Abstract
Epicardial adipose tissue (EAT) is an endocrine and paracrine organ constituted by a layer of adipose tissue directly located between the myocardium and visceral pericardium. Under physiological conditions, EAT exerts protective effects of brown-like fat characteristics, metabolizing excess fatty acids, and secreting anti-inflammatory and anti-fibrotic cytokines. In certain pathological conditions, EAT acquires a proatherogenic transcriptional profile resulting in increased synthesis of biologically active adipocytokines with proinflammatory properties, promoting oxidative stress, and finally causing endothelial damage. The role of EAT in heart failure (HF) has been mainly limited to HF with preserved ejection fraction (HFpEF) and related to the HFpEF obese phenotype. In HFpEF, EAT seems to acquire a proinflammatory profile and higher EAT values have been related to worse outcomes. Less data are available about the role of EAT in HF with reduced ejection fraction (HFrEF). Conversely, in HFrEF, EAT seems to play a nutritive role and lower values may correspond to the expression of a catabolic, adverse phenotype. As of now, there is evidence that the beneficial systemic cardiovascular effects of sodium-glucose cotransporter-2 receptors-inhibitors (SGLT2-i) might be partially mediated by inducing favorable modifications on EAT. As such, EAT may represent a promising target organ for the development of new drugs to improve cardiovascular prognosis. Thus, an approach based on detailed phenotyping of cardiac structural alterations and distinctive biomolecular pathways may change the current scenario, leading towards a precision medicine model with specific therapeutic targets considering different individual profiles. The aim of this review is to summarize the current knowledge about the biomolecular pathway of EAT in HF across the whole spectrum of ejection fraction, and to describe the potential of EAT as a therapeutic target in HF.
1. Introduction
Epicardial adipose tissue (EAT) is a peculiar fat depot located between the myocardium and the visceral layer of the epicardium. Interestingly, EAT evolved from brown adipose tissue and shares the same embryologic origin as omental and mesenteric fat [1].
EAT works as a lipid-storing depot, but it has been also identified as an endocrine organ secreting hormones, part of the immune signalling network, and as an inflammatory tissue secreting cytokines and chemokines [2]. Notably, due to its close proximity and shared microvascular network, the EAT-myocardium microenvironment can be a place of intensive exchange of paracrine regulators or metabolic substrates, and it may serve as a transducer of systemic metabolic state, inflammation, immunologic response, or neurohumoral activation on the cardiac muscle [3,4]. In normal conditions, EAT plays several physiologic roles. Firstly, it works as a mechanical protectant for the surrounding coronary arteries, buffering them against the torsion induced by the arterial pulse wave and cardiac contraction [5]. Furthermore, it has a fundamental thermogenic function, serving as a protector against myocardial hypothermia [6]. EAT also houses a wide range of immune cells, mostly M2-like macrophages (with a 4:1 M2:M1 ratio), but also eosinophils and regulatory T cells which secrete interleukin (IL)-4/IL-13 and IL-10, respectively, thus polarizing macrophages towards an anti-inflammatory phenotype [7]. Finally, EAT plays a key role in the myocardial energy metabolism. Importantly, free fatty acids (FFAs) oxidation accounts for about 50–70% of the myocardial energy production, representing the most important energy source in a healthy heart [8]. As such, epicardial fat has been proposed as a buffer between the myocardium and the local vasculature, possibly protecting the heart against excessively high levels of FFAs [9,10]. Furthermore, the high lipolytic activity of EAT suggests that it may also play a role as a ready source of FFAs to meet increased myocardial energy demand through the β-oxidation and oxidative phosphorylation in mitochondria, and, thus, nourish the healthy myocardium [11].
Furthermore, EAT has been shown to be a highly active biological tissue secreting many adipokines that have the capacity to affect the adjacent myocardium [8]. Among these, adiponectin—an adipocyte-derived cytokine with cardioprotective effects—appears to be most important, particularly due to its anti-atherosclerotic, anti-apoptosis, oxidative stress diminishing, fibrosis-reducing, and anti-congestion properties [12,13]. Several other adipokines have been described to be secreted by EAT, such as interleukin (IL)-1β, -6, -8 and -10, tumor necrosis factor α (TNF-α), monocyte chemo-attractive protein 1 (MCP-1), leptin, and plasminogen activator inhibitor 1 (PAI-1) [14].
1.1. The Role of Epicardial Adipose Tissue in Cardiovascular Disease
Systemic inflammation and metabolic disorders cause EAT proliferation and deranged adipogenesis [15]. As EAT expands, it becomes hypoxic and dysfunctional [16], resulting in a shift in its metabolic profile with the activation of stress pathways such as endoplasmic reticulum stress, oxidative stress, and inflammasome [17]. As a consequence, adipose tissue produces several inflammatory mediators (i.e., IL1-β, IL-6; IL-8, IL-10; TGF-β, TNF-α, MCP-1, PAI-I), and induces adiponectin production drops and leptin increases. This last event results in a significant increase in immune cells in the EAT (PMID: 16751422) mediated by their membrane leptin receptor (LEP-R) [18,19]. Upregulation of the immune response signalling and pro-inflammatory state in the EAT might, in ultimate analysis, influence the development of cardiovascular disease, namely coronary artery disease (CAD) [20,21], cardiac arrhythmias [22,23], and heart failure (HF) [24,25]. The EAT of HF patients was characterized by pronounced immune activation, mainly dominated by the accumulation of T lymphocytes. Indeed, in the EAT of HF patients, these immune cells were highly expanded and in particular constituted by clonally expanded IFN-γ+ effector memory T lymphocytes [26]. The established local proinflammatory environment is deemed to cause myocardial microvascular dysfunction and fibrosis [15], responsible in turn for the development of ventricular hypertrophy, diastolic dysfunction, conduction abnormalities, and increased cardiac filling pressures [27,28,29,30], all of which are highly prevalent in HF. In this setting, increased EAT thickness may also change myocardial substrate utilization, with an increased reliance on FFAs oxidation for energy and a concomitant impaired oxygen use, contributing to a reduction in cardiac reserve and aerobic capacity [31]. Myocardial metabolic remodeling with a switch from FFAs to energetically more effective substrates, such as ketone bodies, is usually needed to preserve myocardial efficiency [32].
However, the available—although limited—literature suggests that the EAT may exert a different pathophysiological effect in HF in relation to the ejection fraction status. In this review, we summarize the currently available evidence regarding the role of EAT in the development of HF, highlighting the different pathophysiological pathways in HF with reduced (HFrEF), mildly reduced (HFmrEF), and preserved (HFpEF) ejection fraction.
1.2. Quantification of Epicardial Adipose Tissue
Transthoracic echocardiography is a simple, cost-effective, and readily available method which allows the measurement of EAT thickness. EAT thickness is measured as the echo-free space between the outer wall of the right ventricle and the visceral pericardium in the parasternal long-axis view at end-systole, perpendicularly to the aortic annulus. This point presents with the highest absolute EAT thickness [10,33]. Another measurement is usually performed between the outer wall of the right ventricle and the visceral pericardium in the parasternal short-axis view at end-systole, perpendicularly to the papillary muscles [1]. In some studies, the echocardiographic measurement of EAT thickness has been shown to have an excellent interobserver and intraobserver agreement, whereas other studies found it to be poor [1,34,35]. The most relevant limitation of measuring EAT by transthoracic echocardiography is the impossibility to provide information about the total fat volume. This issue is overcome by cardiac magnetic resonance imaging (CMR). CMR, despite being more time-consuming and expensive, allows a more reliable measurement of the global cardiac EAT volume and, as such, represents the gold-standard for its assessment [36]. Similarly, cardiac computer tomography (CT) has a good spatial resolution and has a highly reproducible correlation coefficient [37]. Moreover, there was a positive correlation between pericoronary adipose tissue assessed by CT and worse clinical outcome independently of calcium score [38]. A recent paper demonstrates an association between EAT volume, CAD extent, and impaired left ventricle global longitudinal strain [39]. CT-radiomic is a new and promising method based on the extraction of mineable data from CT to better define and characterize EAT, which has been shown to have a prognostic value in patients with atrial fibrillation [40].
2. Epicardial Adipose Tissue in Heart Failure with Preserved Ejection Fraction
HFpEF is a heterogenous and incompletely understood syndrome with specific molecular, genetic, and metabolomic features, all of which reflect on vascular and myocardial cell adaptations [41]. HFpEF encompasses different pathophysiological pathways and cardiac structural profiles compared to HFrEF [42]. Therefore, the HF classification criteria are often elusive and mainly based upon ejection fraction rather than on distinct clinical, metabolic, and laboratory phenotypes [43]. Patients affected by metabolic syndrome (i.e., presenting with metabolic alterations such as obesity, diabetes, and arterial hypertension) present with a particular HFpEF phenotype characterized by relatively low levels of natriuretic peptides, impaired renal function, and only modestly increased cardiac volumes [15]. Altered cardiac hemodynamics in obese people with HFpEF are mainly related to impaired ventricular distensibility, which disproportionately increases ventricular filling pressures. There is increasing evidence that obesity induces the dysregulation of the nitric oxide–cyclic guanosine monophosphate–protein kinase G signaling cascade via obesity-induced proinflammatory pathways [44]. Consequently, inflammation leads to endothelial dysfunction, microvascular myocardial alterations, mitochondrial dysfunction, relative hypoxia and, finally, increased myocardial fibrosis [44,45]. In particular, the interplay of cytokines such as TNF-α, IL-1β, IL-10, IL-4, and IL-13 seems to play a crucial role in modulating LV remodeling and myocardial fibrosis and repair in HFpEF [46,47]. EAT may represent the local mediator and promote proinflammatory adverse effects by expressing changes in the inflammasome and developing a proatherogenic transcriptional profile with increased synthesis of biologically active adipokines with proinflammatory properties [48,49]. This may be the case independently of the level of inflammation in other fat depots, supporting the hypothesis of a local, paracrine effect of EAT [17,50]. As such, EAT might promote the secretion of proinflammatory adipokines via deranged adipogenesis and, thus, induce atrial and ventricular fibrosis and alterations of the microcirculation [15]. Additionally, local inflammation and changes in the micro-environment may foster cardiomyocyte dysfunction [15]. This hypothesis is supported by the finding of a direct correlation between intramyocardial adipose tissue, and impaired diastolic function in HFpEF patients, particularly in HFpEF women, regardless of their age, co-morbidities, BMI, and myocardial fibrosis [51]. Furthermore, EAT has been shown to express profibrotic proteins, such as serpine A3, matrix metalloproteinase 14, and inflammatory biomarkers (p53 mRNA), which may work as modulators of HF [48,52].
Higher EAT volumes might negatively affect the left ventricle (LV), impairing its distensibility and leading to the development of HFpEF [53]. In a large meta-analysis investigating 22 studies, a greater EAT was associated with diastolic dysfunction independently of other adiposity measures [28]. In particular, in patients with HFpEF, a positive correlation between increased EAT and a right-sided ventricular constrictive pattern at echocardiography has been observed, thus supporting the hypothesis of a mechanical constrictive effect of EAT on the myocardial distensibility [54]. Similarly, EAT localized near the ventricles has been related to ventricular mass, independently of global measures of adiposity [55].
There is increasing evidence showing that patients with HFpEF have more EAT compared with healthy controls and patients with HFrEF (Figure 1). Moreover, it has been shown that EAT is strongly correlated with increased mortality and new-onset heart failure in both HFpEF and HFmrEF [56,57,58,59]. This association was independent of body mass index (BMI), HF severity, and co-morbidities, thus suggesting a direct role of EAT in the pathophysiology of HFpEF [56,57,58]. Studies investigating the role of EAT in HFpEF are summarized in Table 1.
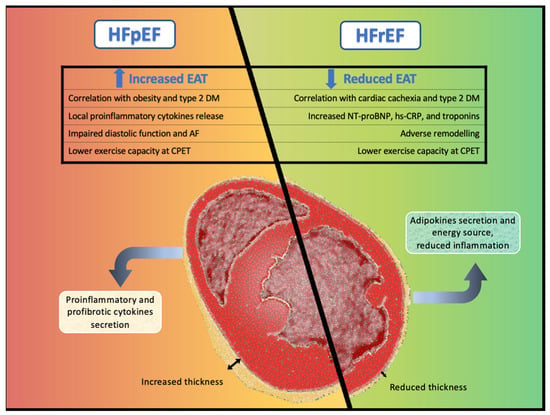
Figure 1.
Central illustration. Different pathological pathways and cardiovascular effects of epicardial adipose tissue in heart failure with preserved ejection fraction and in heart failure with reduced ejection fraction.

Table 1.
Studies investigating the role of EAT in heart failure with preserved and mildly reduced ejection fraction.
2.1. The Obese HFpEF Phenotype
Obesity is a key component in HFpEF, favoring disease progression, and both increasing myocardial load and worsening HFpEF-related comorbidities, such as arterial hypertension [62]. Lately, research has focused on fat distribution and characteristics in HFpEF, suggesting a particular adverse role for visceral fat and EAT, independently of BMI [48]. Although some trials showed that abdominal adiposity is a risk factor for all-cause mortality, there are increasing data suggesting that EAT may be a better biomarker for overall body fat compared to BMI, particularly in HF [63]. Indeed, pericardial adipose tissue and visceral adipose tissue, but not subcutaneous adipose tissue, have been associated with incident HF and, particularly, with HFpEF [25]. Furthermore, a correlation between EAT thickness and visceral adipose tissue as measured by CT has been observed in HFpEF patients [64]. Interestingly, only epi- and pericardial adipose tissue have been related to a higher mortality risk and with adverse cardiovascular events, also in otherwise apparently healthy individuals [25,65]. Although the studies investigating the effects of EAT in HFpEF patients scarcely explored the overall body fat composition, HFpEF patients with an obese phenotype and more EAT present with a higher relative adverse event rate as compared to obese patients with lower EAT mass [58,62].
The relationship between obesity and HFpEF is complex. Increased pericardial fat may exert a direct compressive effect on the right ventricle, thus preventing its distension [31,54]. Obese HFpEF patients display increased EAT thickness and a concentric ventricular remodeling, which in turn are associated with parameters of greater pericardial restraint and ventricular diastolic dysfunction as compared to the non-obese counterparts [31]. On the other hand, a reverse causality may also be hypothesized: local paracrine alteration appearing during the early stages of HF may promote the proliferation of local epicardial adipose tissue [24].
These findings point towards an obesity-related HFpEF phenotype as a separate form of cardiac failure with specific hemodynamic changes and characteristics, which should be specifically addressed.
2.2. EAT and Sex Differences
Clinical studies demonstrated conflicting results regarding the amount of EAT in men compared to women [66,67]. In particular, older women and post-menopausal status showed higher EAT volumes compared to younger women. Interestingly, parameters of LV diastolic dysfunction such as E/e’ were significantly related to increased EAT thickness in women, but not in men. Similar results were found for LV systolic function where S’ reduction was directly correlated with the amount of EAT [68]. Unfortunately, we do not know if the differences are based on overall adiposity and visceral fat distribution, which are likely different between genders [55,56].
2.3. EAT and Exercise Capacity
An increased EAT volume has been associated with a markedly reduced exercise capacity (i.e., reduced capacity of peripheral oxygen extraction), independent of BMI in HFpEF, but not in HfrEF [54,56]. Subclinical impaired exercise capacity may then lead to worse body composition and even more accumulation of EAT [69]. The remark of a greater exercise intolerance has been particularly observed in HfpEF patients with an obese phenotype and increased EAT thickness as compared to their counterparts with normal EAT values, likely due to a concomitant increase in ventricular filling pressures and pulmonary pressures [60]. Peripheral limitation to exercise is a pivotal cause of exercise intolerance in HFpEF patients, and EAT accumulation directly leads to central and peripheral microvascular dysfunction, regardless of BMI [56]. These data further support the emerging paradigm that excess EAT might contribute to the pathophysiology of patients with obesity-related HFpEF.
Although the evidence on differences concerning a reduced exercise capacity in HFpEF patients according to EAT volume are not concordant, different testing modalities and experimental setup might account for different results (e.g., supine position during invasive hemodynamic assessment) [10].
2.4. EAT and Atrial Fibrillation
The expansion of EAT has been associated with both obesity and type 2 diabetes mellitus, two important risk factors for atrial fibrillation [53]. In these settings, EAT-related proinflammatory mediators may induce microvascular dysfunction, leading to intramyocardial fibrosis and electroanatomical remodeling also in the atria, leading to atrial myopathy [15,53]. Atrial fibrillation often represents the first manifestation of an underlying latent HFpEF characterized by atrial myopathy and increased ventricular filling pressures [53]. An increased EAT predicts the incidence of atrial fibrillation in apparently healthy individuals, independently from its localization above the myocardium [70]; although, atrial EAT has been observed to be more increased in HFpEF patients with atrial fibrillation as compared to those without atrial fibrillation [55].
3. Epicardial Adipose Tissue in Heart Failure with Reduced Ejection Fraction
The potential role of EAT in HfrEF has not been clearly understood yet and, up to now, only few studies have been conducted in this setting (Table 2). The majority of observational studies in HfrEF patients examined the distribution of EAT via CMR [36,51,71], whereas only a minority investigated EAT thickness via transthoracic echocardiography [56].

Table 2.
Studies investigating the role of EAT in heart failure with reduced ejection fraction.
Several observations showed that both EAT mass and EAT thickness are significantly reduced in HFrEF patients as compared to both healthy controls and HFpEF patients [36,56,71]. This is seen regardless of the etiology of the underlying cardiomyopathy, but mostly in the presence of known cardiovascular risk factors such as type 2 diabetes mellitus, hypertension, dyslipidemia, or active smoking status [36].
Nevertheless, other studies reported that EAT mass increases in patients with HFrEF [51,71], but with a reduced EAT mass/LV mass ratio and thinner right ventricular EAT thickness as compared to healthy controls and HFpEF patients [36,56,71]. Accordingly, HFrEF patients also display the lowest intramyocardial fat amount as compared to HFpEF patients and to non-HF patients [51]. These findings of a globally reduced EAT mass in patients with HFrEF may be the expression of a particular cachectic phenotype in HFrEF patients characterized by a pathological catabolic state, where the heart fails in sufficiently increasing EAT volume to meet the metabolic requests of a compensatory increased ventricular mass [3,72]. In support of this hypothesis, a reduced EAT in HFrEF patients has been related to worse cardiac function and with adverse myocardial remodeling—in contrast to the previously reported findings in HFpEF patients [56]. This catabolic effect might be driven by increased natriuretic peptides, which have a strong lipolytic effect mostly in HF patients, and this leads to excessive fatty acid mobilization [73].
Thus, a reduced EAT thickness in HFrEF patients might, thus, be a sign of preclinical cardiac cachexia and its assessment might help in individuating those patients with worse cardiovascular prognosis. However, this conclusion is partially speculative and further studies addressing this question are warranted.
Different Role of EAT in HFrEF as Compared to HFpEF
Pugliese and colleagues were the first to describe EAT-related differences between HFrEF and HFpEF in terms of cardiometabolic profile, hemodynamics, and cardiovascular outcome [56]. In HFrEF patients, a reduced EAT thickness was associated with increased NT-proBNP values, higher markers of inflammation (hs-CRP), and myocardial damage (troponins), contrarily to what is observed in HFpEF patients [56]. These data further support the hypothesis of a higher inflammatory status in HFrEF patients with reduced EAT thickness as a direct consequence of cardiac cachexia and catabolic-related adverse effects [3]. Accordingly, in opposition to the previously reported findings in HFpEF patients, HFrEF patients with a reduced EAT thickness had a worse peripheral muscular capacity of oxygen extraction, and presented with cardiopulmonary exercise intolerance as measured by oxygen consumption (peak VO2), regardless of BMI [56]. Increased EAT thickness was associated with better LV global longitudinal strain and left atrial (LA) reservoir function in patients with HFrEF/HFmrEF, but not in patients with HFpEF. Accordingly, EAT thickness > 10 mm was associated with LA dysfunction in HFpEF, but not in HFrEF/HFmrEF [61]. In addition, a decreased EAT in HFrEF has been related with worse cardiovascular outcomes (HF hospitalizations and cardiovascular deaths) after a 21-month follow-up [56].
4. Epicardial Adipose Tissue in Heart Failure with Mildly Reduced Ejection Fraction
Although almost all of the available studies investigated HF patients with a LVEF >40% as a single entity [55,57,58], HFmrEF likely represents a milder phenotype of HFrEF rather than a separate entity [74]. To the best of our knowledge, only one study investigated the amount of pericardial fat (defined as the sum of epicardial and paracardial fat) by CT differentiating HFpEF from HFmrEF [24]. In this large, community-based, prospective cohort study, pericardial fat was strongly associated with an increased risk of HFpEF, but only modestly with HFmrEF and lacking in HFrEF [24]. Interestingly, only a mild association with HFmrEF was observed, whereas no association with HFrEF was found [24]. Although only hypothesis-generating, these data point towards a distinguished phenotype, and further studies specifically focusing on HFmrEF are warranted.
5. Potential of Epicardial Adipose Tissue in Heart Failure as Therapeutic Target
The role of inflammation in HF pathophysiology has been increasingly acknowledged [75].
In this point of view, EAT might play a key role as pathophysiological mediator in the development of HF. In fact, in pathological conditions, EAT acquires a proatherogenic transcriptional profile resulting in the increased synthesis of biologically active adipocytokines with proinflammatory properties, promoting oxidative stress, and finally causing endothelial damage [76]. As previously stated, the EAT amount is increased in HFpEF as compared to HFrEF. This observation might be of particular interest, since therapeutic efforts for disease-modifying drugs in HFpEF may concentrate on reducing the mass and inflammatory state of EAT [77]. Up to now, two anti-inflammatory drugs, i.e., statins and anticytokine agents, have been specifically investigated for their possible effects on adipose tissue inflammation. Statins have known pleiotropic anti-inflammatory properties in patients with systemic inflammatory disorders such as rheumatoid arthritis [78]. First, experimental data in animal HF models demonstrated that the use of rosuvastatin in addition to standard HF therapy resulted in a significant improvement in cardiac remodeling, which has been likely linked to a reduced myocardial inflammation independently from plasma lipid levels [79]. In line, a robust association between statin therapy and both a reduced EAT thickness and lower levels of EAT-secreted inflammatory mediators has been demonstrated [80]. In a large metanalysis of 17 randomized-controlled clinical trials involving patients with dyslipidemia, statins reduced the incidence of HF-related adverse events regardless of the presence of a previous myocardial infarction, possibly indicating a beneficial effect in HFpEF prevention [81]. Conversely, the same positive effects on morbidity and mortality have not been found in patients with HFrEF [82]. Anticytokine drugs, particularly agents against tumor necrosis factor-α and interleukin 1-β, showed a beneficial effect on hypertension-induced cardiac damage in animal models [83]. The effect of the anti-interleukin-1 agent anakinra on systemic inflammation has been studied in HFpEF patients, producing contradictory results [84,85]. In a large randomized controlled trial, canakinumab has been demonstrated to reduce proinflammatory biomarkers as well as the risk of HF hospitalization in patients with HFpEF [86]. However, this study did not investigate separately patients with HFrEF and HFpEF, and included subjects who suffered from a myocardial infarction and had high parameters of systemic inflammation [86]. Thus, the provided data about canakinumab should be considered as exploratory and hypothesis-generating [86]. On the other side, no beneficial effects have been found for tumor necrosis factor-α antagonists in patients with HFrEF, where their use has been linked to an increased risk of adverse outcomes, likely related to sodium retention secondary to increased aldosterone synthesis [87,88].
Inhibitors of sodium-glucose cotransporter-2 receptors (SGLT-2i) represent a pillar of the modern HF therapy due to their beneficial effect on the reduction of adverse cardiovascular events in HF patients across the entire ejection fraction spectrum, and their broad eligibility [89,90,91]. Although their mechanisms of action in HF are still debated, there is increasing evidence that SGLT-2i can reduce cardiac fibrosis and hypertrophy, lowering LV filling pressures in experimental models of HFpEF [92,93]. In diabetic patients with coronary artery disease, dapagliflozin therapy has been demonstrated to reduce EAT mass and inflammatory parameters such as tumor necrosis factor-α and plasminogen activator inhibitor-1 over a period of 6 months [94]. Similar positive effects of SGLT-2i have been reproduced also in non-diabetic patients with HFrEF. Indeed, in the setting of the EMPA-TROPISM study, empagliflozin has been associated with a significant reduction in EAT volume as measured by cardiac magnetic resonance [95]. Interestingly, this reduction in EAT was accompanied by a reduction in myocardial fibrosis and inflammatory biomarkers related to endothelial dysfunction, leukocyte circulation, and cell adhesion molecules, such as E-selectin [95].
Besides the above-mentioned therapies, metformin and glucagon-like peptide-1 (GLP-1) receptor agonists have also been found to reduce EAT inflammation and EAT mass, respectively. However, randomized-controlled trials on the outcome-modifying effect of metformin in HF are lacking. Interestingly, GLP-1 receptor agonists such as liraglutide have been shown to worsen congestion and to increase cardiovascular adverse events in HFrEF independently from diabetes mellitus type 2 [96,97]. In particular, GLP1 analogs may reduce adipogenesis, improve fat utilization, and induce brown fat differentiation in EAT [98]. Iacobellis et al. showed in a small trial that GLP1A was able to reduce EAT volume, but the study was not designed to detect a difference in hard clinical endpoints [99].
Table 3 summarizes current available therapies targeting epicardial adipose tissue in heart failure.

Table 3.
Future perspectives on drugs targeting EAT.
In the recent future, adipose tissue could be a new target for gene therapy as well. The fibroblast growth factor 21 (FGF21) has been identified as a promising therapeutic agent for type 2 diabetes and other metabolic diseases. The overexpression of the FGF21 in the visceral adipose tissue was associated with an improvement in energy homeostasis. A low-dose intraperitoneal injection of engineered serotype adeno-associated viral vector-FGF21 resulted in reduced whole-body adiposity and inflammatory cytokine release, lower insulin resistance, and glycemic processing [100].
Taken together, these studies raise the question of whether EAT volume alone is the right surrogate marker at all, as the metabolic activity of the EAT may be independent of its total volume. Overall, different interventions seem to lead to similar reductions in EAT in both HFpEF and HFrEF, but we need more clinical trials to investigate the different roles of EAT across the spectrum of LVEF and underlying etiologies.
6. Conclusions
Epicardial adipose tissue represents an important determinant for altered cell signals, energetic substrate, and an excessive immune response. Moreover, it shows additional diagnostic and prognostic properties in the HFpEF population, aside from the common markers of inflammation, cardiovascular dysfunction, and fibrosis. On the contrary, EAT is reduced in HFrEF patients, in whom its role is not fully understood and is likely related to a nurturing function. The assessment of EAT in clinical practice could lead to a better understanding of the molecular pathways and biological mechanisms responsible for HF syndromes, where it might represent a promising therapeutic target. In particular, understanding the EAT transcriptional profile in physiological and pathological conditions might represent the keystone to developing disease-modifying therapies in HFpEF, and to deep-phenotyping this not yet fully understood, poly-etiologic clinical syndrome. Until then, EAT remains a risk factor for adverse outcomes and not a treatment target. As effective therapies to reduce the disease burden, particularly of HFpEF, are sparse, EAT may provide novel insights into treatment strategies in the future.
Author Contributions
Conceptualization, V.A.R., M.G., L.M., A.G., M.B.; methodology, V.A.R., M.G., L.M.; writing—original draft preparation, V.A.R., M.G., L.M.; writing—review and editing, V.A.R., M.G., L.M., A.G., M.B.; supervision, M.B.; project administration, V.A.R.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iacobellis, G.; Willens, H.J. Echocardiographic Epicardial Fat: A Review of Research and Clinical Applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319, quiz 417–418. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Janovska, P.; Melenovsky, V.; Svobodova, M.; Havlenova, T.; Kratochvilova, H.; Haluzik, M.; Hoskova, E.; Pelikanova, T.; Kautzner, J.; Monzo, L.; et al. Dysregulation of epicardial adipose tissue in cachexia due to heart failure: The role of natriuretic peptides and cardiolipin. J. Cachexia Sarcopenia Muscle 2020, 11, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Rengo, G.; Pagano, G.; D’Esposito, V.; Passaretti, F.; Caruso, A.; Grimaldi, M.G.; Lonobile, T.; Baldascino, F.; De Bellis, A.; et al. Epicardial adipose tissue has an increased thickness and is a source of inflammatory mediators in patients with calcific aortic stenosis. Int. J. Cardiol. 2015, 186, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Epicardial fat: Properties, function and relationship to obesity. Obes. Rev. 2007, 8, 253–261. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling Protein-1 and Related Messenger Ribonucleic Acids in Human Epicardial and Other Adipose Tissues: Epicardial Fat Functioning as Brown Fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr. Physiol. 2017, 7, 1051–1082. [Google Scholar]
- Talman, A.H.; Psaltis, P.J.; Cameron, J.D.; Meredith, I.T.; Seneviratne, S.K.; Wong, D.T.L. Epicardial adipose tissue: Far more than a fat depot. Cardiovasc. Diagn. Ther. 2014, 4, 416–429. [Google Scholar] [CrossRef]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 94, 225–232. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Blandenier, C.; Iacobellis, G. Epicardial adipose tissue: More than a simple fat deposit? Endocrinol. Nutr. Engl. Ed. 2013, 60, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Sakuma, I.; Shin, E.K.; Koh, K.K. Antiatherosclerotic and Anti-Insulin Resistance Effects of Adiponectin: Basic and Clinical Studies. Prog. Cardiovasc. Dis. 2009, 52, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Monzo, L.; Kotrc, M.; Benes, J.; Sedlacek, K.; Jurcova, I.; Franekova, J.; Jarolim, P.; Kautzner, J.; Melenovsky, V. Clinical and Humoral Determinants of Congestion in Heart Failure: Potential Role of Adiponectin. Kidney Blood Press. Res. 2019, 44, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.E.; Kavey, R.-E.; Quinzi, D. Combined dyslipidemia in obese children: Response to a focused lifestyle approach. J. Clin. Lipidol. 2014, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Papathanassoglou, E.; El-Haschimi, K.; Li, X.C.; Matarese, G.; Strom, T.; Mantzoros, C. Leptin Receptor Expression and Signaling in Lymphocytes: Kinetics During Lymphocyte Activation, Role in Lymphocyte Survival, and Response to High Fat Diet in Mice. J. Immunol. 2006, 176, 7745–7752. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentić, S.; Šestan, M.; Wensveen, T.T.; Polić, B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015, 45, 2446–2456. [Google Scholar] [CrossRef]
- Yerramasu, A.; Dey, D.; Venuraju, S.; Anand, D.V.; Atwal, S.; Corder, R.; Berman, D.S.; Lahiri, A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2011, 220, 223–230. [Google Scholar] [CrossRef]
- Bachar, G.N.; Dicker, D.; Kornowski, R.; Atar, E. Epicardial Adipose Tissue as a Predictor of Coronary Artery Disease in Asymptomatic Subjects. Am. J. Cardiol. 2012, 110, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.K.; Hwang, T.; Liebers, C.S.; Ng, F.S. Epicardial adipose tissue as a mediator of cardiac arrhythmias. Am. J. Physiol. Circ. Physiol. 2022, 322, H129–H144. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Ding, J.; Carr, J.J.; Allison, M.A.; Budoff, M.J.; Tracy, R.P.; Burke, G.L.; McClelland, R.L.; Arai, A.E.; Bluemke, D.A. Pericardial Fat and the Risk of Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2638–2652. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Bush, C.G.; Mongraw-Chaffin, M.; Hall, M.E.; Clark, D., 3rd; Fudim, M.; Correa, A.; Hammill, B.G.; O’Brien, E.; Min, Y.I.; et al. Regional Adiposity and Risk of Heart Failure and Mortality: The Jackson Heart Study. J. Am. Heart Assoc. 2021, 10, e020920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Chen, X.-L.; Tang, T.-T.; Zhang, S.; Li, Q.-L.; Xia, N.; Nie, S.-F.; Zhang, M.; Zhu, Z.-F.; Zhou, Z.-H.; et al. T lymphocyte characteristics and immune repertoires in the epicardial adipose tissue of heart failure patients. Front. Immunol. 2023, 14, 1126997. [Google Scholar] [CrossRef]
- Mancio, J.; Azevedo, D.; Fragao-Marques, M.; Falcao-Pires, I.; Leite-Moreira, A.; Lunet, N.; Fontes-Carvalho, R.; Bettencourt, N. Meta-Analysis of Relation of Epicardial Adipose Tissue Volume to Left Atrial Dilation and to Left Ventricular Hypertrophy and Functions. Am. J. Cardiol. 2019, 123, 523–531. [Google Scholar] [CrossRef]
- Nerlekar, N.; Muthalaly, R.G.; Wong, N.; Thakur, U.; Wong, D.T.L.; Brown, A.J.; Marwick, T.H. Association of Volumetric Epicardial Adipose Tissue Quantification and Cardiac Structure and Function. J. Am. Heart Assoc. 2018, 7, e009975. [Google Scholar] [CrossRef]
- Aydın, H.; Toprak, A.; Deyneli, O.; Yazıcı, D.; Tarçın, O.; Sancak, S.; Yavuz, D.; Akalın, S. Epicardial Fat Tissue Thickness Correlates with Endothelial Dysfunction and Other Cardiovascular Risk Factors in Patients with Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 229–234. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Bell, J.R.; Raaijmakers, A.J.A.; Waddell, H.M.; Wells, S.P.; Bernasochi, G.B.; Montgomery, M.K.; Binny, S.; Watts, T.; Joshi, S.B.; et al. Epicardial Adipose Tissue Accumulation Confers Atrial Conduction Abnormality. J. Am. Coll Cardiol. 2020, 76, 1197–1211. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.; Pislaru, S.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Monzo, L.; Sedlacek, K.; Hromanikova, K.; Tomanova, L.; Borlaug, B.A.; Jabor, A.; Kautzner, J.; Melenovsky, V. Myocardial ketone body utilization in patients with heart failure: The impact of oral ketone ester. Metabolism 2021, 115, 154452. [Google Scholar] [CrossRef] [PubMed]
- Schejbal, V. Epicardial fatty tissue of the right ventricle--morphology, morphometry and functional significance. Pneumologie 1989, 43, 490–499. [Google Scholar] [PubMed]
- Iacobellis, G.; Willens, H.J.; Barbaro, G.; Sharma, A.M. Threshold Values of High-risk Echocardiographic Epicardial Fat Thickness. Obesity 2008, 16, 887–892. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; Ophuis, B.; Saucedo-Orozco, H.; van Empel, V.P.M.; Willems, T.P.; Geelhoed, B.; Rienstra, M.; Westenbrink, B.D. The value of echocardiographic measurement of epicardial adipose tissue in heart failure patients. ESC Heart Fail. 2022, 9, 953–957. [Google Scholar] [CrossRef]
- Doesch, C.; Haghi, D.; Flüchter, S.; Suselbeck, T.; Schoenberg, S.O.; Michaely, H.; Borggrefe, M.; Papavassiliu, T. Epicardial adipose tissue in patients with heart failure. J. Cardiovasc. Magn. Reson. 2010, 12, 40. [Google Scholar] [CrossRef]
- Nakazato, R.; Shmilovich, H.; Tamarappoo, B.K.; Cheng, V.Y.; Slomka, P.J.; Berman, D.S.; Dey, D. Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J. Cardiovasc. Comput. Tomogr. 2011, 5, 172–179. [Google Scholar] [CrossRef]
- Takahashi, D.; Fujimoto, S.; Nozaki, Y.O.; Kudo, A.; Kawaguchi, Y.O.; Takamura, K.; Hiki, M.; Sato, H.; Tomizawa, N.; Kumamaru, K.K.; et al. Validation and clinical impact of novel pericoronary adipose tissue measurement on ECG-gated non-contrast chest CT. Atherosclerosis 2023, 370, 18–24. [Google Scholar] [CrossRef]
- Ishikawa, H.; Otsuka, K.; Kono, Y.; Hojo, K.; Yamaura, H.; Hirata, K.; Kasayuki, N.; Izumiya, Y.; Fukuda, D. Extent of coronary atherosclerosis is associated with deterioration of left ventricular global longitudinal strain in patients with preserved ejection fraction undergoing coronary computed tomography angiography. IJC Heart Vasc. 2023, 44, 101176. [Google Scholar] [CrossRef]
- Ilyushenkova, J.; Sazonova, S.; Popov, E.; Zavadovsky, K.; Batalov, R.; Archakov, E.; Moskovskih, T.; Popov, S.; Minin, S.; Romanov, A. Radiomic phenotype of epicardial adipose tissue in the prognosis of atrial fibrillation recurrence after catheter ablation in patients with lone atrial fibrillation. J. Arrhythmia 2022, 38, 682–693. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Tramonte, F.; Beltrami, M. Laboratory and Metabolomic Fingerprint in Heart Failure with Preserved Ejection Fraction: From Clinical Classification to Biomarker Signature. Biomolecules 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Beltrami, M. Are HFpEF and HFmrEF So Different? The Need to Understand Distinct Phenotypes. Front. Cardiovasc. Med. 2021, 8, 676658. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Shah, S.J. The HFpEF Obesity Phenotype: The Elephant in the Room. J. Am. Coll Cardiol. 2016, 68, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Venkateshvaran, A.; Faxen, U.L.; Hage, C.; Michaelsson, E.; Svedlund, S.; Saraste, A.; Beussink-Nelson, L.; Fermer, M.L.; Gan, L.M.; Tromp, J.; et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: Insights from the PROMIS-HFpEF study. Eur. J. Heart Fail. 2022, 24, 2251–2260. [Google Scholar] [CrossRef]
- Lindner, D.; Zietsch, C.; Tank, J.; Sossalla, S.; Fluschnik, N.; Hinrichs, S.; Maier, L.; Poller, W.; Blankenberg, S.; Schultheiss, H.-P.; et al. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res. Cardiol. 2014, 109, 428. [Google Scholar] [CrossRef]
- Conceicao, G.; Martins, D.; Miranda, I.M.; Leite-Moreira, A.F.; Vitorino, R.; Falcao-Pires, I. Unraveling the Role of Epicardial Adipose Tissue in Coronary Artery Disease: Partners in Crime? Int. J. Mol. Sci. 2020, 21, 8866. [Google Scholar] [CrossRef]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- De Biase, N.; Del Punta, L.; Pugliese, N.R. The dangerous liaison between epicardial adipose tissue and heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2022, 24, 2261–2263. [Google Scholar] [CrossRef]
- McAninch, E.; Fonseca, T.L.; Poggioli, R.; Panos, A.; Salerno, T.A.; Deng, Y.; Li, Y.; Bianco, A.; Iacobellis, G. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity 2015, 23, 1267–1278. [Google Scholar] [CrossRef]
- Wu, C.; Lee, J.; Hsu, J.; Su, M.-Y.; Wu, Y.; Lin, T.; Lan, C.; Hwang, J.; Lin, L. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Guo, Z.; Wang, P.; Zheng, M.; Yang, X.; Liu, Y.; Ma, Z.; Chen, M.; Yang, X. Proteomics of epicardial adipose tissue in patients with heart failure. J. Cell. Mol. Med. 2020, 24, 511–520. [Google Scholar] [CrossRef]
- Packer, M. Disease–treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: The potential mediating influence of epicardial adipose tissue. Cardiovasc. Diabetol. 2019, 18, 121. [Google Scholar] [CrossRef]
- Gorter, T.M.; van Woerden, G.; Rienstra, M.; Dickinson, M.G.; Hummel, Y.M.; Voors, A.A.; Hoendermis, E.S.; van Veldhuisen, D.J. Epicardial Adipose Tissue and Invasive Hemodynamics in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 667–676. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; van Empel, V.P.M.; Hemels, M.E.W.; Hazebroek, E.J.; van Veldhuisen, S.L.; Willems, T.P.; Rienstra, M.; Westenbrink, B.D. Importance of epicardial adipose tissue localization using cardiac magnetic resonance imaging in patients with heart failure with mid-range and preserved ejection fraction. Clin. Cardiol. 2021, 44, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; Paneni, F.; Mazzola, M.; De Biase, N.; Del Punta, L.; Gargani, L.; Mengozzi, A.; Virdis, A.; Nesti, L.; Taddei, S.; et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur. J. Heart Fail. 2021, 23, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; van Veldhuisen, D.J.; Rienstra, M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Manintveld, O.C.; van Empel, V.P.M.; Willems, T.P.; de Boer, R.A.; Rienstra, M.; Westenbrink, B.D.; Gorter, T.M. Epicardial Adipose Tissue and Outcome in Heart Failure with Mid-Range and Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e009238. [Google Scholar] [CrossRef]
- Ying, W.; Sharma, K.; Yanek, L.R.; Vaidya, D.; Schär, M.; Markl, M.; Subramanya, V.; Soleimani, S.; Ouyang, P.; Michos, E.D.; et al. Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2535–2545. [Google Scholar] [CrossRef]
- Koepp, K.E.; Obokata, M.; Reddy, Y.N.; Olson, T.P.; Borlaug, B.A. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 657–666. [Google Scholar] [CrossRef]
- Jin, X.; Hung, C.; Tay, W.T.; Soon, D.; Sim, D.; Sung, K.; Loh, S.Y.; Lee, S.; Jaufeerally, F.; Ling, L.H.; et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur. J. Heart Fail. 2022, 24, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Alpert, M.A.; Arena, R.; Mehra, M.R.; Milani, R.V.; Ventura, H.O. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure. JACC Heart Fail. 2013, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sorimachi, H.; Obokata, M.; Takahashi, N.; Reddy, Y.N.V.; Jain, C.C.; Verbrugge, F.H.; Koepp, K.E.; Khosla, S.; Jensen, M.D.; Borlaug, B.A. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur. Heart J. 2021, 42, 1595–1605. [Google Scholar] [CrossRef]
- Shah, R.V.; Anderson, A.; Ding, J.; Budoff, M.; Rider, O.; Petersen, S.E.; Jensen, M.K.; Koch, M.; Allison, M.; Kawel-Boehm, N.; et al. Pericardial, But Not Hepatic, Fat by CT Is Associated with CV Outcomes and Structure: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc. Imaging 2017, 10, 1016–1027. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Westenbrink, B.D.; de Boer, R.A.; Rienstra, M.; Gorter, T.M. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: Mechanisms, management and modern perspectives. Eur. J. Heart Fail. 2022, 24, 2238–2250. [Google Scholar] [CrossRef]
- Mancio, J.; Pinheiro, M.; Ferreira, W.; Carvalho, M.; Barros, A.; Ferreira, N.; Vouga, L.; Ribeiro, V.G.; Leite-Moreira, A.; Falcao-Pires, I.; et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int. J. Cardiol. 2017, 249, 419–425. [Google Scholar] [CrossRef]
- Kim, S.-A.; Kim, M.-N.; Shim, W.-J.; Park, S.-M. Epicardial adipose tissue is related to cardiac function in elderly women, but not in men. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 41–47. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, H.; Guo, L.; Hong, K. Relationship between epicardial adipose tissue volume and atrial fibrillation: A systematic review and meta-analysis. Herz 2016, 41, 421–427. [Google Scholar] [CrossRef]
- Tromp, J.; Bryant, J.A.; Jin, X.; van Woerden, G.; Asali, S.; Yiying, H.; Liew, O.W.; Ching, J.C.P.; Jaufeerally, F.; Loh, S.Y.; et al. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 835–838. [Google Scholar] [CrossRef]
- Shibata, R.; Sato, K.; Pimentel, D.R.; Takemura, Y.; Kihara, S.; Ohashi, K.; Funahashi, T.; Ouchi, N.; Walsh, K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2–dependent mechanisms. Nat. Med. 2005, 11, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Kotrc, M.; Wedellova, Z.; Jabor, A.; Malek, I.; Kautzner, J.; Kazdova, L.; Melenovsky, V. Lipolytic Effects of B-Type Natriuretic Peptide1–32 in Adipose Tissue of Heart Failure Patients Compared with Healthy Controls. J. Am. Coll. Cardiol. 2011, 58, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart failure with mid-range or mildly reduced ejection fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Iacobellis, G.; Barbaro, G. The Double Role of Epicardial Adipose Tissue as Pro- and Anti-inflammatory Organ. Horm. Metab. Res. 2008, 40, 442–445. [Google Scholar] [CrossRef]
- Miao, C.; Tang, Z.-W.; Nie, F. Epicardial adipose tissue in heart failure: A promising therapeutic target. Int. J. Cardiol. 2023, 371, 297. [Google Scholar] [CrossRef]
- Lv, S.; Liu, Y.; Zou, Z.; Li, F.; Zhao, S.; Shi, R.; Bian, R.; Tian, H. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: A meta-analysis. Ann. Rheum. Dis. 2015, 33, 69–76. [Google Scholar]
- Gómez-Garre, D.; González-Rubio, M.L.; Muñoz-Pacheco, P.; Caro-Vadillo, A.; Aragoncillo, P.; Fernández-Cruz, A. Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur. J. Heart Fail. 2010, 12, 903–912. [Google Scholar] [CrossRef]
- Parisi, V.; Petraglia, L.; D’Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; et al. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef]
- Preiss, D.; Campbell, R.T.; Murray, H.M.; Ford, I.; Packard, C.J.; Sattar, N.; Rahimi, K.; Colhoun, H.M.; Waters, D.D.; LaRosa, J.C.; et al. The effect of statin therapy on heart failure events: A collaborative meta-analysis of unpublished data from major randomized trials. Eur. Heart J. 2015, 36, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, J.; Apetrei, E.; Barrios, V.; Boehm, M.; Cleland, J.G.F.; Cornel, J.H.; Dunselman, P.; Fonseca, C.; Goudev, A.; Grande, P.; et al. Rosuvastatin in Older Patients with Systolic Heart Failure. N. Engl. J. Med. 2007, 357, 2248–2261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Wu, Y.; Jia, L.; Wang, J.; Xie, B.; Hui, M.; Du, J. 5TNF-alpha and IL-1beta neutralization ameliorates angiotensin II-induced cardiac damage in male mice. Endocrinology 2014, 155, 2677–2687. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy with Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef]
- Mann, D.L.; McMurray, J.J.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T.; Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003, 107, 3133–3140. [Google Scholar]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Monzo, L.; Ferrari, I.; Cicogna, F.; Tota, C.; Calo, L. What proportion of patients with heart failure and preserved ejection fraction are eligible for empagliflozin? J. Cardiovasc. Med. 2022, 23, 567–569. [Google Scholar] [CrossRef]
- Monzo, L.; Ferrari, I.; Cicogna, F.; Tota, C.; Calò, L. Sodium–glucose co-transporter-2 inhibitors eligibility in patients with heart failure with reduced ejection fraction. Int. J. Cardiol. 2021, 341, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Shiou, Y.-L.; Jhuo, S.-J.; Chang, C.-Y.; Liu, P.-L.; Jhuang, W.-J.; Dai, Z.-K.; Chen, W.-Y.; Chen, Y.-F.; Lee, A.-S. The sodium–glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc. Diabetol. 2019, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Parajuli, N.; Levasseur, J.L.; Boisvenue, J.; Beker, D.L.; Masson, G.; Fedak, P.W.; Verma, S.; Dyck, J.R. Empagliflozin Prevents Worsening of Cardiac Function in an Experimental Model of Pressure Overload-Induced Heart Failure. JACC Basic Transl. Sci. 2017, 2, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Kishi, S.; Fuse, K.; Fujita, S.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Sato, M.; et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc. Diabetol. 2018, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Requena-Ibanez, J.A.; Santos-Gallego, C.G.; Rodriguez-Cordero, A.; Vargas-Delgado, A.P.; Mancini, D.; Sartori, S.; Atallah-Lajam, F.; Giannarelli, C.; Macaluso, F.; Lala, A.; et al. Mechanistic Insights of Empagliflozin in Nondiabetic Patients with HFrEF: From the EMPA-TROPISM Study. JACC Heart Fail. 2021, 9, 578–589. [Google Scholar] [CrossRef]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability Among Patients with Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hanselmann, A.; Nilsson, B.; Moller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Dozio, E.; Vianello, E.; Malavazos, A.E.; Tacchini, L.; Schmitz, G.; Iacobellis, G.; Corsi Romanelli, M.M. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: A target to modulate cardiovascular risk? Int. J. Cardiol. 2019, 292, 218–224. [Google Scholar] [CrossRef]
- Iacobellis, G.; Fricke, A.C.V. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J. Endocr. Soc. 2020, 4, bvz042. [Google Scholar] [CrossRef]
- Queen, N.J.; Bates, R.; Huang, W.; Xiao, R.; Appana, B.; Cao, L. Visceral adipose tissue-directed FGF21 gene therapy improves metabolic and immune health in BTBR mice. Mol. Ther. Methods Clin. Dev. 2021, 20, 409–422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).