Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress
Abstract
1. Introduction
2. Results
2.1. Stability Evaluation
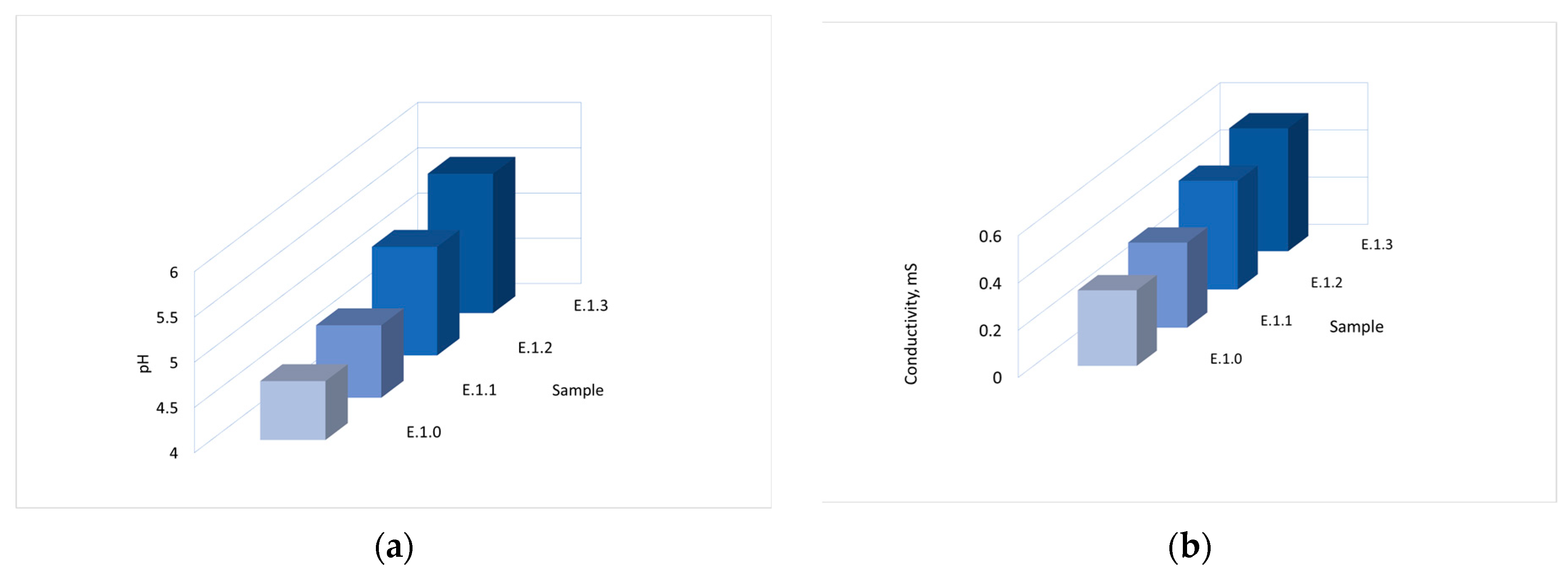
2.1.1. pH Measurements
2.1.2. Conductivity Measurements
2.1.3. Phase Separation
2.2. Antioxidant Activity
2.3. Microbiological Control
2.4. Analysis of Homogeneity and Stability of the Emulsions
2.4.1. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
2.4.2. Microscopic Image Analysis
2.5. Preliminary Results of In Vitro Diffusion Study
3. Discussion
3.1. Stability Evaluation
3.1.1. pH Measurements
3.1.2. Conductivity Measurements
3.1.3. Phase Separation
3.2. Antioxidant Activity
3.3. Microbiological Control
3.4. Analysis of Homogeneity and Stability of the Emulsions
3.4.1. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
3.4.2. Microscopic Image Analysis
3.5. Preliminary Results of In Vitro Diffusion Study
4. Materials and Methods
4.1. Materials
Active Compounds
4.2. Formulation of O/W Emulsions
4.3. Methods
4.3.1. Stability Evaluation
pH Measurements
Conductivity Measurements
Phase Separation
4.3.2. Assessment of Total Phenolic Contents in Emulsions
4.3.3. Determination of Antioxidant Activity of Emulsions
2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Assay (DPPH Method)
2,2′-Azino-bis(3-ethylbenzothiazoline) 6-sulfonic acid) Radical Scavenging Assay (ABTS Method)
4.3.4. Microbiological Control
4.3.5. Analysis of Homogeneity and Stability of the Emulsions
Microscopic Image Analysis
Physical-Chemical Characterization of Emulsions
4.3.6. In Vitro Diffusion Methodology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kerscher, M.; Buntrock, H. Update on cosmeceuticals. J. Dtsch. Dermatol. Gesell 2011, 9, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Araviiskaia, E.; Berardesca, E.; Bieber, T.; Hawk, J.; Sanchez-Viera, M.; Wolkenstein, P. The science of dermocosmetics and its role in dermatology. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.E.; Perju, M.D.; Tămaș, A. Systems theory and the study of cosmetic products. J. Eng. Sci. Innov. 2022, 7, 45–58. [Google Scholar] [CrossRef]
- Larry, E.; Millikan, M.D. Cosmetology, cosmetics, cosmeceuticals: Definitions and regulations. Clin. Dermatol. 2001, 19, 371–374. [Google Scholar]
- Hameed, A.; Fatima, G.R.; Malik, K.; Muqadas, A.; Fazal-ur-Rehman, M. Scope of Nanotechnology in Cosmetics: Dermatology and Skin Care Products. J. Med. Chem. Sci. 2019, 2, 9–16. [Google Scholar]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Klein, K. Combating Oxidative Stress with a Healthy Lifestyle in Antioxidants. In Food, Vitamins and Supplements, 1st ed.; Dasgupta, A., Klein, K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 317–333. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in Dermatology. Redox Rep. Commun. Free Radic. Res. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Martins, T.E.A.; Sales de Oliveira Pinto, C.A.; Costa de Oliveira, A.; Velasco, M.V.R.; Guitiérrez, A.R.G.; Rafael, M.F.C.; Tarazona, J.P.H.; Retuerto-Figueroa, M.G. Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Sci. Pharm. 2020, 88, 27. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Oresajo, C.; Pillai, S.; Yatskayer, M.; Puccetti, G.; McDaniel, D.H. Antioxidants and Skin Aging: A Review. Cosmet. Dermatol. 2009, 22, 563–570. [Google Scholar]
- Pai, V.V.; Shukla, P.; Kikkeri, N.N. Antioxidants in dermatology. Indian Dermatol. Online J. 2014, 5, 210–214. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, J.; Lewis, J. The role of cosmeceuticals in dermatology. In Cosmetic Formulation of Skin Care Products, 1st ed.; Draelos, Z.D., Thaman, L.A., Eds.; Publisher by CRC Press: Boca Raton, FL, USA, 2006; pp. 189–203. [Google Scholar]
- Draelos, Z.D. (Ed.) Cosmetic Dermatology: Products and Procedure, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022. [Google Scholar]
- Rähse, W. (Ed.) Cosmetic Creams Development, Manufacture and Marketing of Effective Skin Care Products; Wiley-VCH Publisher: Weiheim, Germany, 2020. [Google Scholar]
- Djiobie Tchienou, G.E.; Tsatsop Tsague, R.K.; Mbam Pega, T.F.; Bama, V.; Bamseck, A.; Dongmo Sokeng, S.; Ngassoum, M.B. Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients. Cosmetics 2018, 5, 7. [Google Scholar] [CrossRef]
- Tiwari, U.; Ganesan, N.G.; Junnarkar, J.; Rangarajan, V. Toward the formulation of bio-cosmetic nanoemulsions: From plant-derived to microbial-derived ingredients. J. Dispers. Sci. Technol. 2022, 43, 1061–1068. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Noamane, T.; Meryem, B.; Hakiki, M.; Abdellatif, H.; Isao, K.; Marcos, N.; Kenichi, T.; Hiroko, I.; Mitsutoshi, N. Stability characteristics of O/W emulsions prepared using purified glycyrrhizin or a non-purified glycyrrhizin-rich extract from liquorice root (Glycyrrhiza glabra). Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126006. [Google Scholar]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Turcov, D.; Rusu, L.; Zbranca, A.; Suteu, D. New dermatocosmetic formulations using bioactive compounds from indigenous natural sources. Bull. IPI 2020, 66, 67–76. [Google Scholar]
- Mocan, A.; Diuzheva, A.; Badarau, S.; Moldovan, C.; Andruch, V.; Carradori, S.; Campestre, C.; Tartaglia, A.; De Simone, M.; Vodnar, D.; et al. Liquid phase and microwave-assisted extractions for multicomponent phenolic pattern determination of Five Romanian Galium species coupled with bioassays. Molecules 2019, 24, 1226. [Google Scholar] [CrossRef]
- Burlando, B.; Verotta, L.; Cornara, L.; Bottini-Massa, E. Herbal Principles in Cosmetics. In Properties and Mechanisms of Action; CRC Press Taylor & Francis Group: Abingdon, UK, 2010; pp. 9–26. [Google Scholar]
- Poljšak, P.; Kreft, S.; Kočevar-Glavač, N. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Sarkar, R.; Podder, I.; Gokhale, N.; Jagadeesan, S.; Garg, V.K. Use of vegetable oils in dermatology: An overview. Int. J. Dermatol. 2017, 56, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, N.; Glavač, N.K. Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Front. Pharmacol. 2022, 13, 868461. [Google Scholar] [CrossRef] [PubMed]
- De Navarre, M.G. Oils and fats, the historical cosmetics. J. Am. Oil Chem. Soc. 1978, 55, 435–437. [Google Scholar] [CrossRef]
- Hubert, C.; Meriadec, C.; Panizza, P.; Artzner, F.; de Clermont-Gallerande, H. Comparison between a wax/volatile oil mixture and vegetable butters in long lasting make up formula: A rheological and structural study compared to product performance. OCL Oilseeds Fats Crops Lipids 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Fréville, V.; Lavarde, M.; Pensé-Lhéritier, A.M. Caractéristiques sensorielles des corps gras. In Matières Premières Cosmétiques Ingrédients Sensoriels; Grisel, M., Savary, G., Eds.; Cosmetic Valley: Chartres, France, 2020; pp. 29–50. [Google Scholar]
- Ngbolua, K.N.; Bongo Ngiala, G.; Inkoto Liyongo, C.; Masengo Ashande, C.; Lufuluabo Lufuluabo, G.; Mukiza, J.; Mpiana, P.T. A mini-review on the Phytochemistry and Pharmacology of the medicinal plant species Persea americana Mill. (Lauraceae). Discov. Phytomed. 2019, 6, 102–111. [Google Scholar] [CrossRef]
- Chen, Y.; Biresaw, G.; Cermak, S.C.; Isbell, T.A.; Ngo, H.L.; Chen, L.; Durham, A.L. Fatty Acid Estolides: A Review. J. Am. Oil Chem. Soc. 2020, 97, 231–241. [Google Scholar] [CrossRef]
- Adarsh Krishna, T.P.; Edachery, B.; Athalathil, S. Bakuchiol—A natural meroterpenoid: Structure, isolation, synthesis and functionalization approaches. RSC Adv. 2022, 12, 8815–8832. [Google Scholar] [CrossRef]
- Puyana, C.; Chandan, N.; Tsoukas, M. Applications of bakuchiol in dermatology: Systematic review of the literature. J. Cosmet. Dermatol. 2022, 21, 1–8. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Vaughni, A.R.; Nguyen, M.; Reiter, P.; Bosanac, S.; et al. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br. J. Dermatol. 2019, 180, 253–254. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Clares, B.; Morales, M.E.; Cazalla, S.; Gallardo, V. Preparation and stability of cosmetic formulations with an anti-aging peptide. J. Cosmet. Sci. 2007, 58, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics—A review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A. (Ed.) Emulsifiers: Properties, Functions and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015. [Google Scholar]
- Pradilla, F.R.D.; Cruz, J.C.; Alvarez, O. Emerging Emulsifiers: Conceptual Basis for the Identification and Rational Design of Peptides with Surface Activity. Int. J. Mol. Sci. 2021, 22, 4615. [Google Scholar]
- Hu, Y.T.; Ting, Y.; Hu, J.Y.; Hsieh, S.C. Techniques and methods to study functional characteristics of emulsion systems. J. Food Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasa, A.M.; Suteu, D. Antioxidants from Galium verum as Ingredients for the Design of New Dermatocosmetic Products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Blaga, A.C.; Ibanescu, C.; Danu, M.; Trifan, A.; Zbranca, A.; Suteu, D. Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties. Pharmaceutics 2022, 14, 2376. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Apreutesei (Ciuperca), O.T.; Trifan, A.; Puitel, A.C.; Suteu, D. Valorization of Bioactive Compounds from Residual Saffron Biomass (Crocus sativus L.) for Obtaining of High Value Added Dermato-Cosmetic Products. BioResource 2022, 17, 4730–4744. [Google Scholar] [CrossRef]
- Pavun, L.; Ðikanovic, P.; Jelikic-Stankov, M.; Ðurdevic, D.; Uskokovic-Markovic, S. Determination of flavonoids and total polyphenol contents in commercial apple juices. Czech J. Food Sci. 2018, 36, 233–238. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Luca, S.V.; Kulinowski, L.; Ciobanu, C.; Zengin, G.; Czerwinska, M.E.; Granica, S.; Xiao, J.; Skalicka-Wozniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- ISO 18415:2007; Cosmetics—Microbiology—Detection of Specified and Non-Specified Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Turcov, D.; Peptu, A.C.; Barna, A.S.; Zbranca, A.; Suteu, D. In vitro evaluation of the dermatocosmetic emulsions based on Lady’s Bedstraw (Galium verum) alchoolic extracts. In Proceedings of the 10th IEEE International Conference on E-Health and Bioengineering—EHB 2022, Iasi, Romania, 17–18 November 2022. [Google Scholar]
- Bujor, A.; Ochiuz, L.; Sha‘at, M.; Stoleriu, I.; Stamate Iliuta, M.; Luca, S.V.; Miron, A. Chemical, antioxidant and In vitro permeation and penetration studies of extracts obtained from Viburnum opulus and Crataegus pentagyna. Farmacia 2020, 68, 672–678. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Quantification of skin penetration of antioxidants of varying lipophilicity. Int. J. Cosmet. Sci. 2013, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. Review Article. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Vasiljević, D.D.; Parojčić, J.V.; Primorac, M.M.; Vuleta, G.M. Rheological and droplet size analysis of W/O/W multiple emulsions containing low concentrations of polymeric emulsifiers. J. Serb. Chem. Soc. 2009, 74, 801–816. [Google Scholar] [CrossRef]
- Sinha, V.R.; Mumar, A. Multiple Emulsions: An overview of Formulation, Characterization, Stability and Applications. Indian J. Pharm. Sci. 2002, 64, 191–199. [Google Scholar]
- Barna, A.S.; Maxim, C.; Trifan, A.; Șuteu, D.; Blaga, A.C.; Turcov, D. New cosmetic preparations based on N-Prolyl Palmitoyl Tripeptide-56 Acetate and Bakuchiol Complex with anti-skin anti-aging properties. In Proceedings of the Summer Edition, International Conference on Radiation in Various Field of Research—RAD, 10th Jubilee, Herceg Novi, Montenegro, 25–29 July 2022. virtual poster. [Google Scholar]
- Nagarnaik, M.; Sarjoshi, A.; Linge, P.; Bhore, S.; Pandya, G. A microbial study of some cosmetics and raw materials used in personal care products in urban area. Res. J. Top. Cosmet. Sci. 2015, 6, 48–51. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H. An Investigation into Incidences of Microbial Contamination in Cosmeceuticals in the UAE: Imbalances between Preservation and Microbial Contamination. Cosmetics 2020, 7, 92. [Google Scholar] [CrossRef]
- Ibegbulam-Njoku, P.; Chijioke-Osuji, C.C. Microbiological evaluation of cosmetics products sourced in Aba city, Nigeria. Int. J. Sci. Rep. 2016, 2, 74–80. [Google Scholar] [CrossRef]
- Aleem, A.; Khan, M.; Abid, U.; Ishfaq, M.F.; Rouf, A.; Jamshaid, T. Microbial Analysis of Selected Brands of Whitening Creams. Saudi J. Med. Pharm. Sci. 2020, 6, 178–182. [Google Scholar] [CrossRef]
- Luki´c, M.; Pantelic, I.; Savic, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Bluemke, A.; Ring, A.P.; Immeyer, J.; Hoff, A.; Eisenberg, T.; Gerwat, W.; Meyer, F.; Breitkreutz, S.; Klinger, L.M.; Brandner, J.M.; et al. Multidirectional activity of bakuchiol against cellular mechanisms of facial ageing—Experimental evidence for a holistic treatment approach. Int. J. Cosmet. Sci. 2022, 44, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lee, S.; Choi, W.H.; Lee, Y.; Jo, Y.O.; Ha, T.Y. Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica. J. Med. Food. 2010, 13, 1019–1023. [Google Scholar] [CrossRef]
- Cariola, A.; El Chami, M.; Granatieri, J.; Valgimigli, L. Anti-tyrosinase and antioxidant activity of meroterpene bakuchiol from Psoralea corylifolia (L.). Food Chem. 2023, 405, 134953. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Halina, E.; Ercisli, S.; Szopa, A. Characteristics of bakuchiol—The compound with high biological activity and the main source of its acquisition—Cullen corylifolium (L.) Medik. Nat. Prod. Res. 2021, 35, 5828–5842. [Google Scholar] [CrossRef]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Inhibition of mitochondrial lipid peroxidation by Bakuchiol, a meroterpene from Psoralea corylifolia. Planta Med. 2000, 66, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Akhtar, N. Stability of a cosmetic multiple emulsion loaded with green tea extract. Sci. World J. 2013, 2013, 153695. [Google Scholar] [CrossRef]
- Kim, K.M.; Oh, H.M.; Lee, J.H. Controlling the emulsion stability of cosmetics through shear mixing process. Korea Aust. Rheol. J. 2020, 32, 243–249. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering Emulsions: A Novel Tool for Cosmetic Formulators. Cosmetics 2022, 9, 68. [Google Scholar] [CrossRef]





| Dermato-Cosmetic Emulsion Samples | |||
| E.1.0. without active substances | E.1.1. 0.5% Bak 0.5% TPA | E.1.2. 1% Bak + 1% TPA | E.1.3. 1% Bak + 2% TPA |
| Dermato-cosmetic emulsion stability after centrifugation test | |||
 |  |  |  |
| Dermato-cosmetic emulsion stability after vortex test | |||
 |  |  |  |
| Sample | TPC (mg GAE/g Emulsion) | DPPH (mg TE/g Emulsion) | ABTS (mg TE/g Emulsion) |
|---|---|---|---|
| E.1.0. | 0.53 ± 0.09 | 0.61 ± 0.03 | 1.72 ± 0.07 |
| E.1.1. | 6.43 ± 0.25 | 1.81 ± 0.01 | 6.12 ± 0.03 |
| E.1.2. | 10.25 ± 0.94 | 2.23 ± 0.03 | 6.50 ± 0.06 |
| E.1.3. | 11.53 ± 0.88 | 2.10 ± 0.04 | 6.31 ± 0.18 |
| Sample | Total Viable Microbiological Count, CFU/g | Total Viable Bacteria Count, CFU/g | Total Viable Yeast and Mold Count, CFU/g | Presence of Pathogenic Contaminants |
|---|---|---|---|---|
| E 1.0. | 80 | 80 | 0 | absent |
| E 1.1. | 40 | 40 | 0 | absent |
| E 1.2. | 0 | 0 | 0 | absent |
| E 1.3. | 0 | 0 | 0 | absent |
| Dermato-Cosmetic Emulsion Samples | |||
|---|---|---|---|
| E.1.0. | E.1.1. | E.1.2. | E.1.3. |
 |  |  |  |
| % (w/w) | Function | INCI Name of Compounds |
|---|---|---|
| Dispersed Phase 23.0% | Emulsifiant | Polyglyceryl-6 Stearate, Polyglyceryl-6 Behenate |
| Co-emulsifiant | Glyceryl stearate | |
| Oil phase | Euterpe oleracea oil | |
| Oenothera biennis oil | ||
| Punica granatum seed oil | ||
| Continuous Phase 73.2% | Aqueous phase | Rosa damascena hydrosol |
| Thickening agent | Xanthan gum | |
| Phase C 3.8% | Active ingredients | BAK and TPA in the established concentration |
| Preservative | benzyl alcohol, salicylic acid, glycerin, sorbic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barna, A.S.; Maxim, C.; Trifan, A.; Blaga, A.C.; Cimpoesu, R.; Turcov, D.; Suteu, D. Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 7004. https://doi.org/10.3390/ijms24087004
Barna AS, Maxim C, Trifan A, Blaga AC, Cimpoesu R, Turcov D, Suteu D. Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress. International Journal of Molecular Sciences. 2023; 24(8):7004. https://doi.org/10.3390/ijms24087004
Chicago/Turabian StyleBarna, Ana Simona, Claudia Maxim, Adriana Trifan, Alexandra Cristina Blaga, Ramona Cimpoesu, Delia Turcov, and Daniela Suteu. 2023. "Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress" International Journal of Molecular Sciences 24, no. 8: 7004. https://doi.org/10.3390/ijms24087004
APA StyleBarna, A. S., Maxim, C., Trifan, A., Blaga, A. C., Cimpoesu, R., Turcov, D., & Suteu, D. (2023). Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress. International Journal of Molecular Sciences, 24(8), 7004. https://doi.org/10.3390/ijms24087004









