MemophenolTM Prevents Amyloid-β Deposition and Attenuates Inflammation and Oxidative Stress in the Brain of an Alzheimer’s Disease Rat
Abstract
:1. Introduction
2. Results
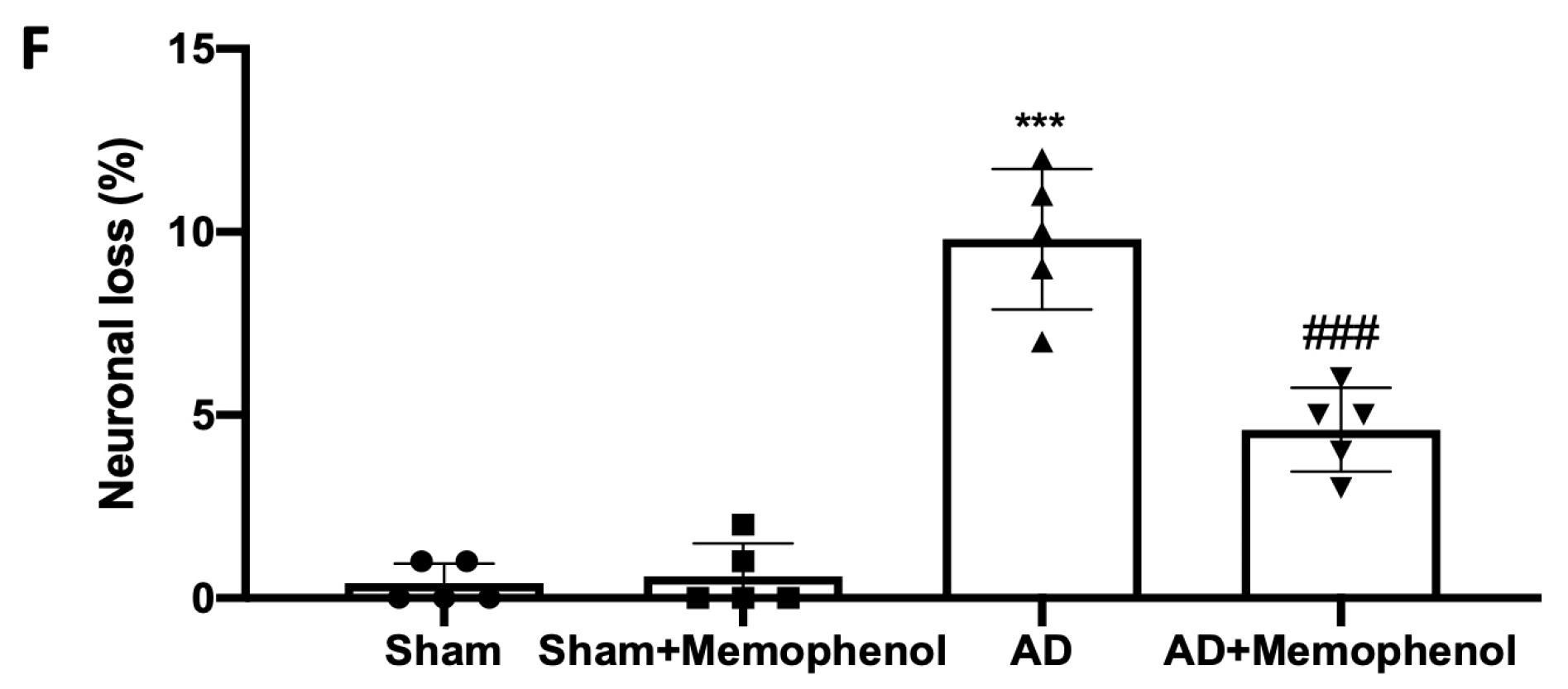
2.1. Effects of MemophenolTM on Behavioral and Histological Alterations
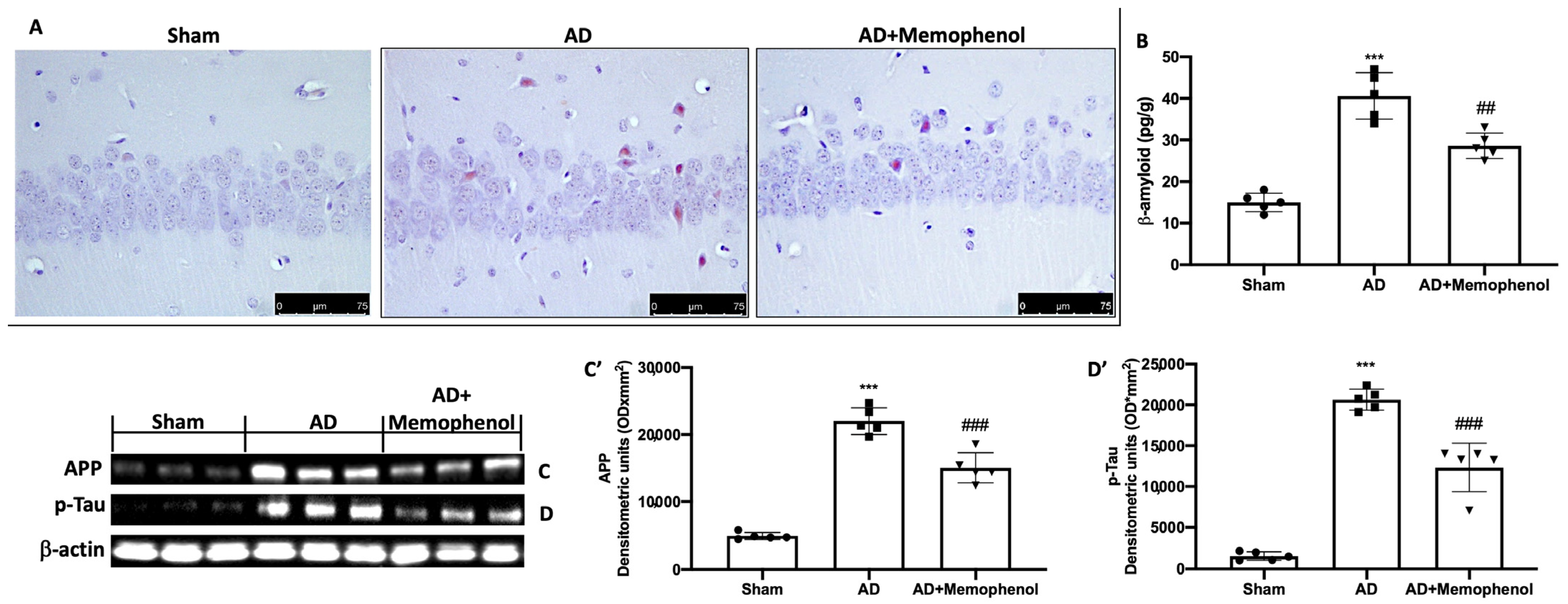
2.2. Effects of MemophenolTM Treatment on Aβ Deposition and APP and p-Tau Over-Expression
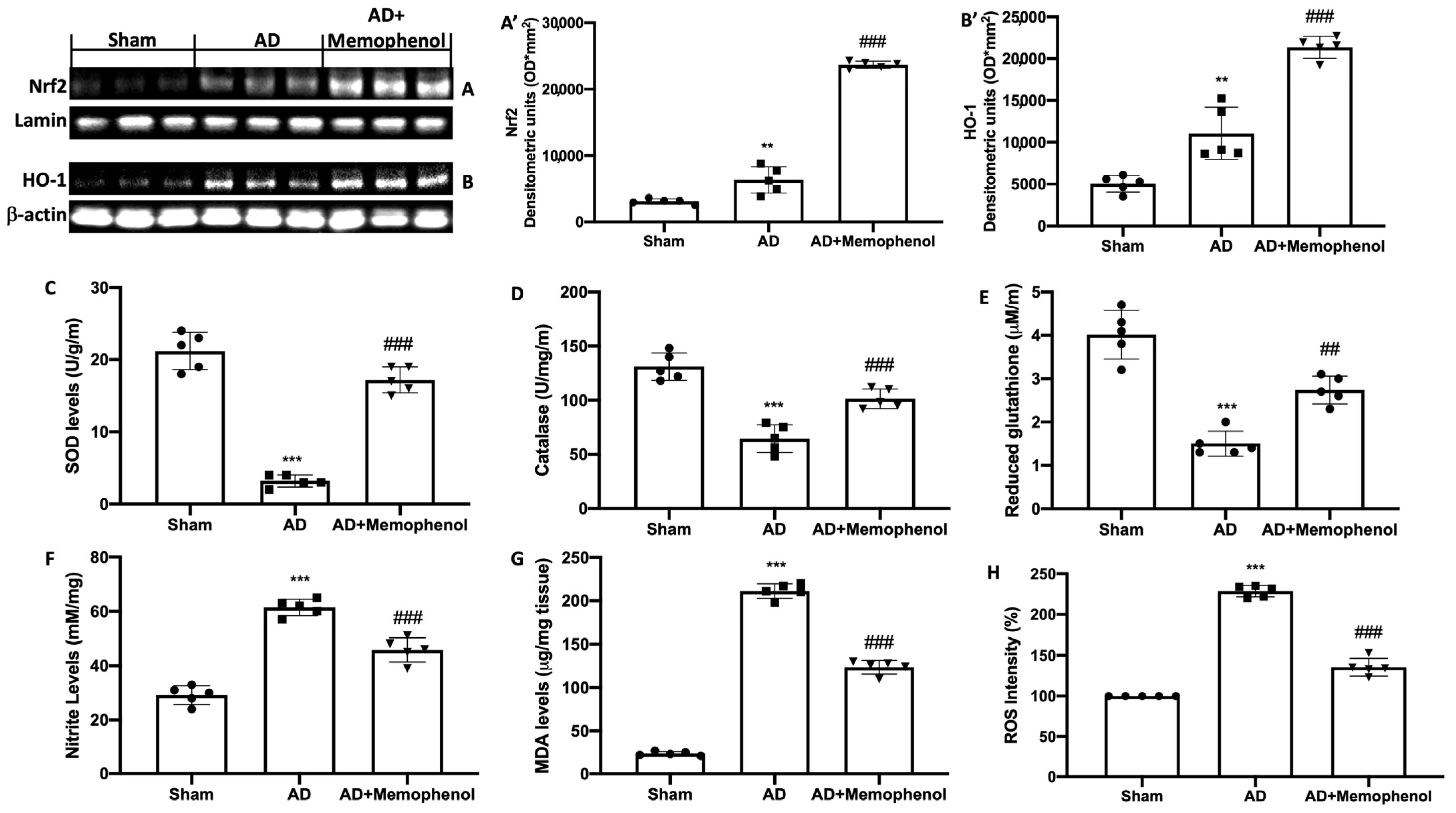
2.3. MemophenolTM Treatment Effects on Oxidative Hippocampal Modifications
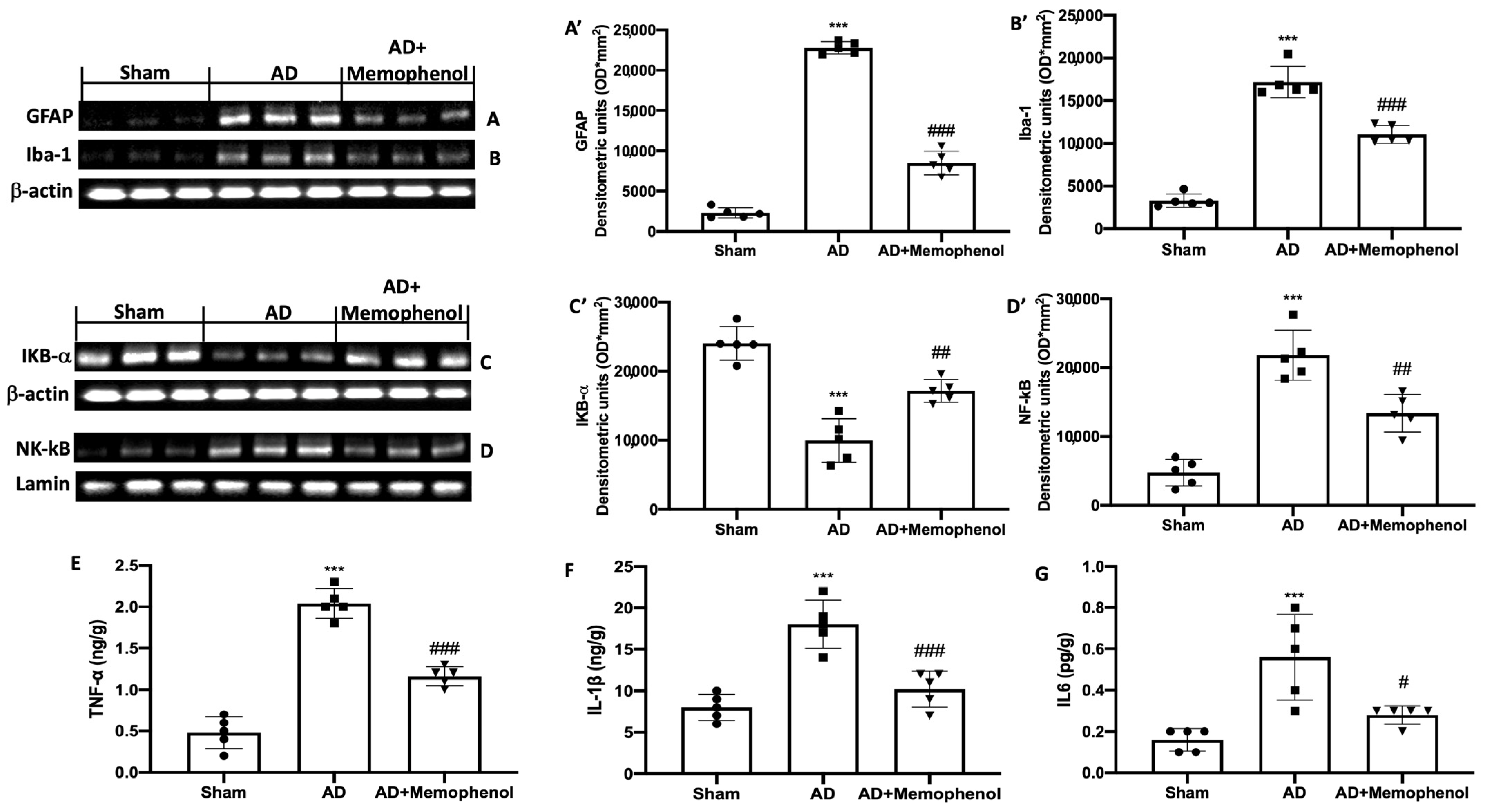
2.4. MemophenolTM Treatment Effects on Pro-Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Tested Product: MemophenolTM
4.2. Animals
4.3. Experimental Protocol
Experimental Groups
- -
- Sham group: saline was administered to the rats;
- -
- Sham + MemophenolTM group: saline was administered to the rats, and MemophenolTM (15 mg/kg) was administered orally for 30 consecutive days;
- -
- AD group: as previously mentioned, the rats were treated with AlCl3 (100 mg/kg, orally) and D-galactose (60 mg/kg, intraperitoneally) for 60 days;
- -
- AD + MemophenolTM group: as previously documented, the rats were treated with AlCl3 (100 mg/kg, orally) and D-galactose (60 mg/kg, intraperitoneally) for 60 days, and MemophenolTM (15 mg/kg) was supplied orally by gavage for 30 consecutive days.
4.4. Behavioral Assessment
4.4.1. MWM
4.4.2. EPM
4.4.3. NOR
4.5. Histological Analysis and Congo Red Staining
4.6. Western Blot Analysis
4.7. Biochemical Analysis
4.7.1. Measurement of SOD Activity
4.7.2. Measurement of CAT Activity
4.7.3. GSH Levels
4.7.4. Measurement of Nitrite Levels
4.7.5. Measurement of MDA
4.7.6. Measurement of ROS
4.7.7. Cytokines and Aβ Measurement
4.8. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Fenech, M. A review of genome mutation and Alzheimer’s disease. Mutagenesis 2007, 22, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seubert, P.; Vigo-Pelfrey, C.; Esch, F.; Lee, M.; Dovey, H.; Davis, D.; Sinha, S.; Schlossmacher, M.; Whaley, J.; Swindlehurst, C.; et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 1992, 359, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Giardina, B.; Colucci, D.; Galtieri, A.; Misiti, F. Amyloid-beta peptide affects the oxygen dependence of erythrocyte metabolism: A role for caspase 3. Int. J. Biochem. Cell Biol. 2007, 39, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Carelli-Alinovi, C.; Pirolli, D.; Giardina, B.; Misiti, F. Protein kinase C mediates caspase 3 activation: A role for erythrocyte morphology changes. Clin. Hemorheol. Microcirc. 2015, 59, 345–354. [Google Scholar] [CrossRef]
- Heneka, M.T.; O’Banion, M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-beta Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [Green Version]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Zhou, H.D.; Zhou, X.F. Clearance of amyloid-beta in Alzheimer’s disease: Progress, problems and perspectives. Drug Discov. Today 2006, 11, 931–938. [Google Scholar] [CrossRef]
- Frozza, R.L.; Lourenco, M.V.; De Felice, F.G. Challenges for Alzheimer’s Disease Therapy: Insights from Novel Mechanisms Beyond Memory Defects. Front. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceuticals and amyloid neurodegenerative diseases: A focus on natural phenols. Expert Rev. Neurother. 2015, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Fumia, A.; Cicero, N.; Gitto, M.; Nicosia, N.; Alesci, A. Role of nutraceuticals on neurodegenerative diseases: Neuroprotective and immunomodulant activity. Nat. Prod. Res. 2022, 36, 5916–5933. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharm. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Ontario, M.L.; Siracusa, R.; Modafferi, S.; Scuto, M.; Sciuto, S.; Greco, V.; Bertuccio, M.P.; Trovato Salinaro, A.; Crea, R.; Calabrese, E.J.; et al. Potential prevention and treatment of neurodegenerative disorders by olive polyphenols and hidrox. Mech. Ageing Dev. 2022, 203, 111637. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox((R)) Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-oxidant Activity of Hidrox((R)) in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef]
- Cordaro, M.; Modafferi, S.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; et al. Natural Compounds Such as Hericium erinaceus and Coriolus versicolor Modulate Neuroinflammation, Oxidative Stress and Lipoxin A4 Expression in Rotenone-Induced Parkinson’s Disease in Mice. Biomedicines 2022, 10, 2505. [Google Scholar] [CrossRef]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensalem, J.; Servant, L.; Alfos, S.; Gaudout, D.; Laye, S.; Pallet, V.; Lafenetre, P. Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice. Front. Behav. Neurosci. 2016, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensalem, J.; Dudonne, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols From Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 996–1007. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonne, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Laye, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [Green Version]
- Markesbery, W.R.; Schmitt, F.A.; Kryscio, R.J.; Davis, D.G.; Smith, C.D.; Wekstein, D.R. Neuropathologic substrate of mild cognitive impairment. Arch. Neurol. 2006, 63, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L.; Crain, B.J.; Pletnikova, O.; Rudow, G.; Iacono, D.; Riudavets, M.A.; Driscoll, I.; et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA). J. Alzheimer’s Dis. 2009, 18, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.H.; Manczak, M.; Mao, P.; Calkins, M.J.; Reddy, A.P.; Shirendeb, U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: Implications for synaptic damage and cognitive decline. J. Alzheimer’s Dis. 2010, 20 (Suppl. S2), S499–S512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; O’Connor, T.; Vassar, R. The contribution of activated astrocytes to Abeta production: Implications for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2011, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Amaral, D.G.; Witter, M.P. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience 1989, 31, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Nakashiba, T.; Young, J.Z.; McHugh, T.J.; Buhl, D.L.; Tonegawa, S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 2008, 319, 1260–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Ahmed, H.I.; Abu-Elfotuh, K. Modeling stages mimic Alzheimer’s disease induced by different doses of aluminum in rats: Focus on progression of the disease in response to time. J. Alzheimer’s Park. Dement. 2016, 1, 2. [Google Scholar]
- Sethi, P.; Jyoti, A.; Singh, R.; Hussain, E.; Sharma, D. Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology 2008, 29, 1069–1079. [Google Scholar] [CrossRef]
- Moumen, R.; Ait-Oukhatar, N.; Bureau, F.; Fleury, C.; Bougle, D.; Arhan, P.; Neuville, D.; Viader, F. Aluminium increases xanthine oxidase activity and disturbs antioxidant status in the rat. J. Trace Elem. Med. Biol. 2001, 15, 89–93. [Google Scholar] [CrossRef]
- Becaria, A.; Bondy, S.C.; Campbell, A. Aluminum and copper interact in the promotion of oxidative but not inflammatory events: Implications for Alzheimer’s disease. J. Alzheimer’s Dis. 2003, 5, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Niu, P.Y.; Niu, Q.; Zhang, Q.L.; Wang, L.P.; He, S.E.; Wu, T.C.; Conti, P.; Di Gioacchino, M.; Boscolo, P. Aluminum impairs rat neural cell mitochondria in vitro. Int. J. Immunopathol Pharm. 2005, 18, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Bal, A.; Gill, K.D. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Res. 2008, 1232, 94–103. [Google Scholar] [CrossRef]
- Kumar, V.; Bal, A.; Gill, K.D. Susceptibility of mitochondrial superoxide dismutase to aluminium induced oxidative damage. Toxicology 2009, 255, 117–123. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Limon-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Mancuso, G.; Midiri, A.; Di Paola, R.; Cuzzocrea, S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1beta and IL-18. Biochem. Pharm. 2018, 155, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Impellizzeri, D.; Fusco, R.; Cordaro, M.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Ultramicronized palmitoylethanolamide (PEA-um((R))) in the treatment of idiopathic pulmonary fibrosis. Pharm. Res. 2016, 111, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Inferrera, A.; Esposito, E.; Di Paola, R.; Cuzzocrea, S. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicol. Appl. Pharm. 2017, 329, 231–240. [Google Scholar] [CrossRef]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharm. 2016, 782, 107–118. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhao, L.; Luo, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Sosna, J.; Philipp, S.; Albay, R., 3rd; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; LaFerla, F.M.; Glabe, C.G. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 11. [Google Scholar] [CrossRef]
- Walton, J.R.; Wang, M.X. APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. J. Inorg. Biochem. 2009, 103, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants 2021, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pi, Z.; Song, F.; Liu, Z. Ginsenosides attenuate d-galactose- and AlCl(3)-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 194, 188–195. [Google Scholar] [CrossRef]
- Mahmoodzadeh, T.; Kashani, M.H.K.; Ramshini, H.; Moslem, A.; Mohammad-Zadeh, M. Effect of Camellia sinensis on spatial memory in a rat model of Alzheimer’s disease. J. Biomed. 2016, 1, e5340. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, O.; Akar, F.; Celikyurt, I.K.; Tanyeri, P.; Ulak, G.; Erden, F. 7-NI and ODQ Disturbs Memory in the Elevated Plus Maze, Morris Water Maze, and Radial Arm Maze Tests in Mice. Drug Target Insights 2015, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Paterniti, I.; Campolo, M.; Siracusa, R.; Cordaro, M.; Di Paola, R.; Calabrese, V.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Liver X receptors activation, through TO901317 binding, reduces neuroinflammation in Parkinson’s disease. PLoS ONE 2017, 12, e0174470. [Google Scholar] [CrossRef] [Green Version]
- Impellizzeri, D.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; Di Paola, R.; et al. Acai Berry Mitigates Vascular Dementia-Induced Neuropathological Alterations Modulating Nrf-2/Beclin1 Pathways. Cells 2022, 11, 2616. [Google Scholar] [CrossRef] [PubMed]
- Mudo, G.; Frinchi, M.; Nuzzo, D.; Scaduto, P.; Plescia, F.; Massenti, M.F.; Di Carlo, M.; Cannizzaro, C.; Cassata, G.; Cicero, L.; et al. Anti-inflammatory and cognitive effects of interferon-beta1a (IFNbeta1a) in a rat model of Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, S.; Gaur, U.; Zheng, W. Artemisinin Improved Neuronal Functions in Alzheimer’s Disease Animal Model 3xtg Mice and Neuronal Cells via Stimulating the ERK/CREB Signaling Pathway. Aging Dis. 2020, 11, 801–819. [Google Scholar] [CrossRef]
- Petrosino, S.; Campolo, M.; Impellizzeri, D.; Paterniti, I.; Allara, M.; Gugliandolo, E.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Esposito, E.; et al. 2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation. Front. Pharm. 2017, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, W.; Xu, F.; Cao, Z.; Jia, F.; Li, Y. Iron Dyshomeostasis Participated in Rat Hippocampus Toxicity Caused by Aluminum Chloride. Biol. Trace Elem. Res. 2020, 197, 580–590. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S. 2-Pentadecyl-2-Oxazoline Reduces Neuroinflammatory Environment in the MPTP Model of Parkinson Disease. Mol. Neurobiol. 2018, 55, 9251–9266. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Interdonato, L.; Crea, R.; Fusco, R.; et al. Hidrox((R)) and Endometriosis: Biochemical Evaluation of Oxidative Stress and Pain. Antioxidants 2021, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Trovato Salinaro, A.; Cordaro, M.; Fusco, R.; Impellizzeri, D.; Interdonato, L.; Scuto, M.; Ontario, M.L.; Crea, R.; Siracusa, R.; et al. Hidrox((R)) and Chronic Cystitis: Biochemical Evaluation of Inflammation, Oxidative Stress, and Pain. Antioxidants 2021, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Paterniti, I.; Siracusa, R.; Impellizzeri, D.; Esposito, E.; Cuzzocrea, S. KU0063794, a Dual mTORC1 and mTORC2 Inhibitor, Reduces Neural Tissue Damage and Locomotor Impairment After Spinal Cord Injury in Mice. Mol. Neurobiol. 2017, 54, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Gugliandolo, E.; Cuzzocrea, S.; Paola, R.D.; et al. Acai (Euterpe Oleraceae Mart.) Seeds Regulate NF-kappaB and Nrf2/ARE Pathways Protecting Lung against Acute and Chronic Inflammation. Cell Physiol. Biochem. 2022, 56, 1–20. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Frinchi, M.; Nuzzo, D.; Jinnah, H.A.; Mudo, G.; Condorelli, D.F.; Caciagli, F.; Ciccarelli, R.; Di Iorio, P.; Mule, F.; et al. Altered gastrointestinal motility in an animal model of Lesch-Nyhan disease. Auton. Neurosci. 2018, 210, 55–64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Impellizzeri, D.; Tomasello, M.; Cordaro, M.; D’Amico, R.; Fusco, R.; Abdelhameed, A.S.; Wenzel, U.; Siracusa, R.; Calabrese, V.; Cuzzocrea, S.; et al. MemophenolTM Prevents Amyloid-β Deposition and Attenuates Inflammation and Oxidative Stress in the Brain of an Alzheimer’s Disease Rat. Int. J. Mol. Sci. 2023, 24, 6938. https://doi.org/10.3390/ijms24086938
Impellizzeri D, Tomasello M, Cordaro M, D’Amico R, Fusco R, Abdelhameed AS, Wenzel U, Siracusa R, Calabrese V, Cuzzocrea S, et al. MemophenolTM Prevents Amyloid-β Deposition and Attenuates Inflammation and Oxidative Stress in the Brain of an Alzheimer’s Disease Rat. International Journal of Molecular Sciences. 2023; 24(8):6938. https://doi.org/10.3390/ijms24086938
Chicago/Turabian StyleImpellizzeri, Daniela, Mario Tomasello, Marika Cordaro, Ramona D’Amico, Roberta Fusco, Ali S. Abdelhameed, Uwe Wenzel, Rosalba Siracusa, Vittorio Calabrese, Salvatore Cuzzocrea, and et al. 2023. "MemophenolTM Prevents Amyloid-β Deposition and Attenuates Inflammation and Oxidative Stress in the Brain of an Alzheimer’s Disease Rat" International Journal of Molecular Sciences 24, no. 8: 6938. https://doi.org/10.3390/ijms24086938
APA StyleImpellizzeri, D., Tomasello, M., Cordaro, M., D’Amico, R., Fusco, R., Abdelhameed, A. S., Wenzel, U., Siracusa, R., Calabrese, V., Cuzzocrea, S., & Di Paola, R. (2023). MemophenolTM Prevents Amyloid-β Deposition and Attenuates Inflammation and Oxidative Stress in the Brain of an Alzheimer’s Disease Rat. International Journal of Molecular Sciences, 24(8), 6938. https://doi.org/10.3390/ijms24086938











