The Transfer of the Hepatocyte Growth Factor Gene by Macrophages Ameliorates the Progression of Peritoneal Fibrosis in Mice
Abstract
1. Introduction
2. Results
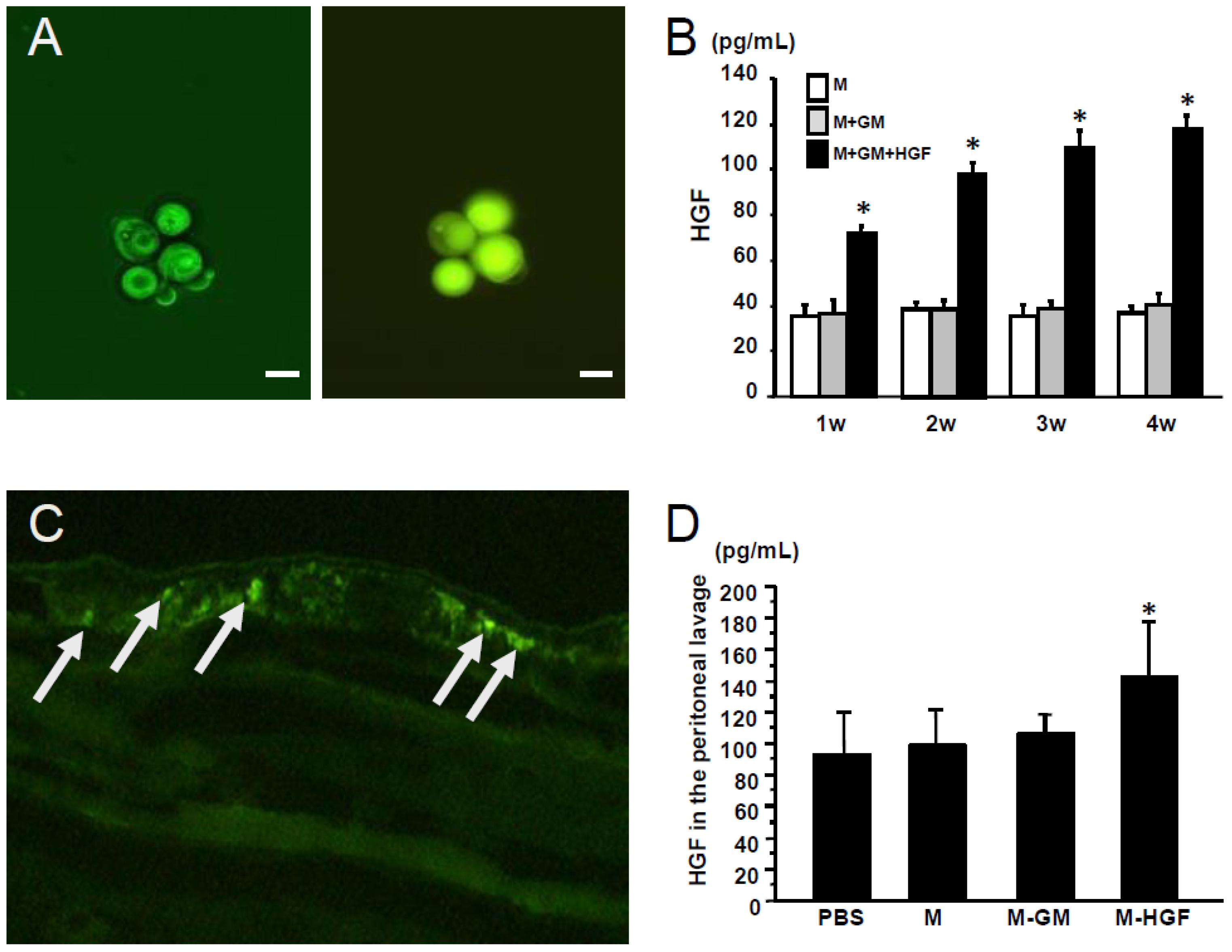
2.1. Confirmation of Gene Expression in the Macrophages In Vitro and In Vivo
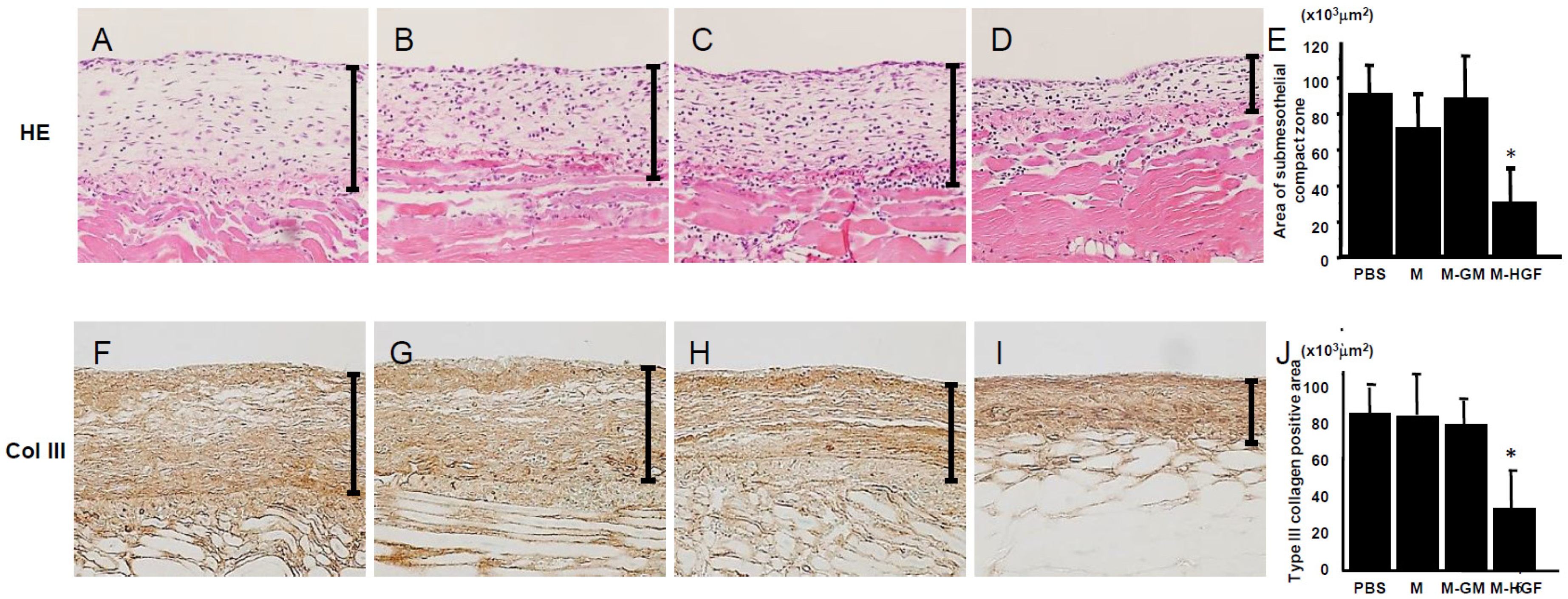
2.2. M-HGF Attenuates Peritoneal Fibrotic Thickening Induced by CG
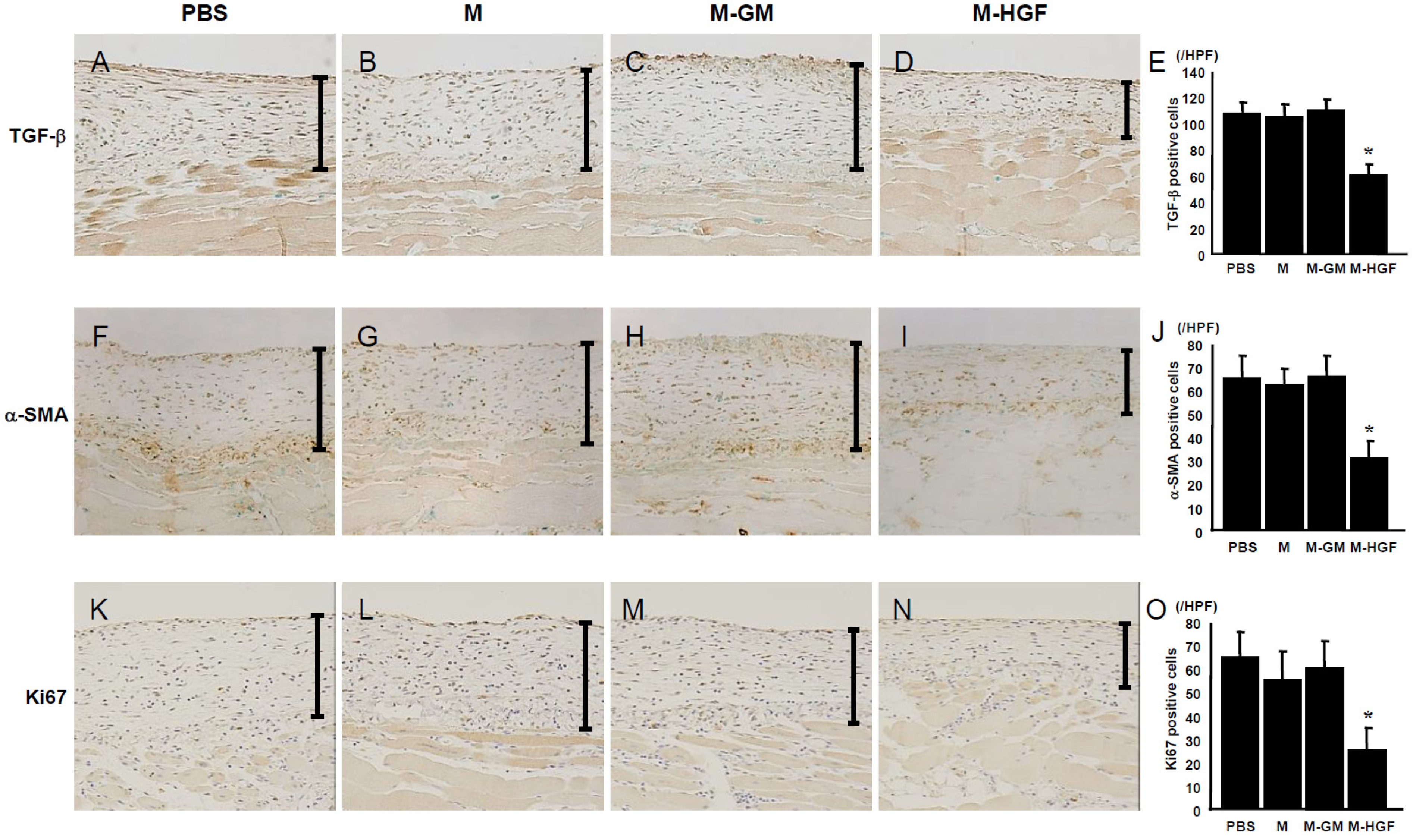
2.3. M-HGF Suppresses TGF-β Expression, Reduces the Number of Myofibroblasts and Suppresses Cell Proliferation
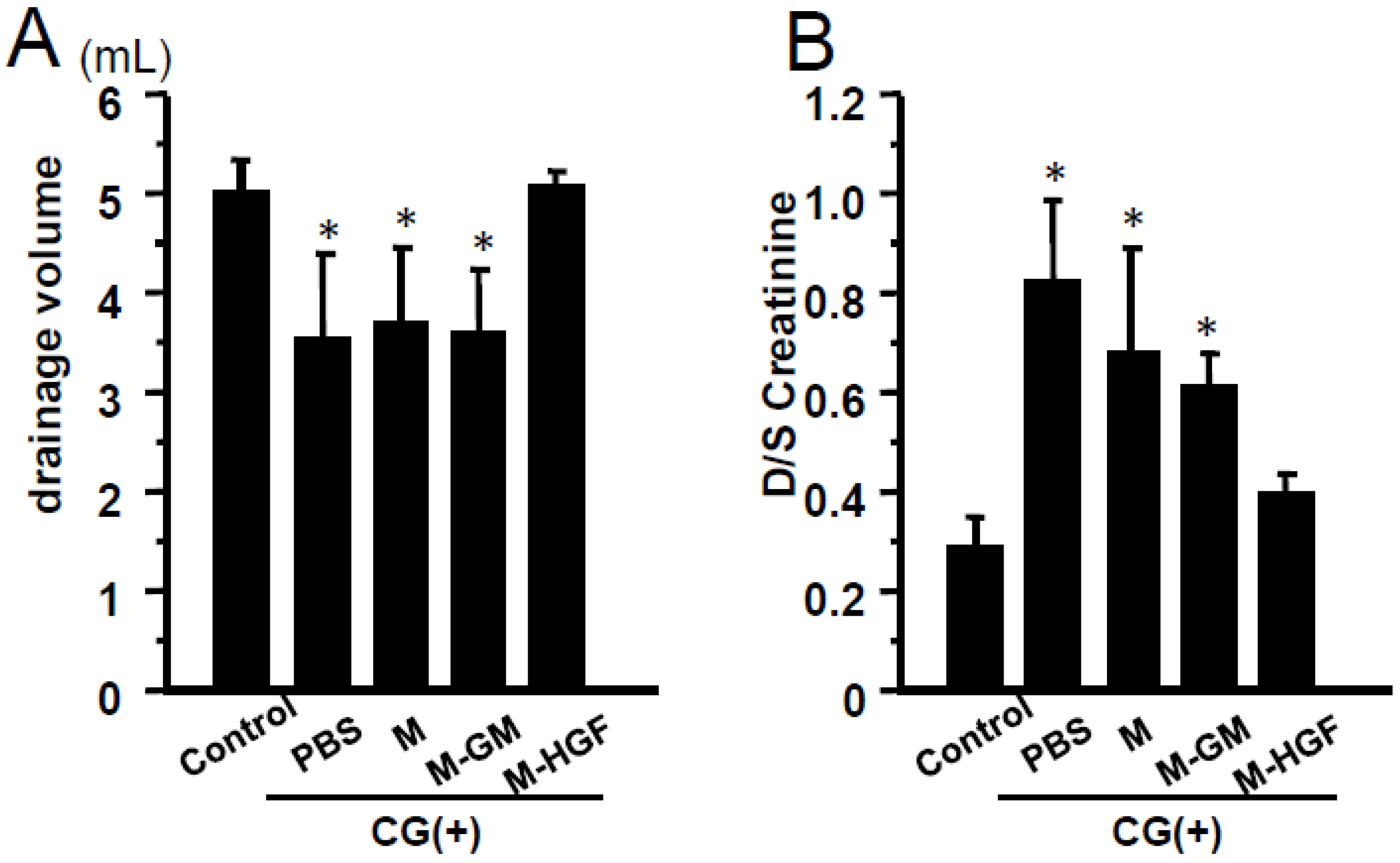
2.4. M-HGF Attenuates the Peritoneal Hyperpermeability
3. Discussion
4. Material and Methods
4.1. Preparation of CGMs
4.2. Preparation of Peritoneal Macrophages
4.3. Preparation of CGMs Incorporating Plasmids
4.4. Verification of Targeted Gene Expression in Macrophages
4.5. Animals
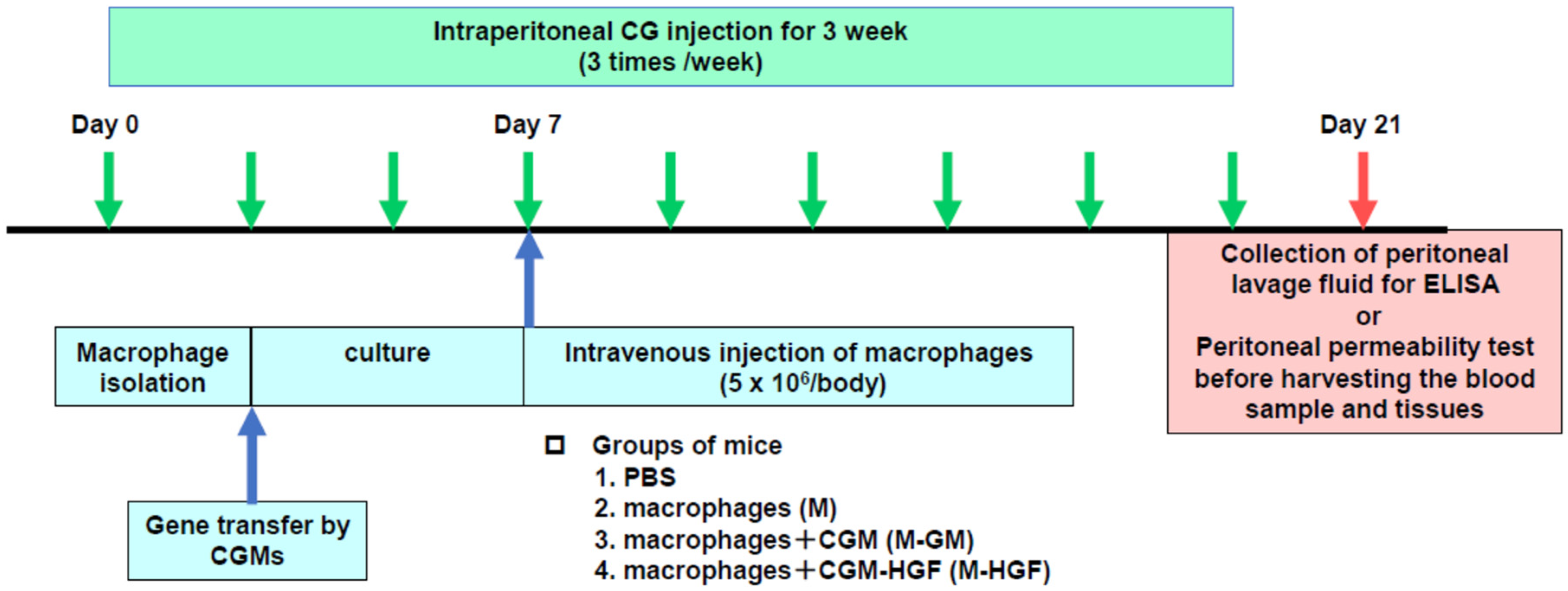
4.6. In Vivo Experimental Protocol
4.7. Histological and Immunohistochemical Examination
4.8. Collection of Peritoneal Lavage Fluid for HGF ELISA
4.9. Evaluation of Peritoneal Permeability
4.10. Data Processing and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gandhi, V.C.; Humayun, H.M.; Ing, T.S.; Daugirdas, J.T.; Jablokow, V.R.; Iwatsuki, S.; Geis, W.P.; Hano, J.E. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch. Intern. Med. 1980, 140, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Nitta, K.; Horita, S.; Yumura, W.; Nihei, H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 1996, 72, 171–176. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef]
- Devuyst, O.; Topley, N.; Williams, J.D. Morphological and functional changes in the dialysed peritoneal cavity: Impact of more biocompatible solutions. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 3), 12–15. [Google Scholar] [CrossRef]
- Nakamura, T.; Nishizawa, T.; Hagiya, M.; Seki, T.; Shimonishi, M.; Sugimura, A.; Tashiro, K.; Shimizu, S. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342, 440–443. [Google Scholar] [CrossRef]
- Motoi, S.; Toyoda, H.; Obara, T.; Ohta, E.; Arita, Y.; Negishi, K.; Moriya, K.; Kuboi, Y.; Soejima, M.; Imai, T.; et al. Anti-apoptotic effects of recombinant human hepatocyte growth factor on hepatocytes were associated with intrahepatic hemorrhage suppression indicated by the preservation of prothrombin time. Int. J. Mol. Sci. 2019, 20, 1821. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.L.; Wang, X.L.; Wang, Y.C.; Wu, H.; Zhou, X.D.; Wang, Z.K.; Wang, Y.C.; Xue, C.S.; Li, B.; Gao, D.L. Anti-inflammatory activities of hepatocyte growth factor in post-ischemic heart failure. Acta Pharmacol. Sin. 2018, 39, 1613–1621. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Wang, H.; Xiong, X.; Liu, X.; Hu, C.; Han, Y.; Lu, Y.; Wu, Z.; Zhang, Q. Plasmid pUDK-HGF encoding human hepatocyte growth factor gene attenuates gentamicin-induced kidney injury in rats. Exp. Toxicol. Pathol. 2013, 65, 541–547. [Google Scholar] [CrossRef]
- Kawaida, K.; Matsumoto, K.; Shimazu, H.; Nakamura, T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc. Natl. Acad. Sci. USA 1994, 91, 4357–4361. [Google Scholar] [CrossRef]
- Amaike, H.; Matsumoto, K.; Oka, T.; Nakamura, T. Preventive effect of hepatocyte growth factor on acute side effects of cyclosporin A in mice. Cytokine 1996, 8, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Kaneda, Y.; Tsutsui, H.; Nakanishi, K.; Sawa, Y.; Morishita, R.; Matsumoto, K.; Nakamura, T.; Takahashi, H.; Okamoto, E.; et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat. Med. 1999, 5, 226–230. [Google Scholar] [CrossRef]
- Mizuno, S.; Kurosawa, T.; Matsumoto, K.; Mizuno-Horikawa, Y.; Okamoto, M.; Nakamura, T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J. Clin. Investig. 1998, 101, 1827–1834. [Google Scholar] [CrossRef]
- Dohi, M.; Hasegawa, T.; Yamamoto, K.; Marshall, B.C. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S.; Matsumoto, K.; Sawa, Y.; Matsuda, H.; Nakamura, T. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J. Clin. Investig. 2000, 106, 1511–1519. [Google Scholar] [CrossRef]
- Li, Y.; Takemura, G.; Kosai, K.; Yuge, K.; Nagano, S.; Esaki, M.; Goto, K.; Takahashi, T.; Hayakawa, K.; Koda, M.; et al. Postinfarction treatment with an adenoviral vector expressing hepatocyte growth factor relieves chronic left ventricular remodeling and dysfunction in mice. Circulation 2003, 107, 2499–2506. [Google Scholar] [CrossRef]
- Chinen, J.; Davis, J.; De Ravin, S.S.; Hay, B.N.; Hsu, A.P.; Linton, G.F.; Naumann, N.; Nomicos, E.Y.; Silvin, C.; Ulrick, J.; et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood 2007, 110, 67–73. [Google Scholar] [CrossRef]
- Aiuti, A.; Cattaneo, F.; Galimberti, S.; Benninghoff, U.; Cassani, B.; Callegaro, L.; Scaramuzza, S.; Andolfi, G.; Mirolo, M.; Brigida, I.; et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009, 360, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Abel, U.; Dal-Cortivo, L.; Caccavelli, L.; et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef]
- Mercuri, E.; Muntoni, F.; Baranello, G.; Masson, R.; Boespflug-Tanguy, O.; Bruno, C.; Corti, S.; Daron, A.; Deconinck, N.; Servais, L.; et al. STR1VE-EU study group. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 832–841. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. Feigin A AAV2-GAD gene therapy for advanced Parkinson’s disease: A double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Nakajima, T.; Taga, N.; Miyauchi, A.; Kato, M.; Matsumoto, A.; Ikeda, T.; Nakamura, K.; Kubota, T.; Mizukami, H.; et al. Gene therapy improves motor and mental function of aromatic L-amino acid decarboxylase deficiency. Brain 2019, 142, 322–333. [Google Scholar] [CrossRef]
- Shigematsu, H.; Yasuda, K.; Iwai, T.; Sasajima, T.; Ishimaru, S.; Ohashi, Y.; Yamaguchi, T.; Ogihara, T.; Morishita, R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010, 17, 1152–1161. [Google Scholar] [CrossRef]
- Viney, N.J.; van Capelleveen, J.C.; Geary, R.S.; Xia, S.; Tami, J.A.; Yu, R.Z.; Marcovina, S.M.; Hughes, S.G.; Graham, M.J.; Crooke, R.M.; et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016, 388, 2239–2253. [Google Scholar] [CrossRef]
- Xia, Z.; Abe, K.; Furusu, A.; Miyazaki, M.; Obata, Y.; Tabata, Y.; Koji, T.; Kohno, S. Suppression of renal tubulointerstitial fibrosis by small interfering RNA targeting heat shock protein 47. Am. J. Nephrol. 2008, 28, 34–46. [Google Scholar] [CrossRef]
- Obata, Y.; Nishino, T.; Kushibiki, T.; Tomoshige, R.; Xia, Z.; Miyazaki, M.; Abe, K.; Koji, T.; Tabata, Y.; Kohno, S. HSP47 siRNA conjugated with cationized gelatin microspheres suppresses peritoneal fibrosis in mice. Acta Biomater. 2012, 8, 2688–2696. [Google Scholar] [CrossRef]
- Veis, A. The physical chemistry of gelatin. Int. Rev. Connect. Tissue Res. 1965, 3, 113–200. [Google Scholar] [CrossRef]
- Kushibiki, T.; Tomoshige, R.; Fukunaka, Y.; Kakemi, M.; Tabata, Y. In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. J. Control. Release 2003, 90, 207–216. [Google Scholar] [CrossRef]
- Mishima, Y.; Miyazaki, M.; Abe, K.; Ozono, Y.; Shioshita, K.; Xia, Z.; Harada, T.; Taguchi, T.; Koji, T.; Kohno, S. Enhanced expression of heat shock protein 47 in rat model of peritoneal fibrosis. Perit. Dial. Int. 2003, 23, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Muta, K.; Nakazawa, Y.; Obata, Y.; Inoue, H.; Torigoe, K.; Nakazawa, M.; Abe, K.; Furusu, A.; Miyazaki, M.; Yamamoto, K.; et al. An inhibitor of Krüppel-like factor 5 suppresses peritoneal fibrosis in mice. Perit. Dial. Int. 2021, 41, 394–403. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J. Hepatocyte growth factor: New arsenal in the fights against renal fibrosis? Kidney Int. 2006, 70, 238–240. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakata, R.; Ueno, T.; Sata, M.; Ueno, H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology 2000, 32, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Plautz, G.; Nabel, E.G.; Nabel, G.J. Introduction of vascular smooth muscle cells expressing recombinant genes in vivo. Circulation 1991, 83, 578–583. [Google Scholar] [CrossRef]
- Ohno, T.; Gordon, D.; San, H.; Pompili, V.J.; Imperiale, M.J.; Nabel, G.J.; Nabel, E.G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science 1994, 265, 781–784. [Google Scholar] [CrossRef]
- Hiltunen, M.O.; Turunen, M.P.; Laitinen, M.; Ylä-Herttuala, S. Insights into the molecular pathogenesis of atherosclerosis and therapeutic strategies using gene transfer. Vasc. Med. 2000, 5, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dichek, D.A.; Anderson, J.; Kelly, A.B.; Hanson, S.R.; Harker, L.A. Enhanced in vivo antithrombotic effects of endothelial cells expressing recombinant plasminogen activators transduced with retroviral vectors. Circulation 1996, 93, 301–309. [Google Scholar] [CrossRef]
- Flugelman, M.Y.; Virmani, R.; Leon, M.B.; Bowman, R.L.; Dichek, D.A. Genetically engineered endothelial cells remain adherent and viable after stent deployment and exposure to flow in vitro. Circ. Res. 1992, 70, 348–354. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Phagocytosis of polymer microspheres by macrophages. Adv. Polym. Sci. 1990, 94, 107–141. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K.; Kanda, M.; Uematsu, M.; Horio, T.; Fukuyama, N.; Hino, J.; Harada-Shiba, M.; Okumura, H.; Tabata, Y.; et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 2003, 108, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, Y.; Iwanaga, K.; Morimoto, K.; Kakemi, M.; Tabata, Y. Controlled release of plasmid DNA from cationized gelatin hydrogels based on hydrogel degradation. J. Control. Release 2002, 80, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Matsumoto, K.; Nakamura, T.; Tabata, Y. Suppression of tumor metastasis by NK4 plasmid DNA released from cationized gelatin. Gene Ther. 2004, 11, 1205–1214. [Google Scholar] [CrossRef]
- Snyder, S.L.; Sobocinski, P.Z. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal. Biochem. 1975, 64, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Nakazawa, M.; Obata, Y.; Nishino, T.; Abe, S.; Nakazawa, Y.; Abe, K.; Furusu, A.; Miyazaki, M.; Koji, T.; Kohno, S. Involvement of leptin in the progression of experimentally induced peritoneal fibrosis in mice. Acta Histochem. Cytochem. 2013, 46, 75–84. [Google Scholar] [CrossRef] [PubMed]
| BW at Day 0 (g) | BW at Day 21 (g) | AST (IU/L) | ALT (IU/L) | BUN (mg/dL) | Cr (mg/dL) | |
|---|---|---|---|---|---|---|
| PBS | 23.1 ± 1.3 | 25.8 ± 2.0 | 54.2 ± 3.8 | 22.8 ± 2.1 | 25.6 ± 4.1 | 0.15 ± 0.01 |
| M | 24.0 ± 0.3 | 25.8 ± 0.2 | 54.8 ± 1.7 | 22.5 ± 1.7 | 24.2 ± 3.3 | 0.22 ± 0.02 |
| M-GM | 24.3 ± 1.0 | 26.1 ± 1.0 | 53.0 ± 2.8 | 23.9 ± 1.8 | 27.1 ± 1.5 | 0.21 ± 0.05 |
| M-HGF | 25.3 ± 1.0 | 26.5 ± 0.9 | 54.0 ± 1.9 | 24.0 ± 2.4 | 23.5 ± 3.5 | 0.20 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obata, Y.; Abe, K.; Miyazaki, M.; Koji, T.; Tabata, Y.; Nishino, T. The Transfer of the Hepatocyte Growth Factor Gene by Macrophages Ameliorates the Progression of Peritoneal Fibrosis in Mice. Int. J. Mol. Sci. 2023, 24, 6951. https://doi.org/10.3390/ijms24086951
Obata Y, Abe K, Miyazaki M, Koji T, Tabata Y, Nishino T. The Transfer of the Hepatocyte Growth Factor Gene by Macrophages Ameliorates the Progression of Peritoneal Fibrosis in Mice. International Journal of Molecular Sciences. 2023; 24(8):6951. https://doi.org/10.3390/ijms24086951
Chicago/Turabian StyleObata, Yoko, Katsushige Abe, Masanobu Miyazaki, Takehiko Koji, Yasuhiko Tabata, and Tomoya Nishino. 2023. "The Transfer of the Hepatocyte Growth Factor Gene by Macrophages Ameliorates the Progression of Peritoneal Fibrosis in Mice" International Journal of Molecular Sciences 24, no. 8: 6951. https://doi.org/10.3390/ijms24086951
APA StyleObata, Y., Abe, K., Miyazaki, M., Koji, T., Tabata, Y., & Nishino, T. (2023). The Transfer of the Hepatocyte Growth Factor Gene by Macrophages Ameliorates the Progression of Peritoneal Fibrosis in Mice. International Journal of Molecular Sciences, 24(8), 6951. https://doi.org/10.3390/ijms24086951






