Conformational Changes of Acyl Carrier Protein Switch the Chain Length Preference of Acyl-ACP Thioesterase ChFatB2
Abstract
:1. Introduction
2. Results
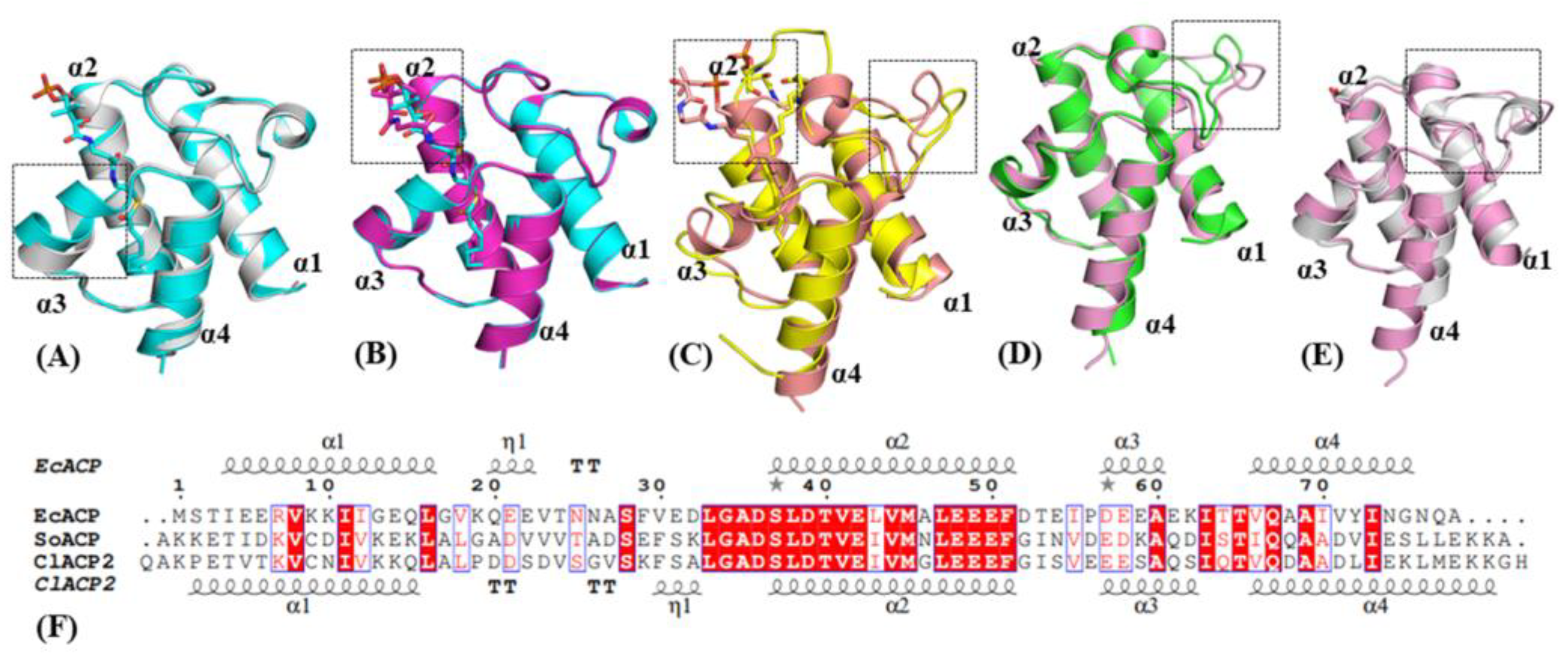
2.1. Structure and Sequence Comparison of ACPs from Different Species
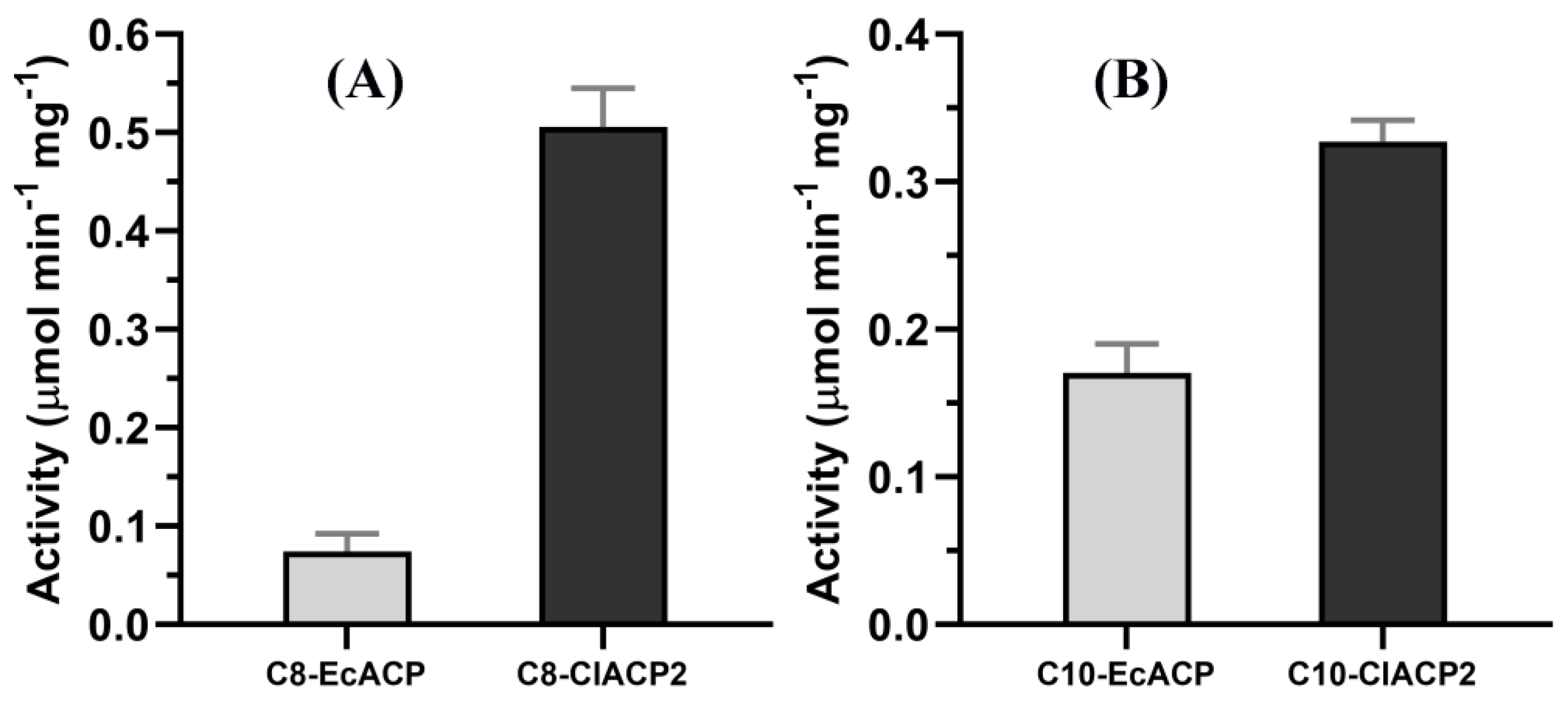
2.2. ChFatB2 Prefers ClACP2 to EcACP as the Acyl Carrier
2.3. Kinetic Parameters of ChFatB2 with Acyl-ACPs
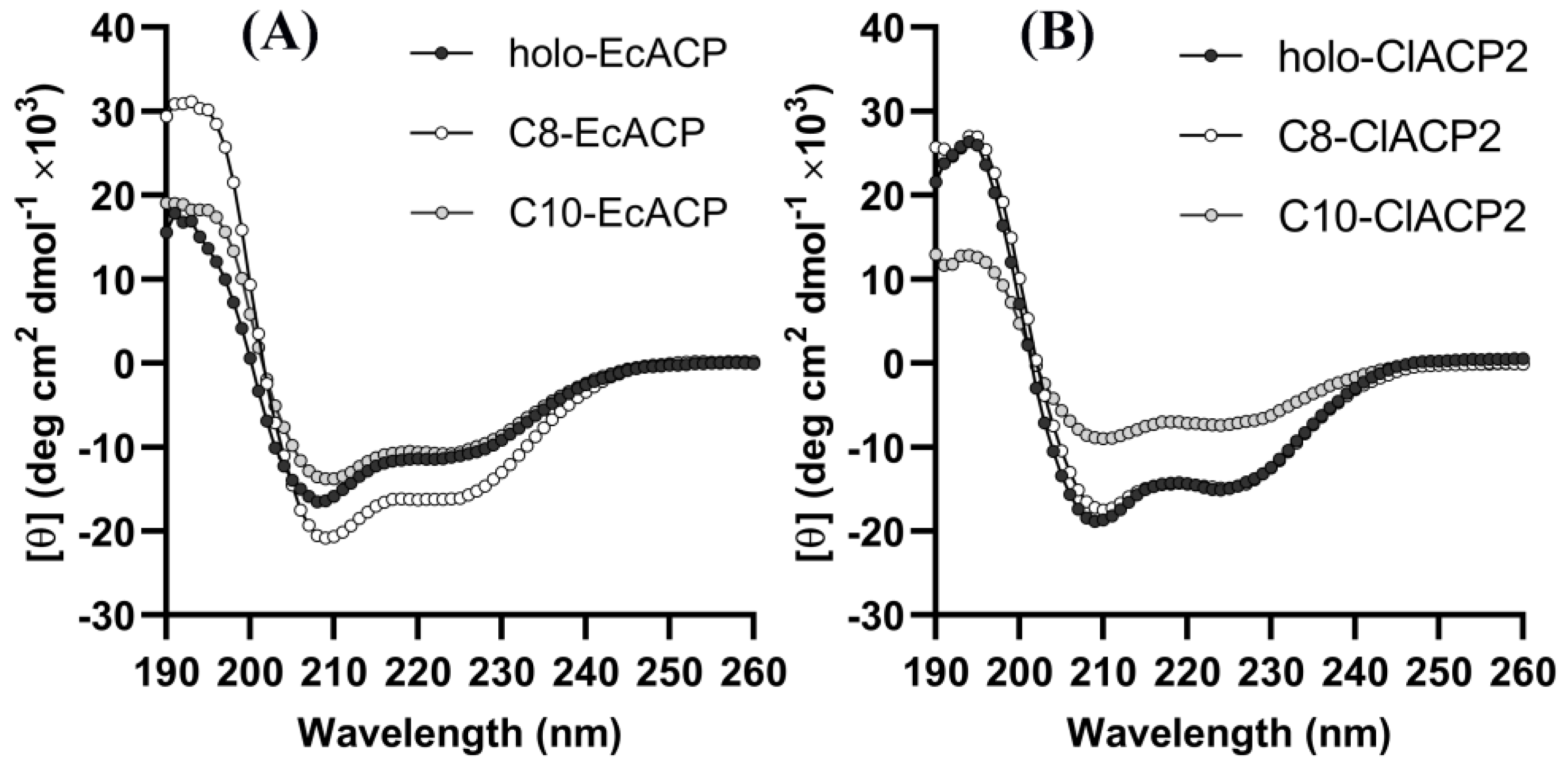
2.4. CD Spectra Results Reveal the Conformational Changes of the Acyl-ACPs
2.5. The Thermal Denaturation Measurements of the ACPs
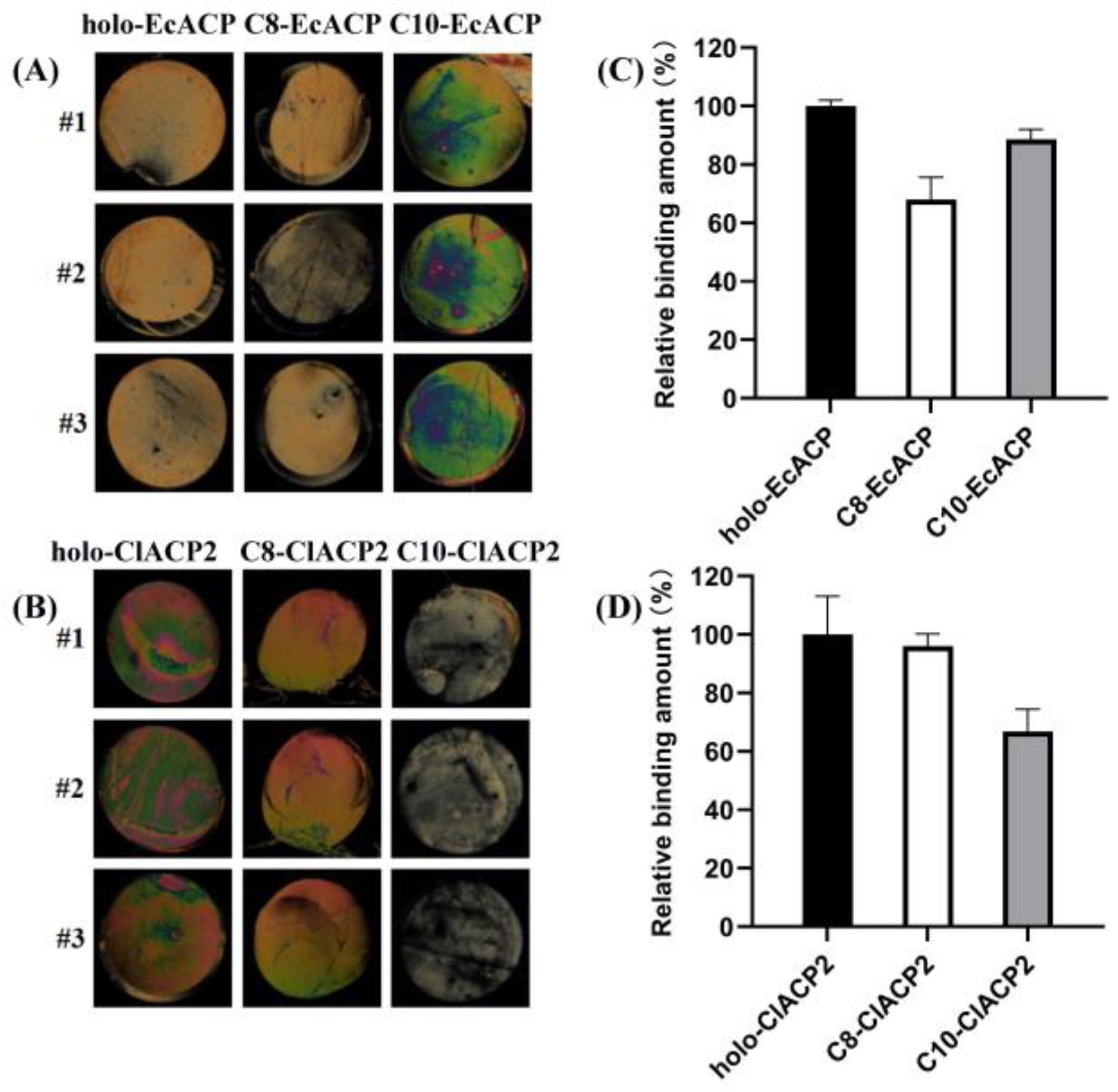
2.6. Binding Affinity of ChFatB2 with the ACPs Determined by Liquid Crystal Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Materials
4.2. Structure and Sequence Comparison of the ACPs
4.3. Protein Expression and Purification
4.4. Synthesis of Acyl-ACPs
4.5. HPLC Analysis of Acyl-ACPs
4.6. Enzymatic Activity for the Thioesterase ChFatB2
4.7. Circular Dichroism Measurements of the ACPs
4.8. The Liquid Crystal Assay of ChFatB2 and the ACPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Q.; Cordell, W.T.; Jindra, M.A.; Courtney, D.K.; Kuckuk, M.K.; Chen, X.Q.; Pfleger, B.F. Metabolic engineering strategies to produce medium-chain oleochemicals via acyl-ACP:CoA transacylase activity. Nat. Commun. 2022, 13, 1619. [Google Scholar] [CrossRef]
- Sarria, S.; Kruyer, N.S.; Peralta-Yahya, P. Microbial synthesis of medium-chain chemicals from renewables. Nat. Biotechnol. 2017, 35, 1158–1166. [Google Scholar] [CrossRef]
- Heil, C.S.; Wehrheim, S.S.; Paithankar, K.S.; Grininger, M. Fatty Acid Biosynthesis: Chain-Length Regulation and Control. Chembiochem 2019, 20, 2298–2321. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Hu, Y.T.; Teixeira, P.G.; Pereira, R.; Chen, Y.; Siewers, V.; Nielsen, J. Multidimensional engineering of Saccharomyces cerevisiae for efficient synthesis of medium-chain fatty acids. Nat. Catal. 2020, 3, 64–74. [Google Scholar] [CrossRef]
- Yan, Q.; Pfleger, B.F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 2020, 58, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.F.; Shi, T.Q.; Lin, L.; Wei, P.; Ledesma-Amaro, R.; Ji, X.J. Engineering Yarrowia lipolytica to Produce Tailored Chain-Length Fatty Acids and Their Derivatives. ACS Synth. Biol. 2022, 11, 2564–2577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.W.; Zhou, Y.J.J.; Krivoruchko, A.; Grininger, M.; Zhao, Z.B.K.; Nielsen, J. Expanding the product portfolio of fungal type I fatty acid synthases. Nat. Chem. Biol. 2017, 13, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Sun, Y.; Wang, X.D.; Xue, J.A.; Wang, J.P.; Jia, X.Y.; Li, R.Z. Identification and Functional Characterization of Acyl-ACP Thioesterases B (GhFatBs) Responsible for Palmitic Acid Accumulation in Cotton Seeds. Int. J. Mol. Sci. 2022, 23, 12805. [Google Scholar] [CrossRef] [PubMed]
- Grisewood, M.J.; Hernandez-Lozada, N.J.; Thoden, J.B.; Gifford, N.P.; Mendez-Perez, D.; Schoenberger, H.A.; Allan, M.F.; Floy, M.E.; Lai, R.Y.; Holden, H.M. Computational Redesign of Acyl-ACP Thioesterase with Improved Selectivity toward Medium-Chain-Length Fatty Acids. ACS Catal. 2017, 7, 3837–3849. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Chen, L.Q.; Hei, M.H.; Liu, T.G.; Feng, Y.; Yang, G.Y. Structure-guided reshaping of the acyl binding pocket of ‘TesA thioesterase enhances octanoic acid production in E. coli. Metab. Eng. 2020, 61, 24–32. [Google Scholar] [CrossRef]
- Jing, F.Y.; Zhao, L.; Yandeau-Nelson, M.D.; Nikolau, B.J. Two distinct domains contribute to the substrate acyl chain length selectivity of plant acyl-ACP thioesterase. Nat. Commun. 2018, 9, 860. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.B.; Wang, Y.Y.; Liu, J.; Liu, Y.H.; Cao, X.P.; Xue, S. Structural Insight into Acyl-ACP Thioesterase toward Substrate Specificity Design. ACS Chem. Biol. 2017, 12, 2830–2836. [Google Scholar] [CrossRef]
- Sarria, S.; Bartholow, T.G.; Verga, A.; Burkart, M.D.; Peralta-Yahya, P. Matching Protein Interfaces for Improved Medium-Chain Fatty Acid Production. ACS Synth. Biol. 2018, 7, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Mains, K.; Peoples, J.; Fox, J.M. Kinetically guided, ratiometric tuning of fatty acid biosynthesis. Metab. Eng. 2022, 69, 209–220. [Google Scholar] [CrossRef]
- Kalinger, R.S.; Rowland, O. Determinants of substrate specificity in a catalytically diverse family of acyl-ACP thioesterases from plants. BMC Plant Biol. 2023, 23, 1. [Google Scholar] [CrossRef]
- Beld, J.; Cang, H.; Burkart, M.D. Visualizing the Chain-Flipping Mechanism in Fatty-Acid Biosynthesis. Angew. Chem. Int. Ed. 2014, 53, 14456–14461. [Google Scholar] [CrossRef] [Green Version]
- Cronan, J.E. The chain-flipping mechanism of ACP (acyl carrier protein)-dependent enzymes appears universal. Biochem. J. 2014, 460, 157–163. [Google Scholar] [CrossRef]
- Blatti, J.L.; Beld, J.; Behnke, C.A.; Mendez, M.; Mayfield, S.P.; Burkart, M.D. Manipulating Fatty Acid Biosynthesis in Microalgae for Biofuel through Protein-Protein Interactions. PLoS ONE 2012, 7, e42949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.W.M.; Lee, Y.K. Expression of the heterologous Dunaliella tertiolecta fatty acyl-ACP thioesterase leads to increased lipid production in Chlamydomonas reinhardtii. J. Biotechnol. 2017, 247, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Roujeinikova, A.; Simon, W.J.; Gilroy, J.; Rice, D.W.; Rafferty, J.B.; Slabas, A.R. Structural studies of fatty acyl-(acyl carrier protein) thioesters reveal a hydrophobic binding cavity that can expand to fit longer substrates. J. Mol. Biol. 2007, 365, 135–145. [Google Scholar] [CrossRef]
- Lennen, R.M.; Braden, D.J.; West, R.M.; Dumesic, J.A.; Pfleger, B.F. A Process for Microbial Hydrocarbon Synthesis: Overproduction of Fatty Acids in Escherichia coli and Catalytic Conversion to Alkanes. Biotechnol. Bioeng. 2010, 106, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.Y.; Cantu, D.C.; Tvaruzkova, J.; Chipman, J.P.; Nikolau, B.J.; Yandeau-Nelson, M.D.; Reilly, P.J. Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC Biochem. 2011, 12, 44. [Google Scholar] [CrossRef] [Green Version]
- Ziesack, M.; Rollins, N.; Shah, A.; Dusel, B.; Webster, G.; Silver, P.A.; Way, J.C. Chimeric Fatty Acyl-Acyl Carrier Protein Thioesterases Provide Mechanistic Insight into Enzyme Specificity and Expression. Appl. Environ. Microbiol. 2018, 84, e02868-17. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.B.; Zhang, Y.X.; Wang, Y.Y.; Liu, J.; Liu, Y.H.; Cao, X.P.; Xue, S. Tuning of acyl-ACP thioesterase activity directed for tailored fatty acid synthesis. Appl. Microbiol. Biotechnol. 2018, 102, 3173–3182. [Google Scholar] [CrossRef]
- Hartono, D.; Xue, C.Y.; Yang, K.L.; Yung, L.Y.L. Decorating Liquid Crystal Surfaces with Proteins for Real-Time Detection of Specific Protein-Protein Binding. Adv. Funct. Mater. 2009, 19, 3574–3579. [Google Scholar] [CrossRef]
- Xue, C.Y.; Khan, S.A.; Yang, K.L. Exploring Optical Properties of Liquid Crystals for Developing Label-Free and High-Throughput Microfluidic Immunoassays. Adv. Mater. 2009, 21, 198–202. [Google Scholar] [CrossRef]
- Xue, C.Y.; Yang, K.L. Dark-to-bright optical responses of liquid crystals supported on solid surfaces decorated with proteins. Langmuir 2008, 24, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.I.; Stockner, T.; Tieleman, D.P.; Vogel, H.J. Molecular Dynamics Simulations of the Apo-, Holo-, and Acyl-forms of Escherichia coli Acyl Carrier Protein. J. Biol. Chem. 2008, 283, 33620–33629. [Google Scholar] [CrossRef] [Green Version]
- Bartholow, T.G.; Sztain, T.; Patel, A.; Lee, D.J.; Young, M.A.; Abagyan, R.; Burkart, M.D. Elucidation of transient protein-protein interactions within carrier protein-dependent biosynthesis. Commun. Biol. 2021, 4, 340. [Google Scholar] [CrossRef] [PubMed]
- Mains, K.; Fox, J.M. Ketosynthase mutants enable short-chain fatty acid biosynthesis in E. coli. Metab. Eng. 2023, 77, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.A.; Choe, K.; Chowdhury, R.; Kong, R.; Ghaffari, S.; Sweedler, J.V.; Pfleger, B.F. Evaluation of strategies to narrow the product chain-length distribution of microbially synthesized free fatty acids. Metab Eng 2023, 77, 21–31. [Google Scholar] [CrossRef]
- Aznar-Moreno, J.A.; Venegas-Caleron, M.; Martinez-Force, E.; Garces, R.; Salas, J.J. Acyl carrier proteins from sunflower (Helianthus annuus L.) seeds and their influence on FatA and FatB acyl-ACP thioesterase activities. Planta 2016, 244, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zornetzer, G.A.; Fox, B.G.; Markley, J.L. Solution structures of spinach acyl carrier protein with decanoate and stearate. Biochemistry 2006, 45, 5217–5227. [Google Scholar] [CrossRef] [Green Version]
- Miyanaga, A.; Iwasawa, S.; Shinohara, Y.; Kudo, F.; Eguchi, T. Structure-based analysis of the molecular interactions between acyltransferase and acyl carrier protein in vicenistatin biosynthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 1802–1807. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Jang, C.H. Highly sensitive and selective glucose sensor based on ultraviolet-treated nematic liquid crystals. Biosens. Bioelectron. 2014, 59, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.D.; Hussain, Z.; Liaqat, U.; Arif, D.; Yang, K.L. Liquid Crystal Based Binding Assay for Detecting HIV-1 Surface Glycoprotein. Front. Chem. 2021, 9, 668870. [Google Scholar] [CrossRef]
- Liu, T.G.; Vora, H.; Khosla, C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab. Eng. 2010, 12, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [Green Version]
- Hickman, T.W.P.; Baud, D.; Benhamou, L.; Hailes, H.C.; Ward, J.M. Characterisation of four hotdog-fold thioesterases for their implementation in a novel organic acid production system. Appl. Microbiol. Biotechnol. 2020, 104, 4397–4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, A.J.; Ramalli, S.G.; Wallace, B.A. DichroWeb, a website for calculating protein secondary structure from circular dichroism spectroscopic data. Protein Sci. 2022, 31, 37–46. [Google Scholar] [CrossRef]

| C8-EcACP | C10-EcACP | C10-EcACP Preference (a) | C8-ClACP2 | C10-ClACP2 | C8-ClACP2 Preference (b) | |
|---|---|---|---|---|---|---|
| Km (µM) | 349.5 ± 66.5 | 185.7 ± 40.5 | - | 216.7 ± 49.9 | 263.1 ± 30.9 | - |
| kcat (min−1) | 1.38 ± 0.09 | 2.16 ± 0.23 | - | 6.36 ± 0.51 | 5.64 ± 0.32 | - |
| kcat/Km (µM−1 min−1) | 3.95 × 10−3 | 11.6 × 10−3 | 2.94 | 29.4 × 10−3 | 21.4 × 10−3 | 1.37 |
| Protein | α-helix (%) | Turns (%) | Unordered (%) | Relative Change (%) (a) |
|---|---|---|---|---|
| holo-EcACP | 54.9 ± 3.1 | 12.1 ± 1.7 | 31.5 ± 2.5 | 0 |
| C8-EcACP | 73.8 ± 1.1 | 8.9 ± 0.9 | 17.2 ± 0.5 | +34.4 |
| C10-EcACP | 51.2 ± 1.3 | 14.3 ± 1.6 | 30.6 ± 2.4 | −6.7 |
| holo-ClACP2 | 68.9 ± 0.1 | 10.1 ± 0.8 | 20.4 ± 1.2 | 0 |
| C8-ClACP2 | 67.3 ± 1.1 | 9.1 ± 1.0 | 23.1 ± 2.2 | −2.3 |
| C10-ClACP2 | 37.5 ± 0.3 | 15.6 ± 1.7 | 33.0 ± 0.9 | −45.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Yang, Y.; Yang, M.; Ren, J.; Xue, C.; Feng, Y.; Xue, S. Conformational Changes of Acyl Carrier Protein Switch the Chain Length Preference of Acyl-ACP Thioesterase ChFatB2. Int. J. Mol. Sci. 2023, 24, 6864. https://doi.org/10.3390/ijms24076864
Yang T, Yang Y, Yang M, Ren J, Xue C, Feng Y, Xue S. Conformational Changes of Acyl Carrier Protein Switch the Chain Length Preference of Acyl-ACP Thioesterase ChFatB2. International Journal of Molecular Sciences. 2023; 24(7):6864. https://doi.org/10.3390/ijms24076864
Chicago/Turabian StyleYang, Tianxiang, Yunlong Yang, Ming Yang, Jiangang Ren, Changying Xue, Yanbin Feng, and Song Xue. 2023. "Conformational Changes of Acyl Carrier Protein Switch the Chain Length Preference of Acyl-ACP Thioesterase ChFatB2" International Journal of Molecular Sciences 24, no. 7: 6864. https://doi.org/10.3390/ijms24076864
APA StyleYang, T., Yang, Y., Yang, M., Ren, J., Xue, C., Feng, Y., & Xue, S. (2023). Conformational Changes of Acyl Carrier Protein Switch the Chain Length Preference of Acyl-ACP Thioesterase ChFatB2. International Journal of Molecular Sciences, 24(7), 6864. https://doi.org/10.3390/ijms24076864








