Metalloporphyrins as Tools for Deciphering the Role of Heme Oxygenase in Renal Immune Injury
Abstract
1. Introduction
2. Effector Mechanisms in Renal Immune Injury
2.1. Complement Activation
2.2. Leukocyte Recruitment and Infiltration
3. Effect of Mps on Effector Mechanisms of Renal Immune Injury
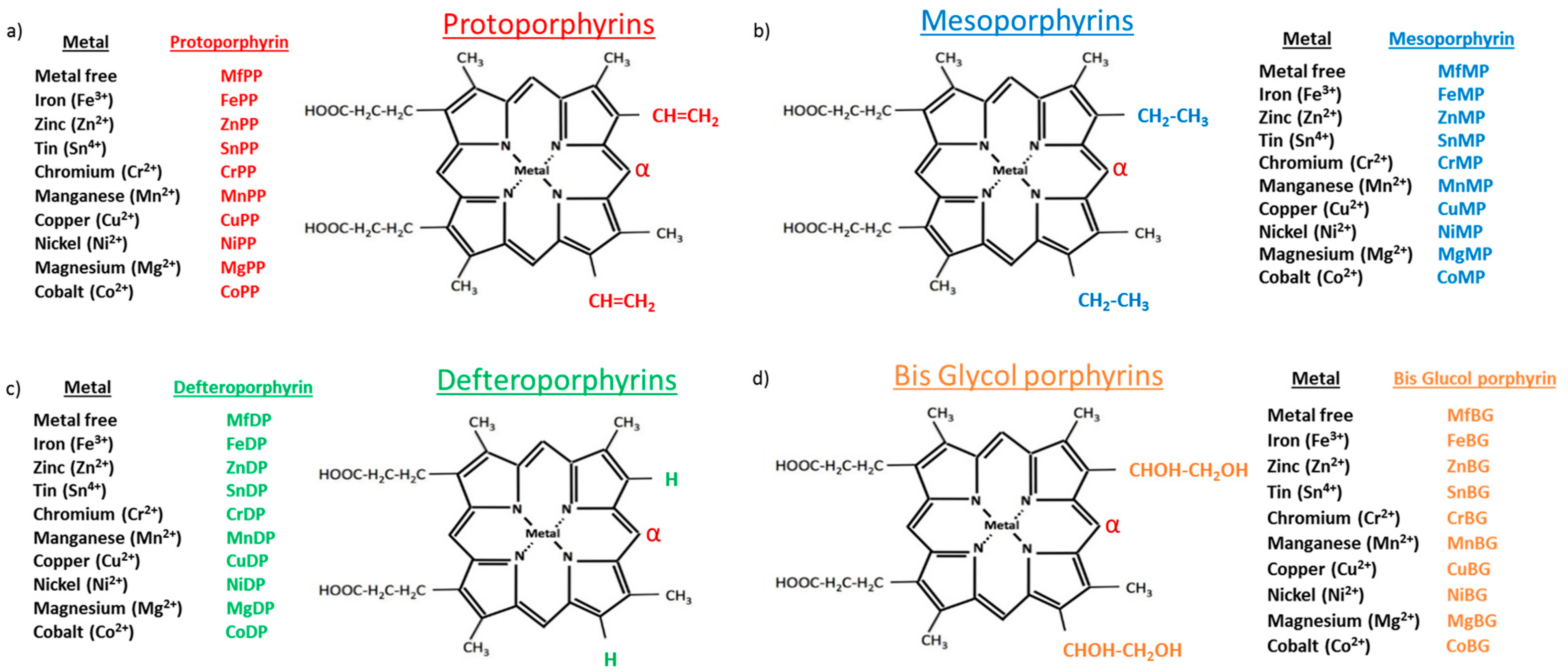
3.1. Mps, HO Activity and HO Isoform Expression
3.2. Role of Mps in Complement Mediated Renal Immune Injury
3.3. Role of Mps in Leukocyte Mediated Renal Immune Injury
4. Use of Mps as Disease Modifying Agents in Renal Immune Injury
5. Mps Toxicity Considerations
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adler, S.; Couser, W. Immunologic mechanisms of renal disease. Am. J. Med. Sci. 1985, 289, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Isome, M.; Fujinaka, H.; Adhikary, L.P.; Kovalenko, P.; El-Shemi, A.G.; Yoshida, Y.; Yaoita, E.; Takeishi, T.; Takeya, M.; Naito, M.; et al. Important role for macrophages in induction of crescentic anti-GBM glomerulonephritis in WKY rats. Nephrol. Dial. Transplant. 2004, 19, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.R.; Tipping, P.G.; Apostolopoulos, J.; Oettinger, C.; D’Souza, M.; Milton, G.; Holdsworth, S.R. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin. Exp. Immunol. 1997, 109, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Main, I.W.; Nikolic-Paterson, D.J.; Atkins, R.C. T cells and macrophages and their role in renal injury. Semin. Nephrol. 1992, 12, 395–407. [Google Scholar]
- Bomback, A.S.; Markowitz, G.S.; Appel, G.B. Complement-Mediated Glomerular Diseases: A Tale of 3 Pathways. Kidney Int. Rep. 2016, 1, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fearn, A.; Sheerin, N.S. Complement activation in progressive renal disease. World J. Nephrol. 2015, 4, 31–40. [Google Scholar] [CrossRef]
- Bomback, A.S. Anti-complement therapy for glomerular diseases. Adv. Chronic Kidney Dis. 2014, 21, 152–158. [Google Scholar] [CrossRef]
- Tipping, P.G.; Kitching, A.R. Glomerulonephritis, Th1 and Th2: What’s new? Clin. Exp. Immunol. 2005, 142, 207–215. [Google Scholar] [CrossRef]
- Ramani, K.; Biswas, P.S. Emerging roles of the Th17/IL-17-axis in glomerulonephritis. Cytokine 2016, 77, 238–244. [Google Scholar] [CrossRef]
- Lema, G.P.; Maier, H.; Nieto, E.; Vielhauer, V.; Luckow, B.; Mampaso, F.; Schlondorff, D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 2001, 12, 1369–1382. [Google Scholar] [CrossRef]
- Urushihara, M.; Kagami, S.; Kuhara, T.; Tamaki, T.; Kuroda, Y. Glomerular distribution and gelatinolytic activity of matrix metalloproteinases in human glomerulonephritis. Nephrol. Dial. Transplant. 2002, 17, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Stewart, K.; Rees, A.J. Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am. J. Pathol. 2000, 156, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Cattell, V. Macrophages in acute glomerular inflammation. Kidney Int. 1994, 45, 945–952. [Google Scholar] [CrossRef]
- Cook, H.T.; Smith, J.; Salmon, J.A.; Cattell, V. Functional characteristics of macrophages in glomerulonephritis in the rat. O2- generation, MHC class II expression, and eicosanoid synthesis. Am. J. Pathol. 1989, 134, 431–437. [Google Scholar] [PubMed]
- Herrnstadt, G.R.; Steinmetz, O.M. The role of Treg subtypes in glomerulonephritis. Cell Tissue Res. 2021, 385, 293–304. [Google Scholar] [CrossRef]
- McKinney, E.F.; Lyons, P.A.; Carr, E.J.; Hollis, J.L.; Jayne, D.R.; Willcocks, L.C.; Koukoulaki, M.; Brazma, A.; Jovanovic, V.; Kemeny, D.M.; et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat. Med. 2010, 16, 586–591, 581p following 591. [Google Scholar] [CrossRef]
- Couzi, L.; Merville, P.; Deminiere, C.; Moreau, J.F.; Combe, C.; Pellegrin, J.L.; Viallard, J.F.; Blanco, P. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 2007, 56, 2362–2370. [Google Scholar] [CrossRef]
- Chen, A.; Lee, K.; Guan, T.; He, J.C.; Schlondorff, D. Role of CD8+ T cells in crescentic glomerulonephritis. Nephrol. Dial. Transplant. 2020, 35, 564–572. [Google Scholar] [CrossRef]
- Fujinaka, H.; Yamamoto, T.; Feng, L.; Nameta, M.; Garcia, G.; Chen, S.; El-shemi, A.A.; Ohshiro, K.; Katsuyama, K.; Yoshida, Y.; et al. Anti-perforin antibody treatment ameliorates experimental crescentic glomerulonephritis in WKY rats. Kidney Int. 2007, 72, 823–830. [Google Scholar] [CrossRef]
- Scheffschick, A.; Fuchs, S.; Malmstrom, V.; Gunnarsson, I.; Brauner, H. Kidney infiltrating NK cells and NK-like T-cells in lupus nephritis: Presence, localization, and the effect of immunosuppressive treatment. Clin. Exp. Immunol. 2022, 207, 199–204. [Google Scholar] [CrossRef]
- Rickassel, C.; Gnirck, A.C.; Shaikh, N.; Adamiak, V.; Waterholter, A.; Tanriver, Y.; Neumann, K.; Huber, T.B.; Gasteiger, G.; Panzer, U.; et al. Conventional NK Cells and Type 1 Innate Lymphoid Cells Do Not Influence Pathogenesis of Experimental Glomerulonephritis. J. Immunol. 2022, 208, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Vreman, H.J.; Wong, R.J.; Stevenson, D.K. Alternative metalloporphyrins for the treatment of neonatal jaundice. J. Perinatol. 2001, 21 (Suppl. S1), S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. The heme oxygenase system: Update 2005. Antioxid. Redox Signal. 2005, 7, 1761–1766. [Google Scholar] [CrossRef]
- Bianchetti, C.M.; Yi, L.; Ragsdale, S.W.; Phillips, G.N., Jr. Comparison of apo- and heme-bound crystal structures of a truncated human heme oxygenase-2. J. Biol. Chem. 2007, 282, 37624–37631. [Google Scholar] [CrossRef]
- Wong, R.J.; Vreman, H.J.; Schulz, S.; Kalish, F.S.; Pierce, N.W.; Stevenson, D.K. In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J. Perinatol. 2011, 31 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef]
- Yang, G.; Nguyen, X.; Ou, J.; Rekulapelli, P.; Stevenson, D.K.; Dennery, P.A. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood 2001, 97, 1306–1313. [Google Scholar] [CrossRef]
- Argyris, E.G.; Vanderkooi, J.M.; Venkateswaran, P.S.; Kay, B.K.; Paterson, Y. The connection domain is implicated in metalloporphyrin binding and inhibition of HIV reverse transcriptase. J. Biol. Chem. 1999, 274, 1549–1556. [Google Scholar] [CrossRef]
- La, P.; Fernando, A.P.; Wang, Z.; Salahudeen, A.; Yang, G.; Lin, Q.; Wright, C.J.; Dennery, P.A. Zinc protoporphyrin regulates cyclin D1 expression independent of heme oxygenase inhibition. J. Biol. Chem. 2009, 284, 36302–36311. [Google Scholar] [CrossRef]
- Wijnsma, K.L.; Veissi, S.T.; de Wijs, S.; van der Velden, T.; Volokhina, E.B.; Wagener, F.; van de Kar, N.; van den Heuvel, L.P. Heme as Possible Contributing Factor in the Evolvement of Shiga-Toxin Escherichia coli Induced Hemolytic-Uremic Syndrome. Front. Immunol. 2020, 11, 547406. [Google Scholar] [CrossRef]
- Muller-Eberhard, U.; Javid, J.; Liem, H.H.; Hanstein, A.; Hanna, M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 1968, 32, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Frimat, M.; Tabarin, F.; Dimitrov, J.D.; Poitou, C.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 2013, 122, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Gerogianni, A.; Dimitrov, J.D.; Zarantonello, A.; Poillerat, V.; Chonat, S.; Sandholm, K.; McAdam, K.E.; Ekdahl, K.N.; Mollnes, T.E.; Mohlin, C.; et al. Heme Interferes With Complement Factor I-Dependent Regulation by Enhancing Alternative Pathway Activation. Front. Immunol. 2022, 13, 901876. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Spiller, O.B.; St John, P.L.; Haas, M.; Hack, B.K.; Ren, G.; Cunningham, P.N.; Doshi, M.; Abrahamson, D.R.; Morgan, B.P.; et al. Decay-accelerating factor expression in the rat kidney is restricted to the apical surface of podocytes. Kidney Int. 2002, 62, 2010–2021. [Google Scholar] [CrossRef]
- Duann, P.; Lin, P.H.; Lianos, E.A. Data on characterization of metalloporphyrin-mediated HO-1 and DAF induction in rat glomeruli and podocytes. Data Brief 2019, 22, 279–285. [Google Scholar] [CrossRef]
- Detsika, M.G.; Duann, P.; Atsaves, V.; Papalois, A.; Lianos, E.A. Heme Oxygenase 1 Up-Regulates Glomerular Decay Accelerating Factor Expression and Minimizes Complement Deposition and Injury. Am. J. Pathol. 2016, 186, 2833–2845. [Google Scholar] [CrossRef]
- Shan, Y.; Lambrecht, R.W.; Donohue, S.E.; Bonkovsky, H.L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006, 20, 2651–2653. [Google Scholar] [CrossRef]
- Sardana, M.K.; Kappas, A. Dual control mechanism for heme oxygenase: Tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc. Natl. Acad. Sci. USA 1987, 84, 2464–2468. [Google Scholar] [CrossRef]
- Abate, A.; Zhao, H.; Wong, R.J.; Stevenson, D.K. The role of Bach1 in the induction of heme oxygenase by tin mesoporphyrin. Biochem. Biophys. Res. Commun. 2007, 354, 757–763. [Google Scholar] [CrossRef]
- Olonisakin, T.F.; Suber, T.; Gonzalez-Ferrer, S.; Xiong, Z.; Penaloza, H.F.; van der Geest, R.; Xiong, Y.; Osei-Hwedieh, D.O.; Tejero, J.; Rosengart, M.R.; et al. Stressed erythrophagocytosis induces immunosuppression during sepsis through heme-mediated STAT1 dysregulation. J. Clin. Investig. 2021, 131, e137468. [Google Scholar] [CrossRef]
- Stenzel, K.H.; Rubin, A.L.; Novogrodsky, A. Mitogenic and co-mitogenic properties of hemin. J. Immunol. 1981, 127, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Novogrodsky, A.; Suthanthiran, M.; Stenzel, K.H. Immune stimulatory properties of metalloporphyrins. J. Immunol. 1989, 143, 3981–3987. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Oh, G.S.; Choi, B.M.; Chae, S.C.; Kim, Y.M.; Chung, K.R.; Chung, H.T. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol. 2004, 172, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, S.; Zhai, Y.; Bo, Q.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: Inhibition of type-1 interferon signaling. Transplantation 2007, 83, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Choi, A.M.; Li, C. Carbon monoxide inhibits IL-17-induced IL-6 production through the MAPK pathway in human pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L268–L273. [Google Scholar] [CrossRef]
- Pae, H.O.; Oh, G.S.; Choi, B.M.; Chae, S.C.; Chung, H.T. Differential expressions of heme oxygenase-1 gene in CD25- and CD25+ subsets of human CD4+ T cells. Biochem. Biophys. Res. Commun. 2003, 306, 701–705. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Choi, B.M.; Pae, H.O.; Jeong, Y.R.; Kim, Y.M.; Chung, H.T. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem. Biophys. Res. Commun. 2005, 327, 1066–1071. [Google Scholar] [CrossRef]
- Xia, Z.W.; Xu, L.Q.; Zhong, W.W.; Wei, J.J.; Li, N.L.; Shao, J.; Li, Y.Z.; Yu, S.C.; Zhang, Z.L. Heme oxygenase-1 attenuates ovalbumin-induced airway inflammation by up-regulation of foxp3 T-regulatory cells, interleukin-10, and membrane-bound transforming growth factor- 1. Am. J. Pathol. 2007, 171, 1904–1914. [Google Scholar] [CrossRef]
- Wu, C.C.; Lu, K.C.; Chen, J.S.; Hsieh, H.Y.; Lin, S.H.; Chu, P.; Wang, J.Y.; Sytwu, H.K.; Lin, Y.F. HO-1 induction ameliorates experimental murine membranous nephropathy: Anti-oxidative, anti-apoptotic and immunomodulatory effects. Nephrol. Dial. Transplant. 2008, 23, 3082–3090. [Google Scholar] [CrossRef]
- Quiza, C.G.; Leenaerts, P.L.; Hall, B.M. The role of T cells in the mediation of glomerular injury in Heymann’s nephritis in the rat. Int. Immunol. 1992, 4, 423–432. [Google Scholar] [CrossRef]
- Penny, M.J.; Boyd, R.A.; Hall, B.M. Permanent CD8(+) T cell depletion prevents proteinuria in active Heymann nephritis. J. Exp. Med. 1998, 188, 1775–1784. [Google Scholar] [CrossRef]
- Chora, A.A.; Fontoura, P.; Cunha, A.; Pais, T.F.; Cardoso, S.; Ho, P.P.; Lee, L.Y.; Sobel, R.A.; Steinman, L.; Soares, M.P. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Investig. 2007, 117, 438–447. [Google Scholar] [CrossRef]
- Cheng, C.; Noorderloos, M.; van Deel, E.D.; Tempel, D.; den Dekker, W.; Wagtmans, K.; Duncker, D.J.; Soares, M.P.; Laman, J.D.; Duckers, H.J. Dendritic cell function in transplantation arteriosclerosis is regulated by heme oxygenase 1. Circ. Res. 2010, 106, 1656–1666. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Hu, X.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhu, P.; Li, X.K. 5-aminolevulinic acid combined with sodium ferrous citrate ameliorated lupus nephritis in a mouse chronic graft-versus-host disease model. Int. Immunopharmacol. 2021, 96, 107626. [Google Scholar] [CrossRef]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef]
- Liu, S.C.; Zhai, S.; Palek, J. Detection of hemin release during hemoglobin S denaturation. Blood 1988, 71, 1755–1758. [Google Scholar] [CrossRef]
- Mosley, K.; Wembridge, D.E.; Cattell, V.; Cook, H.T. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int. 1998, 53, 672–678. [Google Scholar] [CrossRef]
- van Swelm, R.P.L.; Beurskens, S.; Dijkman, H.; Wiegerinck, E.T.G.; Roelofs, R.; Thevenod, F.; van der Vlag, J.; Wetzels, J.F.M.; Swinkels, D.W.; Smeets, B. Kidney tubule iron loading in experimental focal segmental glomerulosclerosis. Sci. Rep. 2022, 12, 1199. [Google Scholar] [CrossRef]
- Yokoyama, T.; Shimizu, M.; Ohta, K.; Yuno, T.; Okajima, M.; Wada, T.; Toma, T.; Koizumi, S.; Yachie, A. Urinary heme oxygenase-1 as a sensitive indicator of tubulointerstitial inflammatory damage in various renal diseases. Am. J. Nephrol. 2011, 33, 414–420. [Google Scholar] [CrossRef]
- Morgan, W.T.; Liem, H.H.; Sutor, R.P.; Muller-Ebergard, U. Transfer of heme from heme-albumin to hemopexin. Biochim. Biophys. Acta 1976, 444, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kino, K.; Mizumoto, K.; Watanabe, J.; Tsunoo, H. Immunohistochemical studies on hemoglobin-haptoglobin and hemoglobin catabolism sites. J. Histochem. Cytochem. 1987, 35, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Estep, R.; Robinson, B.; Wong, S.W. Nonhuman primate models of human immunology. Antioxid. Redox Signal. 2011, 14, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Hvidberg, V.; Maniecki, M.B.; Jacobsen, C.; Hojrup, P.; Moller, H.J.; Moestrup, S.K. Identification of the receptor scavenging hemopexin-heme complexes. Blood 2005, 106, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef]
- Du, Q.; Tsuboi, N.; Shi, Y.; Ito, S.; Sugiyama, Y.; Furuhashi, K.; Endo, N.; Kim, H.; Katsuno, T.; Akiyama, S.; et al. Transfusion of CD206(+) M2 Macrophages Ameliorates Antibody-Mediated Glomerulonephritis in Mice. Am. J. Pathol. 2016, 186, 3176–3188. [Google Scholar] [CrossRef]
- Sheng, H.; Chaparro, R.E.; Sasaki, T.; Izutsu, M.; Pearlstein, R.D.; Tovmasyan, A.; Warner, D.S. Metalloporphyrins as therapeutic catalytic oxidoreductants in central nervous system disorders. Antioxid. Redox Signal. 2014, 20, 2437–2464. [Google Scholar] [CrossRef]
- Paez-Ribes, M.; Gonzalez-Gualda, E.; Doherty, G.J.; Munoz-Espin, D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 2019, 11, e10234. [Google Scholar] [CrossRef]
- Bissell, D.M.; Anderson, K.E.; Bonkovsky, H.L. Porphyria. N. Engl. J. Med. 2017, 377, 862–872. [Google Scholar] [CrossRef]
- Wilson, H.M.; Welikson, R.E.; Luo, J.; Kean, T.J.; Cao, B.; Dennis, J.E.; Allen, M.D. Can Cytoprotective Cobalt Protoporphyrin Protect Skeletal Muscle and Muscle-derived Stem Cells From Ischemic Injury? Clin. Orthop. Relat. Res. 2015, 473, 2908–2919. [Google Scholar] [CrossRef][Green Version]
- Schulz, S.; Wong, R.J.; Vreman, H.J.; Stevenson, D.K. Metalloporphyrins—An update. Front. Pharmacol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B Biol. 1992, 14, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Kappas, A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics 2004, 113, 119–123. [Google Scholar] [CrossRef]
- Berglund, L.; Angelin, B.; Hultcrantz, R.; Einarsson, K.; Emtestam, L.; Drummond, G.; Kappas, A. Studies with the haeme oxygenase inhibitor Sn-protoporphyrin in patients with primary biliary cirrhosis and idiopathic haemochromatosis. Gut 1990, 31, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, R.A.; Kappas, A. Pharmacokinetics of tin-mesoporphyrin in man and the effects of tin-chelated porphyrins on hyperexcretion of heme pathway precursors in patients with acute inducible porphyria. Hepatology 1989, 9, 882–888. [Google Scholar] [CrossRef]
- Datta, P.K.; Dhupar, S.; Lianos, E.A. Regulatory effects of inducible nitric oxide synthase on cyclooxygenase-2 and heme oxygenase-1 expression in experimental glomerulonephritis. Nephrol. Dial. Transplant. 2006, 21, 51–57. [Google Scholar] [CrossRef][Green Version]
- Datta, P.K.; Duann, P.; Lianos, E.A. Long-term effect of heme oxygenase (HO)-1 induction in glomerular immune injury. J. Lab. Clin. Med. 2006, 147, 150–155. [Google Scholar] [CrossRef]
- Dercho, R.A.; Nakatsu, K.; Wong, R.J.; Stevenson, D.K.; Vreman, H.J. Determination of in vivo carbon monoxide production in laboratory animals via exhaled air. J. Pharmacol. Toxicol. Methods 2006, 54, 288–295. [Google Scholar] [CrossRef]
- Mackern-Oberti, J.P.; Obreque, J.; Mendez, G.P.; Llanos, C.; Kalergis, A.M. Carbon monoxide inhibits T cell activation in target organs during systemic lupus erythematosus. Clin. Exp. Immunol. 2015, 182, 1–13. [Google Scholar] [CrossRef]
- Nakahira, K.; Kim, H.P.; Geng, X.H.; Nakao, A.; Wang, X.; Murase, N.; Drain, P.F.; Wang, X.; Sasidhar, M.; Nabel, E.G.; et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 2006, 203, 2377–2389. [Google Scholar] [CrossRef]
- Volin, L.; Rasi, V.; Vahtera, E.; Tenhunen, R. Heme arginate: Effects on hemostasis. Blood 1988, 71, 625–628. [Google Scholar] [CrossRef]
- Datta, P.K.; Koukouritaki, S.B.; Hopp, K.A.; Lianos, E.A. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J. Am. Soc. Nephrol. 1999, 10, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.S.; Kim, R.I.; Kim, C. Carbon Monoxide Regulates Macrophage Differentiation and Polarization toward the M2 Phenotype through Upregulation of Heme Oxygenase 1. Cells 2021, 10, 3444. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, Y.; Fan, C.; Feng, C.; Wang, H.; Bai, F.; Zuo, J.; Tang, W. Heme supplementation ameliorates lupus nephritis through rectifying the disorder of splenocytes and alleviating renal inflammation and oxidative damage. Int. Immunopharmacol. 2021, 94, 107482. [Google Scholar] [CrossRef]
- Takeda, Y.; Takeno, M.; Iwasaki, M.; Kobayashi, H.; Kirino, Y.; Ueda, A.; Nagahama, K.; Aoki, I.; Ishigatsubo, Y. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin. Exp. Immunol. 2004, 138, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Pogu, J.; Remy, S.; Brau, F.; Pogu, S.; Maquigneau, M.; Fonteneau, J.F.; Poirier, N.; Vanhove, B.; Blancho, G.; et al. Inhibition of effector antigen-specific T cells by intradermal administration of heme oxygenase-1 inducers. J. Autoimmun. 2017, 81, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Eremina, V.; Wong, M.A.; Cui, S.; Schwartz, L.; Quaggin, S.E. Glomerular-specific gene excision in vivo. J. Am. Soc. Nephrol. 2002, 13, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Duann, P.; Lianos, E.A. GEC-targeted HO-1 expression reduces proteinuria in glomerular immune injury. Am. J. Physiol. Ren. Physiol. 2009, 297, F629–F638. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Makri, P.; Detsika, M.G.; Tsirogianni, A.; Lianos, E.A. Glomerular Epithelial Cells-Targeted Heme Oxygenase-1 Over Expression in the Rat: Attenuation of Proteinuria in Secondary But Not Primary Injury. Nephron 2016, 133, 270–278. [Google Scholar] [CrossRef]
- Pimstone, N.R.; Engel, P.; Tenhunen, R.; Seitz, P.T.; Marver, H.S.; Schmid, R. Inducible heme oxygenase in the kidney: A model for the homeostatic control of hemoglobin catabolism. J. Clin. Investig. 1971, 50, 2042–2050. [Google Scholar] [CrossRef]
- Shepard, M.; Dhulipala, P.; Kabaria, S.; Abraham, N.G.; Lianos, E.A. Heme oxygenase-1 localization in the rat nephron. Nephron 2002, 92, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Cuitino, L.; Obreque, J.; Gajardo-Meneses, P.; Villarroel, A.; Crisostomo, N.; San Francisco, I.F.; Valenzuela, R.A.; Mendez, G.P.; Llanos, C. Heme-Oxygenase-1 Is Decreased in Circulating Monocytes and Is Associated With Impaired Phagocytosis and ROS Production in Lupus Nephritis. Front. Immunol. 2019, 10, 2868. [Google Scholar] [CrossRef]
- Poulaki, E.; Detsika, M.G.; Fourtziala, E.; Lianos, E.A.; Gakiopoulou, H. Podocyte-targeted Heme Oxygenase (HO)-1 overexpression exacerbates age-related pathology in the rat kidney. Sci. Rep. 2020, 10, 5719. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M.; Stewart, K.N.; Brown, P.A.; Anegon, I.; Chettibi, S.; Rees, A.J.; Kluth, D.C. Bone-marrow-derived macrophages genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol. Ther. 2002, 6, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Weis, N.; Weigert, A.; von Knethen, A.; Brune, B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol. Biol. Cell 2009, 20, 1280–1288. [Google Scholar] [CrossRef]
- Kishimoto, D.; Kirino, Y.; Tamura, M.; Takeno, M.; Kunishita, Y.; Takase-Minegishi, K.; Nakano, H.; Kato, I.; Nagahama, K.; Yoshimi, R.; et al. Dysregulated heme oxygenase-1(low) M2-like macrophages augment lupus nephritis via Bach1 induced by type I interferons. Arthritis Res. Ther. 2018, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Canonne-Hergaux, F. Erythrophagocytosis and recycling of heme iron in normal and pathological conditions; regulation by hepcidin. Transfus. Clin. Biol. 2005, 12, 123–130. [Google Scholar] [CrossRef]
- Kappas, A.; Drummond, G.S.; Henschke, C.; Valaes, T. Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics 1995, 95, 468–474. [Google Scholar] [CrossRef]
- Kappas, A.; Drummond, G.S.; Galbraith, R.A. Prolonged clinical use of a heme oxygenase inhibitor: Hematological evidence for an inducible but reversible iron-deficiency state. Pediatrics 1993, 91, 537–539. [Google Scholar] [CrossRef]
- Duckham, J.M.; Lee, H.A. The treatment of refractory anaemia of chronic renal failure with cobalt chloride. Q. J. Med. 1976, 45, 277–294. [Google Scholar]
- Alexander, C.S. Cobalt-beer cardiomyopathy. A clinical and pathologic study of twenty-eight cases. Am. J. Med. 1972, 53, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, U.O. Case of cobalt poisoning. Br. Med. J. 1967, 1, 544–545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keegan, G.M.; Learmonth, I.D.; Case, C.P. Orthopaedic metals and their potential toxicity in the arthroplasty patient: A review of current knowledge and future strategies. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 567–573. [Google Scholar] [CrossRef]
- Rosenberg, D.W. Pharmacokinetics of cobalt chloride and cobalt-protoporphyrin. Drug Metab. Dispos. 1993, 21, 846–849. [Google Scholar] [PubMed]
- Sandberg, S.; Romslo, I. Porphyrin-induced photodamage at the cellular and the subcellular level as related to the solubility of the porphyrin. Clin. Chim. Acta Int. J. Clin. Chem. 1981, 109, 193–201. [Google Scholar] [CrossRef]

| Study | Summary of Findings | Reference Number |
|---|---|---|
| Mosley K. et.al., 1998 | Hemin treatment in a rat nephrotoxic nephritis model ameliorates disease via induction of HO-1 and reduction in glomerular macrophage infiltration | [58] |
| Data P.K et.al., 1999 | Hemin treatment in a rat model of anti-glomerular basement membrane antibody-mediated glomerulonephritis reduces proteinuria via HO-1 induction and attenuation of iNOs expression. | [82] |
| Datta P.K. et.al., 2006 | Long term hemin treatment attenuates proteinuria in a rat model of anti-glomerular basement membrane antibody-mediated glomerulonephritis. | [77] |
| Kang I.-S. et.al., 2021 | Hemin treatment increased macrophage differentiation whereas ZnPP reduced macrophage differentiation in CORM mediated macrophage differentiation. | [83] |
| Wu B. et.al., 2021 | Hemin treatment ameliorated lupus disease in lupus prone mice. | [84] |
| Takeda Y. et.al., 2004 | Hemin treatment suppressed lupus nephritis via HO-1 induction and reduction in iNOs synthesis in lupus mice. | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lianos, E.A.; Detsika, M.G. Metalloporphyrins as Tools for Deciphering the Role of Heme Oxygenase in Renal Immune Injury. Int. J. Mol. Sci. 2023, 24, 6815. https://doi.org/10.3390/ijms24076815
Lianos EA, Detsika MG. Metalloporphyrins as Tools for Deciphering the Role of Heme Oxygenase in Renal Immune Injury. International Journal of Molecular Sciences. 2023; 24(7):6815. https://doi.org/10.3390/ijms24076815
Chicago/Turabian StyleLianos, Elias A., and Maria G. Detsika. 2023. "Metalloporphyrins as Tools for Deciphering the Role of Heme Oxygenase in Renal Immune Injury" International Journal of Molecular Sciences 24, no. 7: 6815. https://doi.org/10.3390/ijms24076815
APA StyleLianos, E. A., & Detsika, M. G. (2023). Metalloporphyrins as Tools for Deciphering the Role of Heme Oxygenase in Renal Immune Injury. International Journal of Molecular Sciences, 24(7), 6815. https://doi.org/10.3390/ijms24076815




