Diagnostic Aqueous Humor Proteome Predicts Metastatic Potential in Uveal Melanoma
Abstract
:1. Introduction
2. Results
2.1. Patient Clinical Characteristics and Demographics
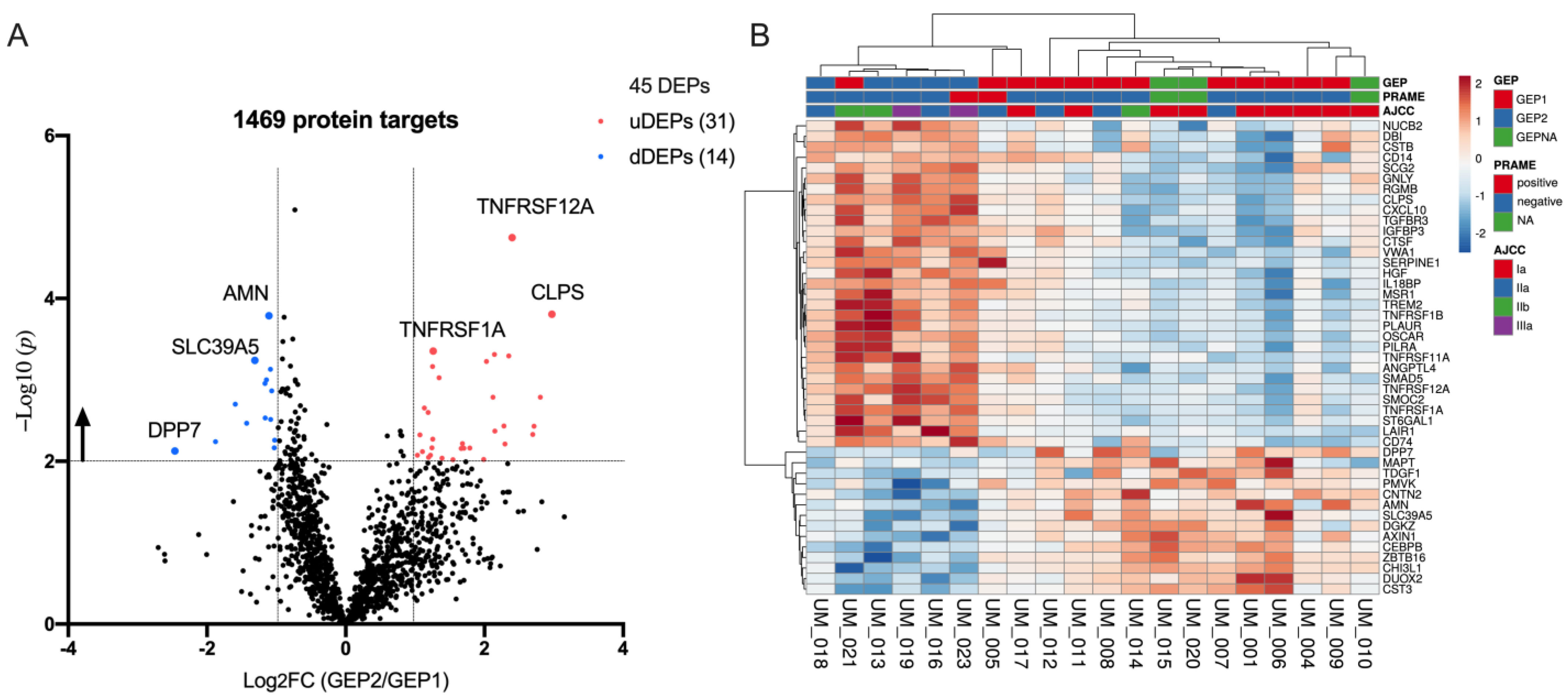
2.2. Target Protein Data Distribution
2.3. Differentially Expression Proteins between GEP2 and GEP1 AH Samples
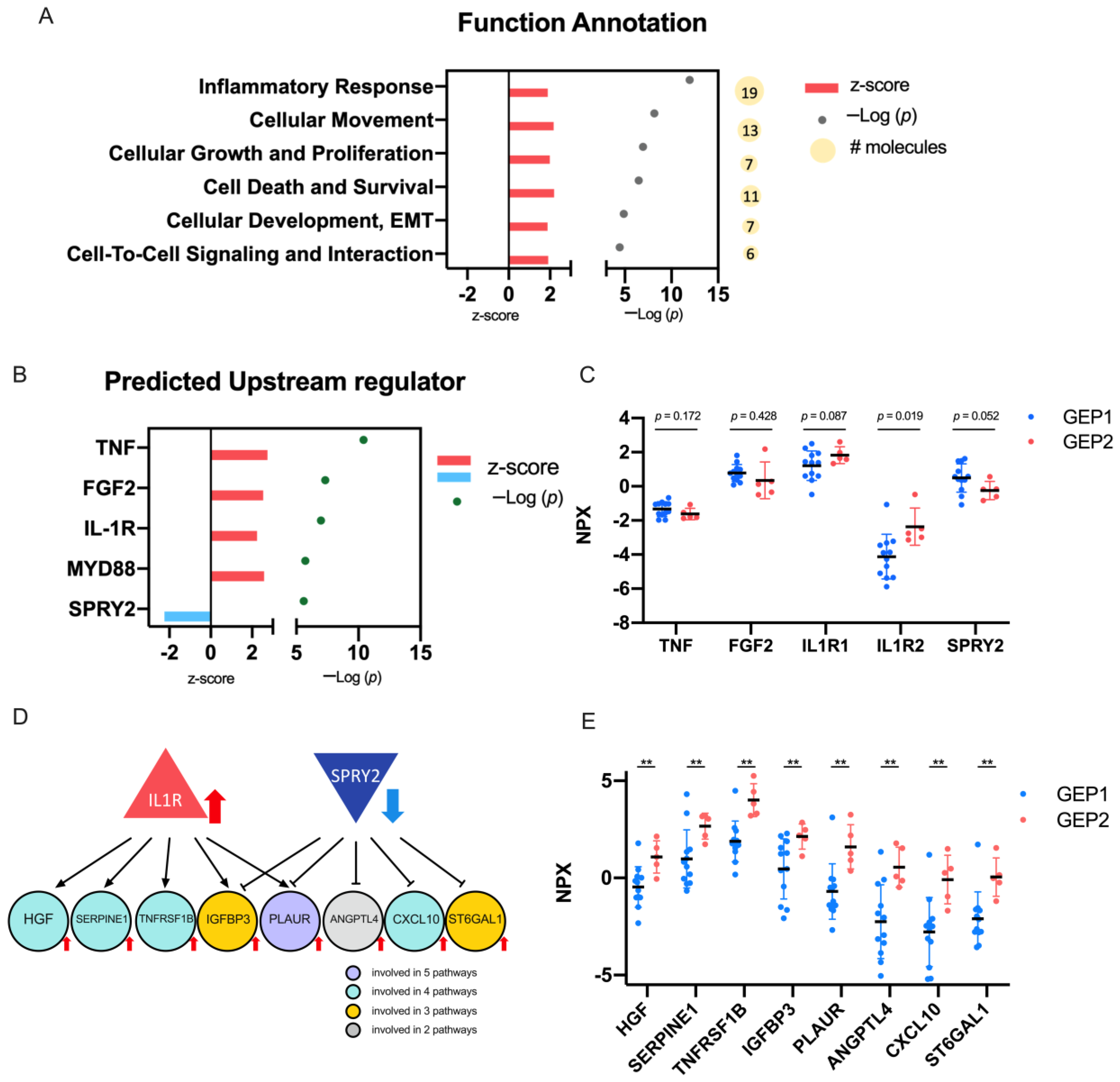
2.4. Biological Significance of the DEPs
3. Discussion
4. Materials and Methods
4.1. Patient Clinical Characteristics and Demographics
4.2. Specimen Collection and Storage
4.3. Quantification of Protein Expression by Proximity Extension Assay (PEA)
4.4. Gene Ontology Analysis and Annotations
4.5. Statistical Analysis
4.6. Analysis of Somatic Copy Number Alterations (SCNAs)
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal Melanoma. Nat. Rev. Dis. Primer 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [Green Version]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group Report No. 1: Prospective Validation of a Multi-Gene Prognostic Assay in Uveal Melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, Z.M.; Augsburger, J.J. Independent Prognostic Significance of Gene Expression Profile Class and Largest Basal Diameter of Posterior Uveal Melanomas. Am. J. Ophthalmol. 2016, 162, 20–27.e1. [Google Scholar] [CrossRef] [PubMed]
- Petrausch, U.; Martus, P.; Tönnies, H.; Bechrakis, N.E.; Lenze, D.; Wansel, S.; Hummel, M.; Bornfeld, N.; Thiel, E.; Foerster, M.H.; et al. Significance of Gene Expression Analysis in Uveal Melanoma in Comparison to Standard Risk Factors for Risk Assessment of Subsequent Metastases. Eye Lond. Engl. 2008, 22, 997–1007. [Google Scholar] [CrossRef]
- Worley, L.A.; Onken, M.D.; Person, E.; Robirds, D.; Branson, J.; Char, D.H.; Perry, A.; Harbour, J.W. Transcriptomic versus Chromosomal Prognostic Markers and Clinical Outcome in Uveal Melanoma. Clin. Cancer Res. 2007, 13, 1466–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Paez-Escamilla, M.; Walter, S.D.; Tarlan, B.; Decatur, C.L.; Perez, B.M.; Harbour, J.W. Gene Expression Profiling and PRAME Status Versus Tumor-Node-Metastasis Staging for Prognostication in Uveal Melanoma. Am. J. Ophthalmol. 2018, 195, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene Expression Profiling in Uveal Melanoma Reveals Two Molecular Classes and Predicts Metastatic Death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [Green Version]
- Onken, M.D.; Worley, L.A.; Tuscan, M.D.; Harbour, J.W. An Accurate, Clinically Feasible Multi-Gene Expression Assay for Predicting Metastasis in Uveal Melanoma. J. Mol. Diagn. 2010, 12, 461–468. [Google Scholar] [CrossRef]
- Chappell, M.C.; Char, D.H.; Cole, T.B.; Harbour, J.W.; Mishra, K.; Weinberg, V.K.; Phillips, T.L. Uveal Melanoma: Molecular Pattern, Clinical Features, and Radiation Response. Am. J. Ophthalmol. 2012, 154, 227–232.e2. [Google Scholar] [CrossRef] [Green Version]
- Correa, Z.M.; Augsburger, J.J. Sufficiency of FNAB Aspirates of Posterior Uveal Melanoma for Cytologic versus GEP Classification in 159 Patients, and Relative Prognostic Significance of These Classifications. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Plasseraud, K.M.; Cook, R.W.; Tsai, T.; Shildkrot, Y.; Middlebrook, B.; Maetzold, D.; Wilkinson, J.; Stone, J.; Johnson, C.; Oelschlager, K.; et al. Clinical Performance and Management Outcomes with the DecisionDx-UM Gene Expression Profile Test in a Prospective Multicenter Study. J. Oncol. 2016, 2016, 5325762. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.D.; Chao, D.L.; Feuer, W.; Schiffman, J.; Char, D.H.; Harbour, J.W. Prognostic Implications of Tumor Diameter in Association With Gene Expression Profile for Uveal Melanoma. JAMA Ophthalmol. 2016, 134, 734–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plasseraud, K.M.; Wilkinson, J.K.; Oelschlager, K.M.; Poteet, T.M.; Cook, R.W.; Stone, J.F.; Monzon, F.A. Gene Expression Profiling in Uveal Melanoma: Technical Reliability and Correlation of Molecular Class with Pathologic Characteristics. Diagn. Pathol. 2017, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaberg, T.M.; Cook, R.W.; Oelschlager, K.; Maetzold, D.; Rao, P.K.; Mason, J.O. Current Clinical Practice: Differential Management of Uveal Melanoma in the Era of Molecular Tumor Analyses. Clin. Ophthalmol. 2014, 8, 2449–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davanzo, J.M.; Binkley, E.M.; Bena, J.F.; Singh, A.D. Risk-Stratified Systemic Surveillance in Uveal Melanoma. Br. J. Ophthalmol. 2019, 103, 1868–1871. [Google Scholar] [CrossRef] [PubMed]
- Velez, G.; Nguyen, H.V.; Chemudupati, T.; Ludwig, C.A.; Toral, M.; Reddy, S.; Mruthyunjaya, P.; Mahajan, V.B. Liquid Biopsy Proteomics of Uveal Melanoma Reveals Biomarkers Associated with Metastatic Risk. Mol. Cancer 2021, 20, 39. [Google Scholar] [CrossRef]
- Augsburger, J.J.; Corrêa, Z.M.; Augsburger, B.D. Frequency and Implications of Discordant Gene Expression Profile Class in Posterior Uveal Melanomas Sampled by Fine Needle Aspiration Biopsy. Am. J. Ophthalmol. 2015, 159, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Heiferman, M.J.; Mahajan, V.B.; Mruthyunjaya, P. Proteomics in Uveal Melanoma. Curr. Opin. Ophthalmol. 2022, 33, 202–210. [Google Scholar] [CrossRef]
- Wierenga, A.P.A.; Cao, J.; Mouthaan, H.; van Weeghel, C.; Verdijk, R.M.; van Duinen, S.G.; Kroes, W.G.M.; Dogrusöz, M.; Marinkovic, M.; van der Burg, S.S.H.; et al. Aqueous Humor Biomarkers Identify Three Prognostic Groups in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4740–4747. [Google Scholar] [CrossRef] [Green Version]
- Im, D.; Peng, C.-C.; Xu, L.; Kim, M.E.; Ostrow, D.; Yellapantula, V.; Bootwalla, M.; Biegel, J.A.; Gai, X.; Kuhn, P.; et al. Potential of Aqueous Humor as a Liquid Biopsy for Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1458. [Google Scholar] [CrossRef]
- Lamas, N.J.; Martel, A.; Nahon-Estève, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhoum, M.F.; Esmaeli, B. Molecular Characteristics of Uveal Melanoma: Insights from the Cancer Genome Atlas (TCGA) Project. Cancers 2019, 11, 1061. [Google Scholar] [CrossRef] [Green Version]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Stuckel, A.J.; Zeng, S.; Lyu, Z.; Zhang, W.; Zhang, X.; Dougherty, U.; Mustafi, R.; Zhang, Q.; Joshi, T.; Bissonnette, M.; et al. Epigenetic DNA Modifications Upregulate SPRY2 in Human Colorectal Cancers. Cells 2021, 10, 2632. [Google Scholar] [CrossRef]
- Berry, J.L.; Xu, L.; Kooi, I.; Murphree, A.L.; Prabakar, R.K.; Reid, M.; Stachelek, K.; Le, B.H.A.; Welter, L.; Reiser, B.J.; et al. Genomic CfDNA Analysis of Aqueous Humor in Retinoblastoma Predicts Eye Salvage: The Surrogate Tumor Biopsy for Retinoblastoma. Mol. Cancer Res. 2018, 16, 1701–1712. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.L.; Xu, L.; Murphree, A.L.; Krishnan, S.; Stachelek, K.; Zolfaghari, E.; McGovern, K.; Lee, T.C.; Carlsson, A.; Kuhn, P.; et al. Potential of Aqueous Humor as a Surrogate Tumor Biopsy for Retinoblastoma. JAMA Ophthalmol. 2017, 135, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baslan, T.; Kendall, J.; Rodgers, L.; Cox, H.; Riggs, M.; Stepansky, A.; Troge, J.; Ravi, K.; Esposito, D.; Lakshmi, B.; et al. Genome-Wide Copy Number Analysis of Single Cells. Nat. Protoc. 2012, 7, 1024–1041, Correction in Nat. Protoc. 2016, 11, 616. [Google Scholar] [CrossRef]
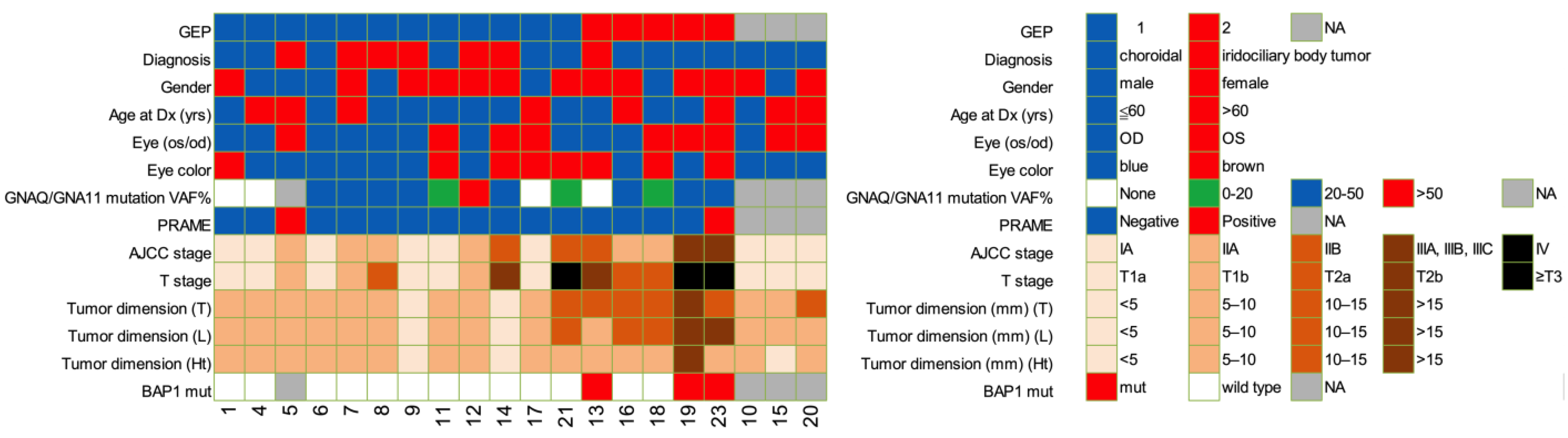

| Characteristic | GEP1, n = 12 | GEP2, n = 5 | p |
|---|---|---|---|
| Sex (Fisher), n (%) | 0.600 | ||
| Females | 7 (58.3) | 4 (80) | |
| Males | 5 (41.7) | 1 (20) | |
| Eye (Fisher), n (%) | 0.620 | ||
| OD | 7 (58.3) | 2 (40.0) | |
| OS | 5 (41.7) | 3 (60.0) | |
| Age at diagnosis, mean (± SD) (MWU) | 53.3 (15.5) | 62.8 (12.7) | 0.183 |
| Eye Color (Fisher), n (%) | 0.620 | ||
| Light (blue, gray, green, hazel) | 7 (58.3) | 2 (40.0) | |
| Dark (brown) | 5 (41.7) | 3 (60.0) | |
| Anterior Involvement (Iris and/or ciliary) (Fisher), n (%) | 0.338 | ||
| Yes | 6 (50.0) | 1 (20.0) | |
| No | 6 (50.0) | 4 (80.0) | |
| AJCC Stage (Linear-by-Linear association), n (%) | 0.012 | ||
| I | 6 (50.0) | 0 (0) | |
| IIA | 4 (33.3) | 2 (40.0) | |
| IIB | 2 (16.7) | 1 (20.0) | |
| IIIA, IIIB, IIIC | 0 (0) | 2 (40.0) | |
| IV | 0 (0) | 0 (0) | |
| PRAME Status, known in 17 cases (Fisher), n (%) | 0.515 | ||
| Negative | 11 (91.7) | 4 (80.0) | |
| Positive | 1 (8.3) | 1 (20.0) | |
| GEP Class, known in 17 cases (Fisher), n (%) | <0.001 | ||
| 1 | 12 (100.0) | 0 (0) | |
| 2 | 0 (0) | 5 (80.0) | |
| Tumor Stage (Linear-by-Linear association), n (%) | 0.007 | ||
| T1 | 9 (75.0) | 0 (0) | |
| T2 | 2 (16.7) | 3 (60.0) | |
| T3 | 1 (8.3) | 1 (20.0) | |
| T4 | 0 (0) | 1 (20.0) | |
| BAP1 mutation status, known in 16 cases (Fisher), n (%) | 0.018 | ||
| Mutation | 0 (0) | 3 (60.0) | |
| Wild type | 11 (100.0) | 2 (40.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-C.; Sirivolu, S.; Pike, S.; Kim, M.E.; Reiser, B.; Li, H.-T.; Liang, G.; Xu, L.; Berry, J.L. Diagnostic Aqueous Humor Proteome Predicts Metastatic Potential in Uveal Melanoma. Int. J. Mol. Sci. 2023, 24, 6825. https://doi.org/10.3390/ijms24076825
Peng C-C, Sirivolu S, Pike S, Kim ME, Reiser B, Li H-T, Liang G, Xu L, Berry JL. Diagnostic Aqueous Humor Proteome Predicts Metastatic Potential in Uveal Melanoma. International Journal of Molecular Sciences. 2023; 24(7):6825. https://doi.org/10.3390/ijms24076825
Chicago/Turabian StylePeng, Chen-Ching, Shreya Sirivolu, Sarah Pike, Mary E. Kim, Bibiana Reiser, Hong-Tao Li, Gangning Liang, Liya Xu, and Jesse L. Berry. 2023. "Diagnostic Aqueous Humor Proteome Predicts Metastatic Potential in Uveal Melanoma" International Journal of Molecular Sciences 24, no. 7: 6825. https://doi.org/10.3390/ijms24076825








