitro fertilization treatment. J. Assist. Reprod. Genet. 2014, 31, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Nejabati, H.R.; Mota, A.; Farzadi, L.; Ghojazadeh, M.; Fattahi, A.; Hamdi, K.; Nouri, M. Follicular fluid PlGF/sFlt-1 ratio and soluble receptor for advanced glycation end–products correlate with ovarian sensitivity index in women undergoing A.R.T. J. Endocrinol. Investig. 2017, 40, 207–215. [Google Scholar] [CrossRef]
- Ananth, C.V.; Savitz, D.A.; Luther, E.R. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am. J. Epidemiol. 1996, 144, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.; Irgens, L.M. The effects of smoking and hypertensive disorders on fetal growth. BMC Pregnancy Childbirth 2006, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Owing, J.H. Trends in smoking and Health Research; Nova Biomedical Books: Hauppauge, NY, USA, 2005. [Google Scholar]
- Llurba, E.; Sánchez, O.; Domínguez, C.; Soro, G.; Goya, M.; Alijotas-Reig, J.; Cabero, L. Smoking during Pregnancy: Changes in mid-gestation angiogenic factors in women at risk of developing preeclampsia according to uterine artery Doppler findings. Hypertens. Pregnancy 2013, 32, 50–59. [Google Scholar] [CrossRef]
- Jeyabalan, A.; Powers, R.W.; Durica, A.R.; Harger, G.F.; Roberts, J.M.; Ness, R.B. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am. J. Hypertens. 2008, 21, 943–947. [Google Scholar] [CrossRef] [Green Version]
- Johansson, A.L.; Andersson, T.M.-L.; Hsieh, C.-C.; Jirström, K.; Cnattingius, S.; Fredriksson, I.; Dickman, P.W.; Lambe, M. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int. J. Cancer 2018, 142, 1343–1354. [Google Scholar] [CrossRef] [Green Version]
- Stensheim, H.; Møller, B.; van Dijk, T.; Fosså, S.D. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J. Clin. Oncol. 2009, 27, 45–51. [Google Scholar] [CrossRef]
- Blakely, L.J.; Buzdar, A.U.; Lozada, J.A.; Shullaih, S.A.; Hoy, E.; Smith, T.L.; Hortobagyi, G.N. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer 2004, 100, 465–469. [Google Scholar] [CrossRef]
- Ruan, L.; Zhang, S.; Chen, X.; Liang, W.; Xie, Q. Role of anti-angiogenic factors in the pathogenesis of breast cancer: A review of therapeutic potential. Pathol.-Res. Pract. 2022, 236, 153956. [Google Scholar] [CrossRef]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef]
- Cornish, R.; Staff, A.C.; Boyd, A.; Lawlor, D.A.; Tretli, S.; Bradwin, G.; McElrath, T.F.; Hyer, M.; Hoover, R.N.; Troisi, R. Maternal reproductive hormones and angiogenic factors in pregnancy and subsequent breast cancer risk. Cancer Causes Control 2019, 30, 63–74. [Google Scholar] [CrossRef]
- Innes, K.E.; Byers, T.E. Preeclampsia and Breast Cancer Risk. Epidemiology 1999, 10, 722–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troisi, E.; Attanasio, A.; Matteis, M.; Bragoni, M.; Monaldo, B.C.; Caltagirone, C.; Silvestrini, M. Cerebral hemodynamics in young hypertensive subjects and effects of atenolol treatment. J. Neurol. Sci. 1998, 159, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.I.; Vatten, L.J. Fostervekst, preeklampsi og fremtidig brystkreftrisiko [Fetal growth, pre-eclampsia and adult breast cancer risk]. Tidsskr. Nor. Laegeforen. 2002, 122, 2525–2529. [Google Scholar] [PubMed]
- Omani-Samani, R.; Alizadeh, A.; Almasi-Hashiani, A.; Mohammadi, M.; Maroufizadeh, S.; Navid, B.; Morasae, E.K.; Amini, P. Risk of preeclampsia following assisted reproductive technology: Systematic review and meta-analysis of 72 cohort studies. J. Matern. Neonatal Med. 2020, 33, 2826–2840. [Google Scholar] [CrossRef]
- Falk, R.T.; Staff, A.C.; Bradwin, G.; Karumanchi, S.A.; Troisi, R. A prospective study of angiogenic markers and postmenopausal breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Causes Control 2016, 27, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- PDQ Adult Treatment Editorial Board. Breast Cancer Treatment During Pregnancy (PDQ®): Patient Version. PDQ Cancer Information Summaries, National Cancer Institute (US), 14 December 2022. Available online: https://www.cancer.gov/types/breast/patient/pregnancy-breast-treatment-pdq (accessed on 24 November 2022).
- Saura, C.; Sánchez, O.; Martínez, S.; Domínguez, C.; Dienstmann, R.; Ruíz-Pace, F.; Céspedes, M.C.; Peñuelas, Á.; Cortés, J.; Llurba, E.; et al. Evolution of Angiogenic Factors in Pregnant Patients with Breast Cancer Treated with Chemotherapy. Cancers 2021, 13, 923. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpova, N.S.; Dmitrenko, O.P.; Budykina, T.S. Literature Review: The sFlt1/PlGF Ratio and Pregestational Maternal Comorbidities: New Risk Factors to Predict Pre-Eclampsia. Int. J. Mol. Sci. 2023, 24, 6744. https://doi.org/10.3390/ijms24076744
Karpova NS, Dmitrenko OP, Budykina TS. Literature Review: The sFlt1/PlGF Ratio and Pregestational Maternal Comorbidities: New Risk Factors to Predict Pre-Eclampsia. International Journal of Molecular Sciences. 2023; 24(7):6744. https://doi.org/10.3390/ijms24076744
Chicago/Turabian StyleKarpova, Nataliia Sergeevna, Olga Pavlovna Dmitrenko, and Tatyana Sergeevna Budykina. 2023. "Literature Review: The sFlt1/PlGF Ratio and Pregestational Maternal Comorbidities: New Risk Factors to Predict Pre-Eclampsia" International Journal of Molecular Sciences 24, no. 7: 6744. https://doi.org/10.3390/ijms24076744
APA StyleKarpova, N. S., Dmitrenko, O. P., & Budykina, T. S. (2023). Literature Review: The sFlt1/PlGF Ratio and Pregestational Maternal Comorbidities: New Risk Factors to Predict Pre-Eclampsia. International Journal of Molecular Sciences, 24(7), 6744. https://doi.org/10.3390/ijms24076744




