Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Control and Study Group
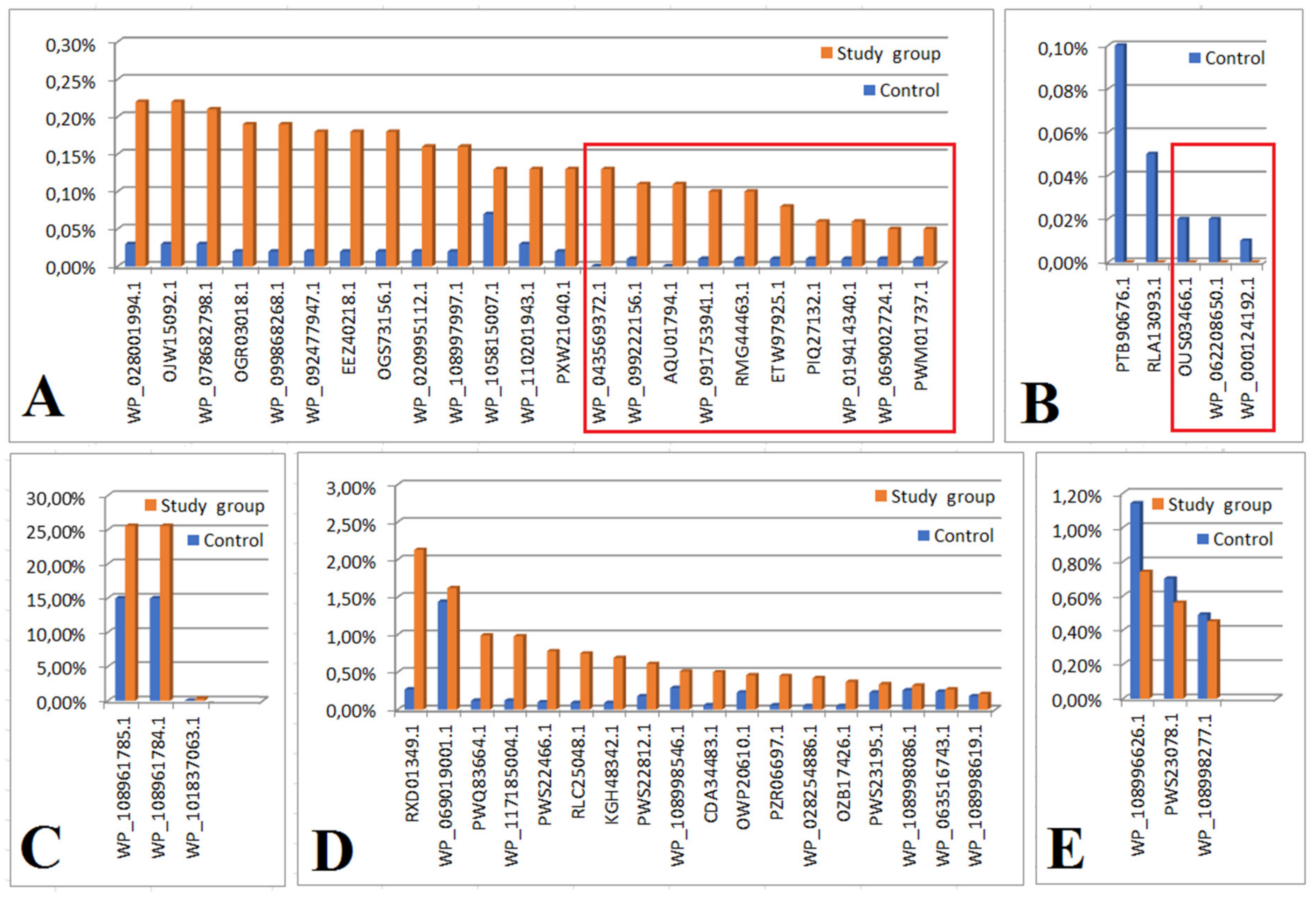
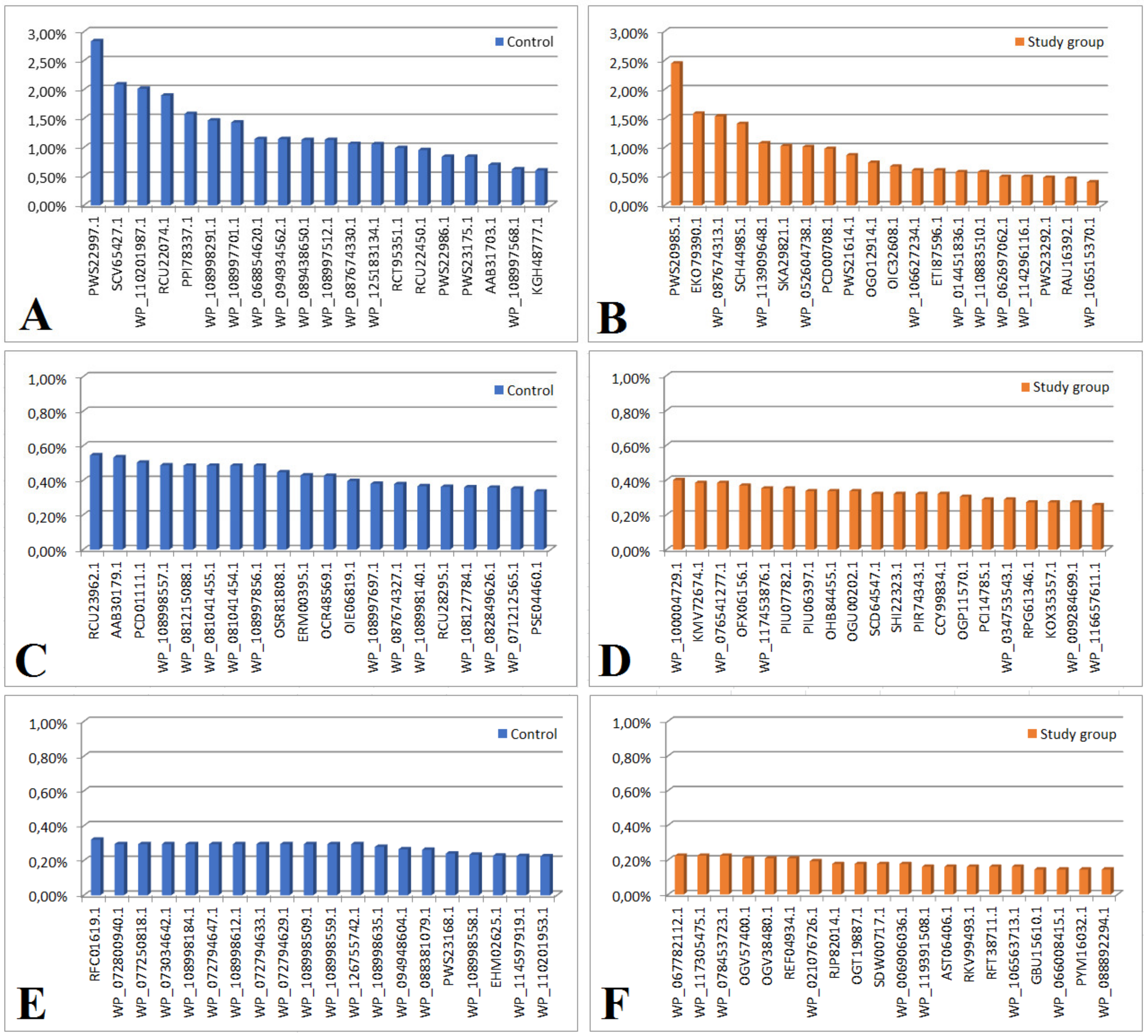
2.2. Analysis of the Bacterial Proteome in the Study and Control Groups
3. Discussion
4. Materials and Methods
4.1. Identification of Proteins Using LC-ESI-MS/MS
4.1.1. Protein Extraction
4.1.2. Mass Spectrometry Examination
4.1.3. Identification Method for Proteins
4.1.4. Quantitative Evaluation
4.2. Statistical Analysis
4.3. Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADABA | circularization of gamma-N-acetyl-alpha: gamma-diaminobutyric acid |
| APGAR | appearance, pulse, grimace, activity, respiration score |
| BMI | body mass index |
| CRL | crown–rump length |
| CPR | cerebroplacental ratio |
| EFW | estimated fetal weight |
| emPAI | Exponentially Modified Protein Abundance Index |
| FGR | fetal growth restriction |
| HMP | Human Microbiome Project |
| NGS | next-generation sequencing |
| OPPsP | opportunistic premise plumbing pathogen |
| PE | preeclampsia |
| PI | pulsation index |
| RI | resistance index |
| TNF | tumor necrosis factor |
| TORCH | toxoplasmosis, other, rubella, cytomegalic and herpes infection |
References
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr. Endocrinol. Rev. 2009, 6 (Suppl. 3), 332–336. [Google Scholar]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Júnior, E.A. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obs. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kamphof, H.D.; Posthuma, S.; Gordijn, S.J.; Wessel, G. Fetal Growth Restriction: Mechanisms, Epidemiology, and Management. Matern.-Fetal Med. 2022, 4, 186–196. [Google Scholar] [CrossRef]
- Chew, L.C.; Verma, R.P. Fetal Growth Restriction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562268/ (accessed on 5 March 2023).
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152 (Suppl. 1), 3–57. [Google Scholar] [CrossRef]
- Maulik, D. Fetal Growth Restriction: The Etiology. Clin. Obstet. Gynecol. 2006, 49, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Borghesi, A.; Tzialla, C.; Stroti, M. IUGR and infections. Early Hum. Dev. 2014, 90 (Suppl. 1), S42–S44. [Google Scholar] [CrossRef]
- Germain, M.; Krohn, M.A.; Hillier, S.L.; Eschenbach, D.A. Genital flora in pregnancy and its association with intrauterine growth retardation. J. Clin. Microbiol. 1994, 32, 2162–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Gonzalez, S.; Ortiz-Arrabal, O.; Torrecillas, A.; Pérez-Cruz, M.; Chueca, N.; Gómez-Roig, M.D.; Gómez-Llorente, C. Study of the Fetal and Maternal Microbiota in Pregnant Women with Intrauterine Growth Restriction and Its Relationship with Inflammatory Biomarkers: A Case-Control Study Protocol (SPIRIT Compliant). Medicine 2020, 99, e22722. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Ruohtula, T.; de Goffau, M.C.; Nieminen, J.K.; Honkanen, J.; Siljander, H.; Hämäläinen, A.; Peet, A.; Tillmann, V.; Ilonen, J.; Niemelä, O.; et al. Maturation of Gut Microbiota and Circulating Regulatory T Cells and Development of IgE Sensitization in Early Life. Front. Immunol. 2019, 10, 2494. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Clark, S.E.; Mulligan, C.E.; Janssen, R.C.; Babcock, L.; Ir, D.; Young, B.; Krebs, N.; Lemas, D.J.; Johnson, L.K.; et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat. Commun. 2018, 9, 4462, Erratum in Nat. Commun. 2019, 10, 2965. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.nature.com/subjects/microbiome (accessed on 30 March 2023).
- Whipps, J.M.; Lewis, K.; Cooke, R.C. Mycoparasitism and plant disease control. In Fungi in Biological Control Systems, ed 2001; Manchester University Press: Manchester, NH, USA, 1988; pp. 161–187. [Google Scholar]
- Bolte, E.E.; Moorshead, D.; Aagaard, K.M. Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med. 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://www.commonfund.nih.gov/hmp (accessed on 30 March 2023).
- Sirota, I.; Zarek, S.M.; Segars, J.H. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin. Reprod. Med. 2014, 32, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Verstraelen, H.; Vieira-Baptista, P.; De Seta, F.; Ventolini, G.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: I. Research Development, Lexicon, Defining "Normal" and the Dynamics Throughout Women’s Lives. J. Low. Genit. Tract Dis. 2022, 26, 73–78. [Google Scholar] [CrossRef]
- Cariño, R., 3rd; Takayasu, L.; Suda, W.; Masuoka, H.; Hirayama, K.; Konishi, S.; Umezaki, M. The search for aliens within us: A review of evidence and theory regarding the fetal microbiome. Crit. Rev. Microbiol. 2022, 48, 611–623. [Google Scholar] [CrossRef]
- Hummel, G.L.; Austin, K.; Cunningham-Hollinger, H.C. Comparing the maternal-fetal microbiome of humans and cattle: A translational assessment of the reproductive, placental, and fetal gut microbiomes. Biol. Reprod. 2022, 107, 371–381. [Google Scholar] [CrossRef]
- Hayward, C.E.; Jones, R.L.; Sibley, C.P. Mechanisms of transfer across the human placenta. In Fetal and Neonatal Physiology, 5th ed.; Polin, R.A., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 121–133. [Google Scholar]
- Gęca, T.; Stupak, A.; Nawrot, R.; Goździcka-Józefiak, A.; Kwaśniewska, A.; Kwaśniewski, W. Placental proteome in late-onset of fetal growth restriction. Mol. Med. Rep. 2022, 26, 356. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Zakis, D.R.; Paulissen, E.; Kornete, L.; Kaan, A.; Nicu, E.A.; Zaura, E. The evidence for placental microbiome and its composition in healthy pregnancies: A systematic review. J. Reprod. Immunol. 2022, 149, 103455. [Google Scholar] [CrossRef] [PubMed]
- Altemani, F.; Barrett, H.L.; Gomez-Arango, L.; Josh, P.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Tyson, G.W.; Nitert, M.D. Pregnant Women Who Develop Preeclampsia Have Lower Abundance of the Butyrate-Producer Coprococcus in Their Gut Microbiota. Pregnancy Hypertens. 2021, 23, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Gao, L.; Zou, X.; Zhang, Y.; Zheng, Z.; Zhang, X.; Li, J.; Tian, Z.; Wang, X.; Gu, J.; et al. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ. Res. 2022, 131, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci. Rep. 2017, 7, 2860. [Google Scholar] [CrossRef] [Green Version]
- Nitert, M.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Wilkinson, S.; Lingwood, B.; Tobin, J.M.; McSweeney, C.; O’Rourke, P.; McIntyre, H.; et al. SPRING: An RCT study of probiotics in the prevention of gestational diabetes mellitus in overweight and obese women. BMC Pregnancy Childbirth 2013, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Den Hollander, W.J.; Schalekamp-Timmermans, S.; Holster, I.L.; Jaddoe, V.W.; Hofman, A.; Moll, H.A.; Perez-Perez, G.I.; Blaser, M.J.; Steegers, E.A.P.; Kuipers, E.J. Helicobacter Pylori Colonization and Pregnancies Complicated by Preeclampsia, Spontaneous Prematurity, and Small for Gestational Age Birth. Helicobacter 2017, 22, e12364. [Google Scholar] [CrossRef] [Green Version]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the Preterm Infant Gut Microbiome: A Research Priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hou, L.; Wang, J.; Xiao, L.; Zhang, J.; Yin, N.; Yao, S.; Cheng, K.; Zhang, W.; Shi, Z.; et al. Unfavourable Intrauterine Environment Contributes to Abnormal Gut Microbiome and Metabolome in Twins. Gut 2022, 71, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Duan, C.; Lin, B.; Li, K.; Gao, J.; Yan, H.; Wang, K.; Zhao, Z. Characteristics of the Gut Microbiota in Pregnant Women with Fetal Growth Restriction. BMC Pregnancy Childbirth 2022, 22, 297. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Benny, P.; Wang, M.; Ma, Y.; Lambertini, L.; Peter, I.; Xu, Y.; Lee, M.J. Intrauterine Growth Restriction Is Associated with Unique Features of the Reproductive Microbiome. Reprod. Sci. 2021, 28, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.uniprot.org/uniprotkb/A0A099D2A0/entry (accessed on 30 March 2023).
- Zhao, L.-X.; Huang, S.-X.; Tang, S.-K.; Jiang, C.-L.; Duan, Y.; Beutler, J.A.; Henrich, C.J.; McMahon, J.B.; Schmid, T.; Blees, J.S.; et al. Actinopolysporins AC and tubercidin as a Pdcd4 stabilizer from the halophilic actinomycetes Actinopolyspora erythraea YIM 90600. J. Nat. Prod. 2011, 74, 1990–1995. [Google Scholar] [CrossRef] [Green Version]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; Van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [Green Version]
- Szwetkowski, K.J.; Falkinham, J.O., III. Methylobacterium spp. as Emerging Opportunistic Premise Plumbing Pathogens. Pathogens 2020, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Gschwind, R.; Fournier, T.; Kennedy, S.; Tsatsaris, V.; Cordier, A.G.; Barbut, F.; Butel, M.J.; Wydawu-Dematteis, S. Evidence for contamination as the origin for bacteria found in human placenta rather than a microbiota. PLoS ONE 2020, 15, e0237232. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: Longitudinal reference ranges and terms for serial measurements. Ultrasound Obs. Gynecol. 2007, 30, 287–296. [Google Scholar] [CrossRef]
- Jugović, D.; Tumbri, J.; Medić, M.; Jukić, M.K.; Kurjak, A.; Arbeille, P.; Salihagić-Kadić, A. New Doppler index for prediction of perinatal brain damage in growth-restricted and hypoxic fetuses. Ultrasound Obs. Gynecol. 2007, 30, 303–311. [Google Scholar] [CrossRef]
- Karpievitch, Y.V.; Polpitiya, A.D.; Anderson, G.A.; Smith, R.D.; Dabney, A.R. Liquid Chromatography Mass Spectrometry-Based Proteomics: Biological and Technological Aspects. Ann. Appl. Stat. 2010, 4, 1797–1823. [Google Scholar] [CrossRef] [PubMed]
- Diffley, J.F.; BStillman, B. Similarity between the transcriptional silencer binding proteins ABF1 and RAP1. Science 1998, 246, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, R.; Kalinowski, A.; Goździcka-Józefiak, A. Proteomic analysis of Chelidonium majus milky sap using two-dimensial gel electrophoresis and tandem mass spectrometry. Phyochemistry 2007, 68, 1612–1622. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
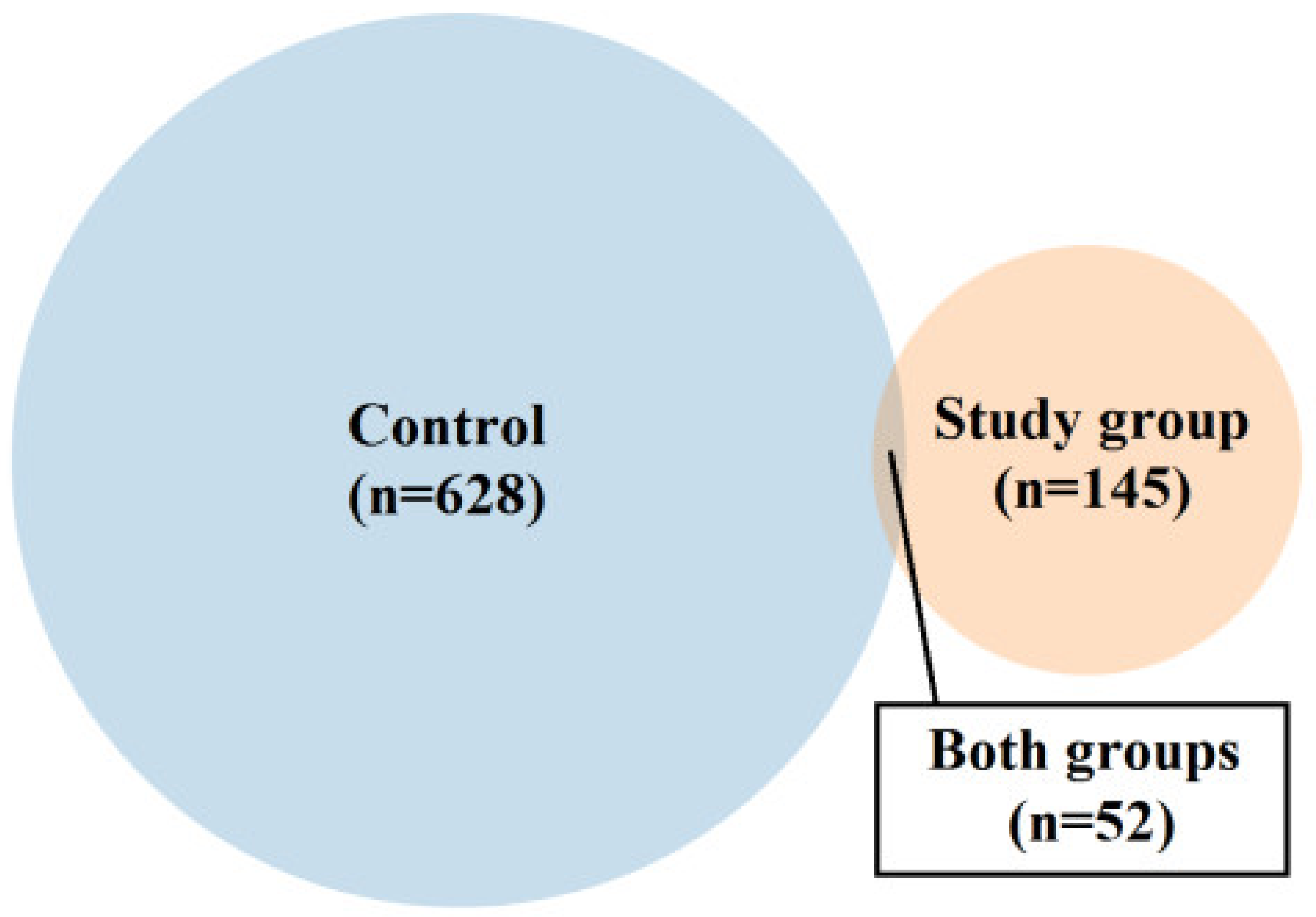
| Control Group N = 18 Median (Range) | Study Group (FGR) N = 18 | p-Value | |
|---|---|---|---|
| Baseline characteristics | |||
| age (years) | 30.2 ± 6.5 | 28.2 ± 5.6 | 0.466 |
| height (m) | 1.7 ± 0.06 | 1.67 ± 0.08 | 0.373 |
| Actual weight (kg) | 80.3 ± 11.3 | 79.5 ± 7 | 0.854 |
| Weight before pregnancy (kg) | 63.6 ± 11.3 | 66.7 ± 6.3 | 0.458 |
| BMI before pregnancy (kg/m2) | 22 ± 3.5 | 23.9 ± 2.1 | 0.154 |
| weight gain (kg) | 14 (12–28) | 12.5 (11–15) | 0.032 * |
| weight of the placenta (g) | 515 ± 46 | 328 ± 53 | <0.001 * |
| Parity | 2 (1–4) | 1 (1–3) | 0.504 |
| Gestation | 2 (1–4) | 1.5 (1–4) | 0.699 |
| Perinatal outcomes | |||
| Gestational age at the delivery (weeks) | 39 (38–41) | 37 (35/4–40) | 0.002 * |
| Fetal weight at birth (g) | 3540 (2910–3890) | 2300 (1385–2570) | <0.001 * |
| neonatal length (cm) | 54 (47–57) | 48 (35–51) | 0.001 * |
| APGAR 1 min (points) | 9 (min. 8–max. 10) | 8 (min. 6–max. 9) | 0.002 * |
| APGAR 5 min (points) | 10 (min. 9–max. 10) | 10 (min. 6–max. 10) | 0.597 |
| Feto-placental Doppler before delivery | |||
| AU PI | 0.77 (0.72–0.91) | 1.11 (0.98–1.9) | <0.001 * |
| MCA PI | 1.44 ± 0.21 | 1.31 ± 0.22 | 0.191 |
| UTA PI | 0.79 ± 0.05 | 0.93 ± 0.17 | 0.025 * |
| CPR | 1.703 (1.48–2.444) | 0.995 (0.737–1.687) | <0.001 * |
| Accession number | Protein (Bacteria) |
| WP_043569372.1 | catalase (Actinopolyspora erythraea) |
| WP_099222156.1 | HTH domain-containing protein (Listeria costaricensis) |
| AQU01794.1 | glucose-6-phosphate isomerase (Escherichia coli) |
| WP_091753941.1 | AAA family ATPase (Methylobacterium sp. ap11) |
| RMG44463.1 | hypothetical protein D6718_10020 (Acidobacteria bacterium) |
| ETW97925.1 | hypothetical protein ETSY1_20835, partial (Candidatus entotheonella factor) |
| PIQ27132.1 | DNA polymerase I (Bacteroidetes bacterium CG18_big_fil_WC_8_21_14_2_50_41_14) |
| WP_019414340.1 | hypothetical protein (Paenisporosarcina sp. TG20) |
| WP_069002724.1 | DUF1631 family protein (Candidatus Thiodiazotropha endoloripes) |
| PWM01737.1 | hypothetical protein DBY05_04045 (Clostridiales bacterium) |
| WP_28001994.1 | shikimate dehydrogenase (Shinorhizobium arboris) |
| GCI 15092.1 | phytoene synthase (Mucilagini bacteria sp.) |
| WP_078682797.1 | dTDP-4dehydrorramnose reductase (Lentisphaerae bacteria GWF-2) |
| OGR030118.1 | hypothetical protein (Pararhizbium haloflavum) |
| WP_92477947.1 | A/G-specific adenine glycolase (Clostridium polysaccharoliticum) |
| EEZ40218.1 | derythrose-4-phosphate dehygrogenase (Photobacterium damselae subs daselae) |
| OGS73156.1 | flagellar biosynthesis protein F1hB (Gallionellales bacterium GWAZ) |
| WP_020995112.1 | hypothetical protein (Oxalobacter formigenes) |
| WP_108997997.1 | 4-hydroxybenzoate-3-monooxygenase (Salinibacterium sp.) |
| WP_105815007.1 | hypothetical protein (Mycobacterium tuberculosis) |
| WP_110201943 | protein disulfide isomerase (Kangiella sp.) |
| PXW21040 | activator of mannose operon (transcriptional terminatol) (Pantoea sp.) |
| Figure 2B | |
| OUS03466.1 | hypothetical protein A9Q86_00710 (Flavobacteriales bacterium 33_180_T64) |
| WP_062208650.1 | tryptophan--tRNA ligase (Aureimonas sp. AU12) |
| WP_000124192.1 | S8 family peptidase (Bacillus cereus) |
| PTB9-676.1 | hypothetical protein (Marvigra lubricoides) |
| RLA13093.1 | uracil DNA glucose (Gammaproteobacteria bacterium) |
| Figure 2C | |
| WP_108961785 | hypothetical protein (E. coli) |
| WP_108961784.1 | hypothetical protein (E. coli) |
| WP_101837063.1 | hypothetical protein (Klebsiella sp.) |
| Figure 2D | |
| RXD 01349.1 | ubiquitin (Splinomonassp) |
| WP_069019000.1 | actin cytoplasmic (Pseudoalteramonas sp.) |
| PWQ83644.1 | 30 Sribosomal oritein S15, partial (Stenoprophomonas maltophilla) |
| WP_1171850004.1 | hypothtical protein (Pseudomonas chorii) |
| PWS22466.1 | hypothtical protein PKP2260 (Enterococcus faecium) |
| RLC25048.1 | hypothetical protein DRX56 (Dettaproteobacteria bacterium) |
| KGH48342.1 | hypothetical protein GS19 (Acinetobacter baumans) |
| PWS22812.1 | hypothetical protein PKP2260 (Enterococcus faecium) |
| CDA34483.1 | Predicted DNA-binding protein withPD1 like DNA binding motif (Firmicutes bacterium CAG-536) |
| OWP2061.01 | hypothetical protein CBF 90 (Microbacterium sp.) |
| PZR06697.1 | hypothetical protein DI536 (Archangium gephora) |
| WP_028254886.1 | BREX-3-SYSTEMP loop-containing protein BrxF (Vellonella magna) |
| OZB17426.1 | ribosomal recycling factor (Hyphomonas sp.) |
| PWS233195.1 | hypothetical protein DKP78 (Enterococcus faecium) |
| WP_108998086.1 | NADP+ isocitrinate dehydrogenase (Escherichia coli) |
| WP_063516743.1 | molecular chaperone Htp G (Lactobacillus harninensis) |
| WP_108998619.1 | lactate dehydrogenase (E. coli) |
| Figure 2E | |
| WP_108996626.1 | hypothetical protein (Klebsiella pneumoniae) |
| PWS23078.1 | hypothetical protein (Enterococcus faecium) |
| WP_108998277.1 | malate dehydrogenase (E. coli) |
| Protein content > 1% | |
| PWS22997.1 | hypothetical protein DKP78_15465, partial (Enterococcus faecium) |
| RCU22074.1 | hypothetical protein DVA69_20680, partial (Acinetobacter baumannii) |
| WP_087674330.1 | MULTISPECIES: peptidylprolyl isomerase (Gammaproteobacteria) |
| SCV65427.1 | Core histone H2A/H2B/H3/H4 (Anaplasma phagocytophilum) |
| WP_110201987.1 | actin, cytoplasmic 2 (Kangiella spongicola) |
| PPI78337.1 | actin, cytoplasmic 2, partial (Marinobacter flavimaris) |
| WP_108998291.1 | 50S ribosomal protein P1 (E. coli) |
| WP_108997701.1 | F0F1 ATP synthase subunit beta (E. coli) |
| WP_068854620.1 | hypothetical protein (Klebsiella pneumoniae) |
| WP_094934562.1 | hypothetical protein (Klebsiella pneumoniae) |
| WP_089438650.1 | actin, cytoplasmic 2 (E. coli) |
| WP_108997512.1 | actin, cytoplasmic 2 (E. coli) |
| WP_125183134.1 | hypothetical protein, partial (Enterobacter hormaechei) |
| Figure 3B Protein content > 1% | |
| PWS20985.1 | hypothetical protein DKP78_25965, partial (Enterococcus faecium) |
| WP_087674313.1 | hypothetical protein (Pseudomonas syringae) |
| SCH44985.1 | Uncharacterized protein (uncultured Clostridium sp.) |
| PCD00708.1 | hypothetical protein CO192_04000, partial (Pseudomonas pelagia) |
| ECO79390.1 | hypothetical protein LEP1GSC068_2346 (Leptospira sp. Fiocruz LV3954) |
| WP_113909648.1 | hypothetical protein (Arcobacter sp. FW59) |
| SKA29821.1 | CheW-like domain-containing protein (Oceanospirillum multiglobuliferum) |
| WP_052604738.1 | hypothetical protein (Acidithrix ferrooxidans) |
| Figure 3C | |
| RCU23962.1 | Glu/Leu/Phe/Val dehydrogenase, partial (Acinetobacter baumannii) |
| AAB30179.1 | p105 = epidermal keratin type 1 intermediate filament protein homolog {29 kda fragment} (Mycoplasma, Peptide Partial, 24 aa) |
| PCD01111.1 | actin, cytoplasmic 2, partial (Pseudomonas pelagia) |
| WP_108998557.1 | pyruvate kinase, partial (E. coli) |
| WP_081215088.1 | hypothetical protein (Lactococcus lactis) |
| WP_081041455.1 | hypothetical protein (Lactococcus lactis) |
| WP_081041454.1 | hypothetical protein (Lactococcus lactis) |
| WP_1089977856.1 | malate dehydrogenase (E. coli) |
| OSR81808.1 | hypothetical protein BV331_05659 (Pseudomonas syringae pv. actinidiae) |
| ERM00395.1 | hypothetical protein Q644_05090 (Ochrobactrum intermedium 229E) |
| OCR48569.1 | hypothetical protein RJ97_26685, partial (Klebsiella pneumoniae) |
| OIE06819.1 | hypothetical protein A7L78_18910 (Acinetobacter baumannii) |
| WP_108997697.1 | fructose-bisphosphate aldolase class I, partial (Escherichia coli) |
| WP_087674327.1 | 50S ribosomal protein L10, partial (Pseudomonas syringae) |
| WP_108998140.1 | nucleoside-diphosphate kinase, partial (Escherichia coli) |
| RCU28295.1 | hypothetical protein DVA69_17570, partial (Acinetobacter baumannlii) |
| WP_108127784.1 | tropomyosin (Saccharospirillum mangrove) |
| WP_082849626.1 | molecular chaperone DnaK (Lactobacillus harbinensis) |
| WP_071212565.1 | 30S ribosomal protein S11, partial (Acinetobacter baumannii) |
| PSE04460.1 | hypothetical protein C7G98_18875, partial (Acinetobacter baumannii) |
| Figure 3D engraving | |
| WP_10004729.1 | integrase, partial (E. coli) |
| KMV72674.1 | hypothetical protein AI28_14165 (bacteria symbiont BFo1 of Frankliniella occidentalis) |
| WP_076541277.1 | DUF3833 domain-containing protein (Shewanella sp. UCD-KL21) |
| OFX06156.1 | ATP:cob (I) alamine adenosyltransferase (Alphaproteobacteria bacterium RIFCSPHIGHO2_12_FULL_63_12) |
| WP_117453876.1 | MULTISPECIES: hypothetical protein (Absiella) |
| PIU07782.1 | hydrolase (Methylobacterium sp. CG09_land_8_20_14_0_10_71_15) |
| PIU06397.1 | hypothetical protein COT56_11130 (Methylobacterium sp. CG09_land_8_20_14_0_10_71_15) |
| OHB84455.1 | hypothetical protein A3J73_04470 (Planctomycetes bacterium RIFCSPHIGHO2_02_FULL_38_41) |
| OGU00202.1 | thioredoxin peroxidase (Geobacteraceae bacterium GWC2_48_7) |
| SCD64547.1 | transcriptional regulator, TetR family (Streptomyces sp. di50b) |
| SHI22323.1 | NlpC/P60 family protein (Leeuwenhoekiella palythoae) |
| PIR74343.1 | hypothetical protein COU35_02740 (Candidatus Magasanikbacteria bacterium CG10_big_fil_rev_8_21_14_0_10_47_10) |
| CCY99834.1 | putative uncharacterized protein (Clostridium sp. CAG:793) |
| OGP11570.1 | metal-dependent hydrolase (Deltaproteobacteria bacterium GWA2_43_19) |
| PCI14785.1 | cell division ATP-binding protein FtsE (Thiotrichales bacterium) |
| WP_034753543.1 | phosphoglycolate phosphatase (Janthinobacterium liquid) |
| RPG61346.1 | lipoyl (octoyl) transferase LipB (Flavobacteriaceae bacterium TMED206) |
| KOX35357.1 | HAD family hydrolase (Saccharothrix sp. NRRL B-16348) |
| WP_009284699.1 | 3-hydroxyacyl-CoA dehydrogenase (Fibrisoma limit) |
| WP_116657611.1 | hydroxyacylglutathione hydrolase (Pseudomonas sp. NDM) |
| Figure 3E | |
| RFC01619.1 | hypothetical protein DDJ49_30220, partial (Klebsiella pneumoniae) |
| WP_07280094.1 | hypothetical protein (E. coli) |
| WP_077250818.1 | hypothetical protein (E. coli) |
| WP_073034642.1 | hypothetical protein (E. coli) |
| WP_108998184.1 | hypothetical protein (E. coli) |
| WP_072794647.1 | hypothetical protein (E. coli) |
| WP_108998612.1 | hypothetical protein (E. coli) |
| WP_072794633.1 | hypothetical protein (E. coli) |
| WP_072794629.1 | hypothetical protein (E. coli) |
| WP_108998509.1 | hypothetical protein (E. coli) |
| WP_108998559.1 | hypothetical protein (E. coli) |
| WP_126755742.1 | hypothetical protein (E. coli) |
| WP_108998635.1 | 60S ribosomal protein L22 (E. coli) |
| WP_094948604.1 | MULTISPECIES: translation elongation factor EF-1 subunit alpha (Enterobacteriaceae) |
| WP_888381079.1 | hypothetical protein (Microbacterium sp. AISO3) |
| PWS23168.1 | hypothetical protein DKP78_14555, partial (Enterococcus faecium) |
| WP_108998558.1 | 30S ribosomal protein S19e (E. coli) |
| EHM02625.1 | hypothetical protein HMPREF9946_00894 (Acetobacteraceae bacterium AT-5844) |
| WP_114597919.1 | tubulin beta chain (Microbacterium arborescens) |
| WP_110201953.1 | hypothetical protein (Kangiella spongicola) |
| Figure 3F | |
| WP_067782112.1 | hypothetical protein (Actinomyces vulturis) |
| WP_117305475.1 | MinD/ParA family protein (Bacillus sp. V59.32a) |
| WP_078453723.1 | molecular chaperone DnaK, partial (Solemya velum gill symbiont) |
| OGV57400.1 | hypothetical protein A2X49_01235 (Lentisphaerae bacteria GWF2_52_8) |
| OGV38480.1 | transcriptional regulator (Lentisphaerae bacteria GWF2_49_21) |
| REF04934.1 | LacI family transcriptional regulator (Microbacterium chocolate) |
| WP_021076726.1 | magnesium chelate ATPase subunit I (Bradyrhizobium sp. MOS004) |
| RJP82014.1 | MCE family protein (Desulfobacteraceae bacterium) |
| OGT19887.1 | histidinol -phosphate transaminase (Gammaproteobacteria bacterium RBG_16_57_12) |
| SDW00717.1 | hypothetical protein SAMN04487912_10192 (Arthrobacter sp. cf158) |
| WP_0069060036.1 | phage major capsid protein (Shuttleworthia satelles) |
| WP_1193915508.1 | pyrophosphate--fructose-6-phosphate 1-phosphotransferase (Phyllobacteriaceae bacterium SYSU D60012) |
| AST06406.1 | MFS transporter (Anoxybacillus flavithermus) |
| RKV99493.1 | type II secretion system F family protein (Candidatus Saccharimonas sp.) |
| RTF38711.1 | hypothetical protein CG399_02610, partial (Bifidobacteriaceae bacterium NR015) |
| WP_106563713.1 | ABC transporter permease (Labedella gwakjiensis) |
| GBU15610.1 | integrase (Polaromonas sp.) |
| WP_066008415.1 | tRNA (guanosine(46)-N7) -methyltransferase TrmB (Campylobacter ornithocola) |
| PYM16032.1 | homoserine dehydrogenase (Verrucomicrobia bacterium) |
| WP_088892294.1 | polysaccharide pyruvyl transferase family protein (Leptolyngbya ohadii) |
| Bacteria Species | Domain | Phylum | Class | Order/Genus | Access Number |
|---|---|---|---|---|---|
| Actinopolyspora erythraea | Bacteria | Actinomycetota | Actinomycetia | Actinosporaceae | WP_043569372.1 |
| Listeria costaricensis | Firmicutes | Listeria | Bacilli | Listeriacea | WP_0999222156.1 |
| Escherichia colli | proteobacteria | Pseudomonadot | Gammaproteobacteria | Enterobacteriacea | AQU017941.1 |
| Methylobacterium sp. ap11 | Bacteria | Methylobacterium | Aphaproteobacteria | Methylobacteriaceae | WP_091753941.1 |
| Acidobacteria bacterium | Bacteria | Acidobacteriota | Acidobacteria | Acidobacteriales | RMG44463.1 |
| candidate entotheonella factor | Bacteria | Tetomicrobia | entotheonella | ETW97925.1 | |
| Bacteroidet bacterium | Bacteria | Bacteroid | Saprospira | Bacteroides | PIQ27132.1 |
| Paenisporasarcina sp. TG20 | Bacteria | Paenisporosarcin | Bacilli | Planococcaceae | WP_019414340.1 |
| candidate Thiodiazotropha endoloripes | Nomenclatural status: not validly published | WP_0690002724.1 | |||
| Clostridiales bacterium | Bacteria | Eubacteriales | Clostrid | Clostridaceae | PWM01737.1 |
| Bacteria Species | Domain | Phylum | Class | Order/Genus | Access Number |
|---|---|---|---|---|---|
| Flavobacteriales bacterium 33_180_T64 | Bacteria | Bacteroides | Flavobacteria | Flavobacteriales | OUS03466.1 |
| Aureimonas sp. AU12 | Bacteria | Pseudomonadot | Alphaproteo bacteria | Hyphomicrobiales | WP_062208650.1 |
| Bacillus cerus | Bacteria | Bacillot | Bacilli | Bacillales | WP_000124192.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stupak, A.; Gęca, T.; Kwaśniewska, A.; Mlak, R.; Piwowarczyk, P.; Nawrot, R.; Goździcka-Józefiak, A.; Kwaśniewski, W. Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies. Int. J. Mol. Sci. 2023, 24, 6922. https://doi.org/10.3390/ijms24086922
Stupak A, Gęca T, Kwaśniewska A, Mlak R, Piwowarczyk P, Nawrot R, Goździcka-Józefiak A, Kwaśniewski W. Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies. International Journal of Molecular Sciences. 2023; 24(8):6922. https://doi.org/10.3390/ijms24086922
Chicago/Turabian StyleStupak, Aleksandra, Tomasz Gęca, Anna Kwaśniewska, Radosław Mlak, Paweł Piwowarczyk, Robert Nawrot, Anna Goździcka-Józefiak, and Wojciech Kwaśniewski. 2023. "Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies" International Journal of Molecular Sciences 24, no. 8: 6922. https://doi.org/10.3390/ijms24086922
APA StyleStupak, A., Gęca, T., Kwaśniewska, A., Mlak, R., Piwowarczyk, P., Nawrot, R., Goździcka-Józefiak, A., & Kwaśniewski, W. (2023). Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies. International Journal of Molecular Sciences, 24(8), 6922. https://doi.org/10.3390/ijms24086922





