Obstetric Neuropathy in Diabetic Patients: The “Double Hit Hypothesis”
Abstract
:1. Introduction
2. The Mechanisms of Diabetic Neuropathies Are Complex
2.1. Macrosomic Babies Play a Role in Causing Diabetic Neuropathy and Obstetric Neurological Injury
2.2. Mechanism of Injury for Obstetric Lower Extremity Neuropathies
2.2.1. Vaginal Delivery can Cause Various obstetrical Neurological Syndromes
2.2.2. Obstetric Lesions of Direct Branches of the Lumbosacral Plexus
2.2.3. Obstetric Lesions of Nerves That Supply the Leg and Foot
3. Obstetrical Neuropathies
3.1. Compressive Neuropathies
3.2. Diabetes-Potentiated Compression Injuries
4. How Diabetes Mellitus Produces Nerve Damage That can Be Potentiated by Any Injury Occurring during Delivery
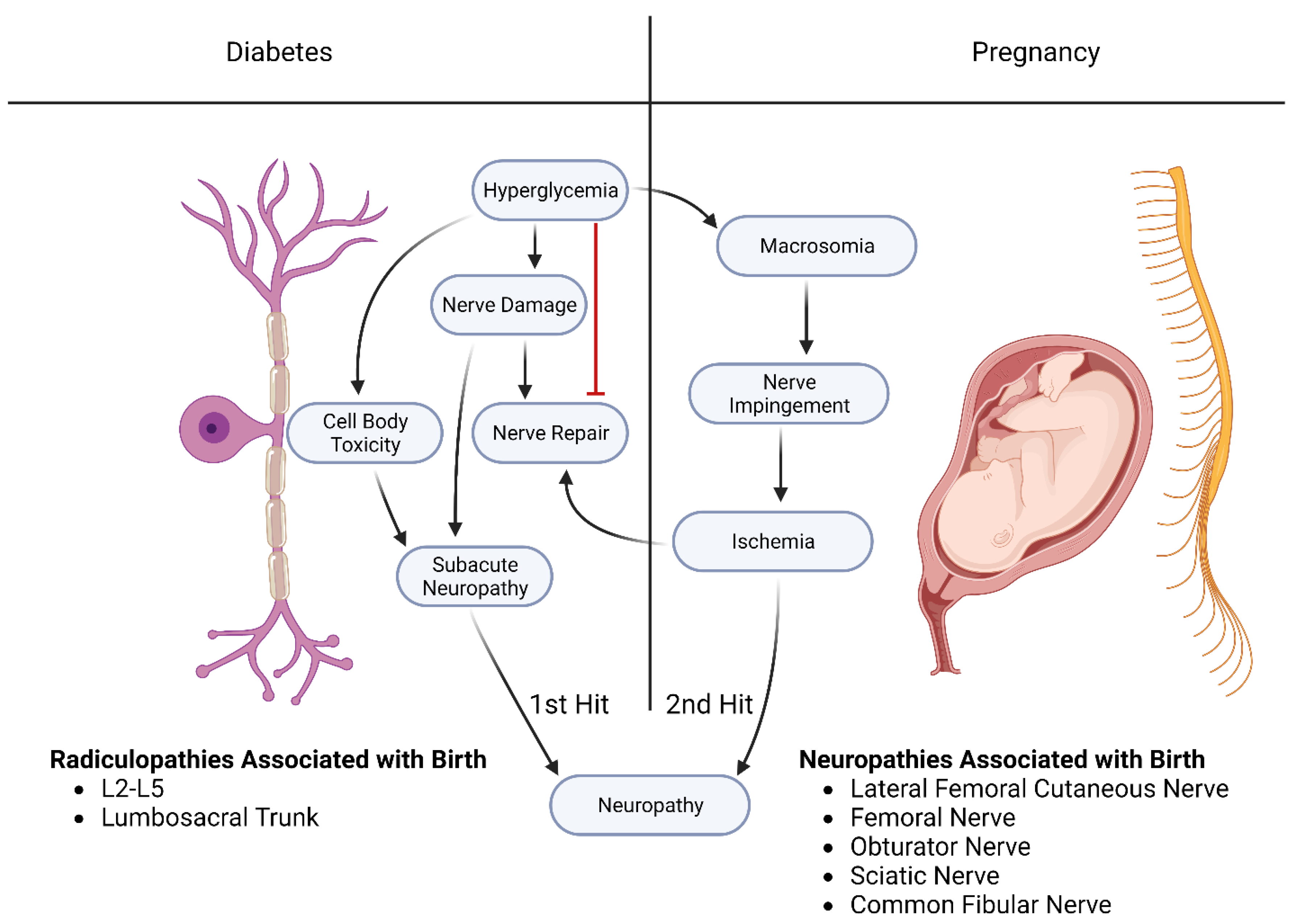
4.1. Hypothesis: Diabetes Mellitus Produces Nerve Damage That can Be Potentiated by Any Injury Occurring during Delivery
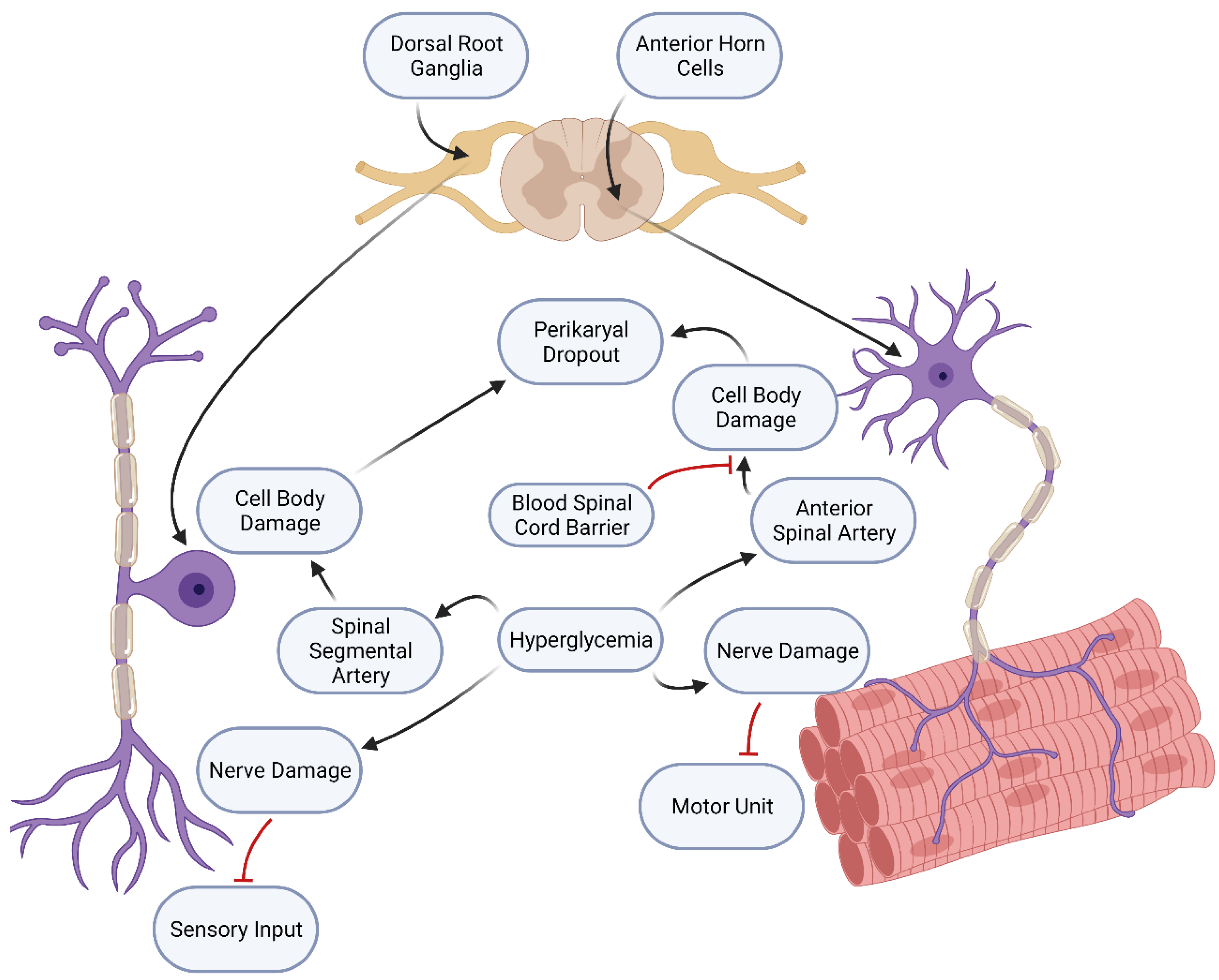
4.1.1. Diabetes Mellitus Produces a More Severe Neuropathy through a Two-Fold Pathological Process
4.1.2. Support for the Two-Hit Hypothesis
4.1.3. A Second Example of a Two-Hit Process Involves the Neuronal Damage Caused by Diabetes
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sempere-Bigorra, M.; Julián-Rochina, I.; Cauli, O. Differences and Similarities in Neuropathy in Type 1 and 2 Diabetes: A Systematic Review. J. Pers. Med. 2021, 11, 230. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes mellitus prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic peripheral neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, E.L.; Callaghan, B.C.; Pop-Bosul, R.P. Diabetic Neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Price, R.S.; Feldman, E. Diagnostic and therapeutic advances: Distal symmetric polyneuropathy. JAMA 2015, 314, 2172–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, E.L.; Nave, K.-A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinik, A.; Mehrabyan, A.; Colen, L.; Boulton, A. Focal Entrapment Neuropathies in Diabetes. Diabetes Care 2004, 27, 1783–1788. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Ito, H.; Sekine, A.; Katano, Y.; Nishimura, T.; Kato, Y.; Takeda, J.; Seishima, M.; Matsuoka, T. Sonographic Evaluation of the Peripheral Nerve in Diabetic Patients: The relationship between nerve conduction studies, echo intensity and cross-sectional area. J. Ultrasound Med. 2010, 29, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Dellon, A.L. Susceptibility of nerve in diabetes to compression: Implications for pain treatment. Plast. Reconstr. Surg. 2014, 134, 142S–150S. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Normahani, P.; Lane, T.; Hohenschurz-Schmidt, D.; Oliver, N.; Davies, A.H. Pathogenesis of Distal Symmetrical Polyneuropathy in Diabetes. Life 2022, 12, 1074. [Google Scholar] [CrossRef]
- Macaré van Maurik, J.F.; Schouten, M.E.; ten Katen, I.; van Hal, M.; Peters, E.J.; Kon, M. Ultrasound findings after surgical compression of the tarsal tunnel in patients with painful diabetic polyneuropathy: A prospective randomized study. Diabetes Care 2014, 37, 3762–3772. [Google Scholar] [CrossRef] [Green Version]
- Thaisetthawatkul, P.; Dyck, P.J.B. Treatment of Diabetic and Nondiabetic Lumbosacral Radiculoplexus Neuropathy. Curr. Treat. Options Neurol. 2010, 12, 95–99. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Ang, L.; Holmes, C.; Gallagher, K.; Feldman, E.L. Inflammation as a therapeutic target for diabetic neuropathies. Curr. Diabetes Rep. 2016, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, V.; Krishnan, A.; Koshy, K.; Martinez, J.A.; Cheng, C.; Almquist, C.; Zochodne, D.W. Regeneration of diabetic axons is enhanced by selective knockdown of the PTEN gene. Brain 2014, 137, 1051–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rask-Madsen, C.; King, G.L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, K.J.; Webber, C.A.; Martinez, J.A.; Singh, B.; Zochodne, D.W. PTEN Inhibition to Facilitate Intrinsic Regenerative Outgrowth of Adult Peripheral Axons. J. Neurosci. 2010, 30, 9306–9315. [Google Scholar] [CrossRef]
- Ramji, N.; Toth, C.; Kennedy, J.; Zochodne, D.W. Does diabetes mellitus target motor neurons? Neurobiol. Dis. 2007, 26, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.M.; Zochodne, D.W. Experimental Diabetic Neuropathy With Spontaneous Recovery: Is there irreparable damage? Diabetes 2005, 54, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.M.; Zochodne, D.W. Impaired peripheral nerve regeneration in diabetes mellitus. J. Peripher. Nerv. Syst. 2005, 10, 144–157. [Google Scholar] [CrossRef]
- Wang, P.H.; Yang, C.-C.; Su, W.-R.; Wu, P.-T.; Cheng, S.-C.; Jou, I.-M. Effects of decompression on behavioral, electrophysiologic, and histomorphologic recovery in a chronic sciatic nerve compression model of streptozotocin-induced diabetic rats. J. Pain Res. 2017, 10, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.M.; Zochodne, D.W. Influence of Experimental Diabetes on the Microcirculation of Injured Peripheral Nerve. Diabetes 2002, 51, 2233–2240. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.J.; Yoon, Y.-S. Emerging therapy for diabetic neuropathy: Cell therapy targeting vessels and nerves. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2010, 2, 18–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Mabie, W.C. Peripheral Neuropathies During Pregnancy. Clin. Obstet. Gynecol. 2005, 48, 57–66. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Contreras, R.; Chen, W.; Sacks, D.A. Trends in the Prevalence of Preexisting Diabetes and Gestational Diabetes Mellitus Among a Racially/Ethnically Diverse Population of Pregnant Women, 1999–2005. Diabetes Care 2008, 31, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Montag, T.W.; Mead, P.B. Postpartum femoral neuropathy. J. Reprod/ Med/ 1981, 26, 563–566. [Google Scholar] [PubMed]
- Peirce, C.; O’Brien, C.; O’Herlihy, C. Postpartum femoral neuropathy following spontaneous vaginal delivery. J. Obstet. Gynaecol. 2010, 30, 203–204. [Google Scholar] [CrossRef]
- Kc, K.; Shakya, S.; Zhang, H. Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 14–20. [Google Scholar] [CrossRef]
- Hemachandra, A.; Ellis, D.; Lloyd, C.E.; Orchard, T.J. The Influence of Pregnancy on IDDM Complications. Diabetes Care 1995, 18, 950–954. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, M.A.; Chang, L.Y.; Salajegheh, M.K. Postpartum Spinal Cord, Root, Plexus and Peripheral Nerve Injuries Involving the Lower Extremities: A practical approach. Anesth Analg. Obstet. Anesthesia Dig. 2015, 120, 141–148. [Google Scholar] [CrossRef]
- Wong, C.A.; Scavone, B.M.; Dugan, S.; Smith, J.C.; Prather, H.; Ganchiff, J.N.; McCarthy, R.J. Incidence of Postpartum Lumbosacral Spine and Lower Extremity Nerve Injuries. Obstet. Gynecol. 2003, 101, 279–288. [Google Scholar] [CrossRef]
- Zakowski, M.I. Obstetric-related Neurological Complications. Int. Anesthesiol. Clin. 2014, 52, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Van Diver, T.; Camann, W. Meralgia paresthetica in the parturient. Int. J. Obstet. Anesthesia 1995, 4, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Grothaus, M.C.; Holt, M.; Mekhail, A.O.; Ebraheim, N.A.; Yeasting, R.A. Lateral femoral cutaneous nerve: An anatomic study. Clin. Orthop. Relat. Res. 2005, 437, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.-L.; Hale, J.; Madhavan, A.; Ringler, M.D.; Toledano, M.; Naddaf, E. Ischiofemoral impingement syndrome provoked by labor: An unusual case of complete sciatic mononeuropathy. Neuroradiol. J. 2022, 36, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Swetenburg, J.; Blackwell, T.A.; Reynolds, Z.; Black, A.C., Jr. Posterior cutaneous neuropathy in piriformis syndrome: A vascular hypothesis. Med. Hypotheses 2020, 144, 109924. [Google Scholar] [CrossRef]
- Bunch, K.; Hope, E. An Uncommon Case of Bilateral Peroneal Nerve Palsy following Delivery: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2014, 2014, 746480. [Google Scholar] [CrossRef] [Green Version]
- Brannigan, T.H. Neuropathic pain. In Merritt’s Neurology, 12th ed; Rowland, L.P., Pedley, T.A., Eds.; Lippincott, Williams, and Wilkins: Philadelphia, Pennsylvania, 2010; p. 840. [Google Scholar]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Kim, B.R.; Ha, D.-H.; Kim, J.K.; Kim, Y.H. Comparison of MR findings of acute traumatic peripheral nerve injury and acute compressive neuropathy in a rat model. PLoS ONE 2020, 15, e0240911. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Belfiore, L.; Chu, T.-H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2021, 13. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. Elife 2021, 10, e62232. [Google Scholar] [CrossRef] [PubMed]
- Molinari, W.J.; Elfar, J.C. The double crush syndrome. J. Hand Surg. 2013, 38, 799–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.M.; Zochodne, D.W. The regenerative deficit of peripheral nerves in experimental diabetes: Its extent, timing and possible mechanisms. Brain 2000, 123, 2118–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zochodne, D.W. Diabetes and failure of axon regeneration in peripheral neurons. Expert Rev. Endocrinol. Metab. 2010, 5, 7–14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.T.; Zaferanieh, M.H.; Black, A.C., Jr.; Hamedi, K.R.; Goodwin, R.L.; Nathaniel, T.I. Obstetric Neuropathy in Diabetic Patients: The “Double Hit Hypothesis”. Int. J. Mol. Sci. 2023, 24, 6812. https://doi.org/10.3390/ijms24076812
Nguyen DT, Zaferanieh MH, Black AC Jr., Hamedi KR, Goodwin RL, Nathaniel TI. Obstetric Neuropathy in Diabetic Patients: The “Double Hit Hypothesis”. International Journal of Molecular Sciences. 2023; 24(7):6812. https://doi.org/10.3390/ijms24076812
Chicago/Turabian StyleNguyen, Dieu Thao, Mohammad Hooshmand Zaferanieh, Asa C. Black, Jr., Kamron Reza Hamedi, Richard L. Goodwin, and Thomas I. Nathaniel. 2023. "Obstetric Neuropathy in Diabetic Patients: The “Double Hit Hypothesis”" International Journal of Molecular Sciences 24, no. 7: 6812. https://doi.org/10.3390/ijms24076812
APA StyleNguyen, D. T., Zaferanieh, M. H., Black, A. C., Jr., Hamedi, K. R., Goodwin, R. L., & Nathaniel, T. I. (2023). Obstetric Neuropathy in Diabetic Patients: The “Double Hit Hypothesis”. International Journal of Molecular Sciences, 24(7), 6812. https://doi.org/10.3390/ijms24076812






