RAGE–TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages
Abstract
:1. Introduction
2. Results
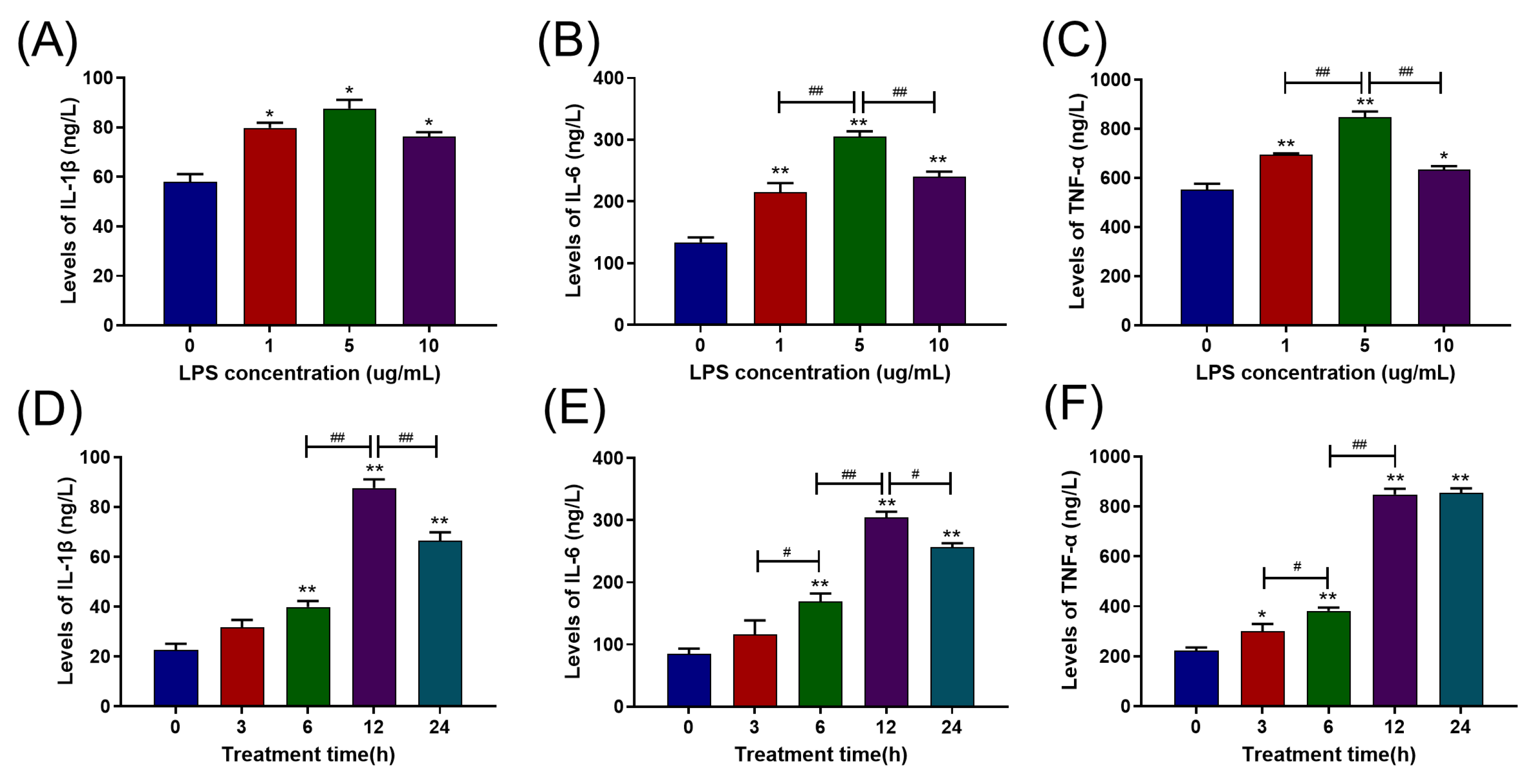
2.1. LPS Increased Pro-Inflammatory Cytokine Release in Primary BAMs in a Dose- and Time-Dependent Manner
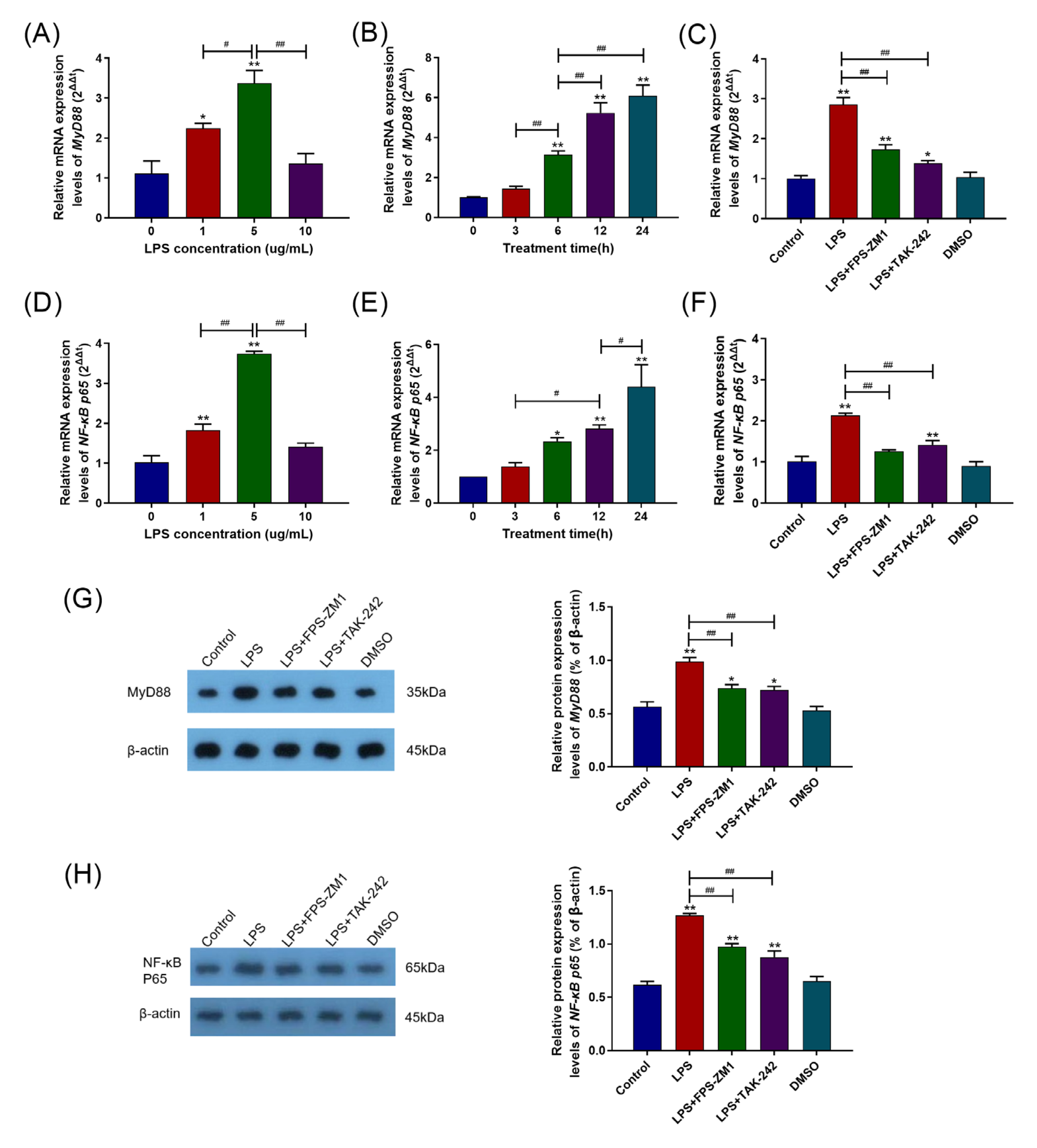
2.2. TLR4, RAGE, and Their Interaction Were Involved in the Inflammatory Response Caused by LPS
2.3. RAGE and TLR4 Synergistically Activate the MyD88/NF-κB Signaling Pathway in the Inflammation Response Caused by LPS
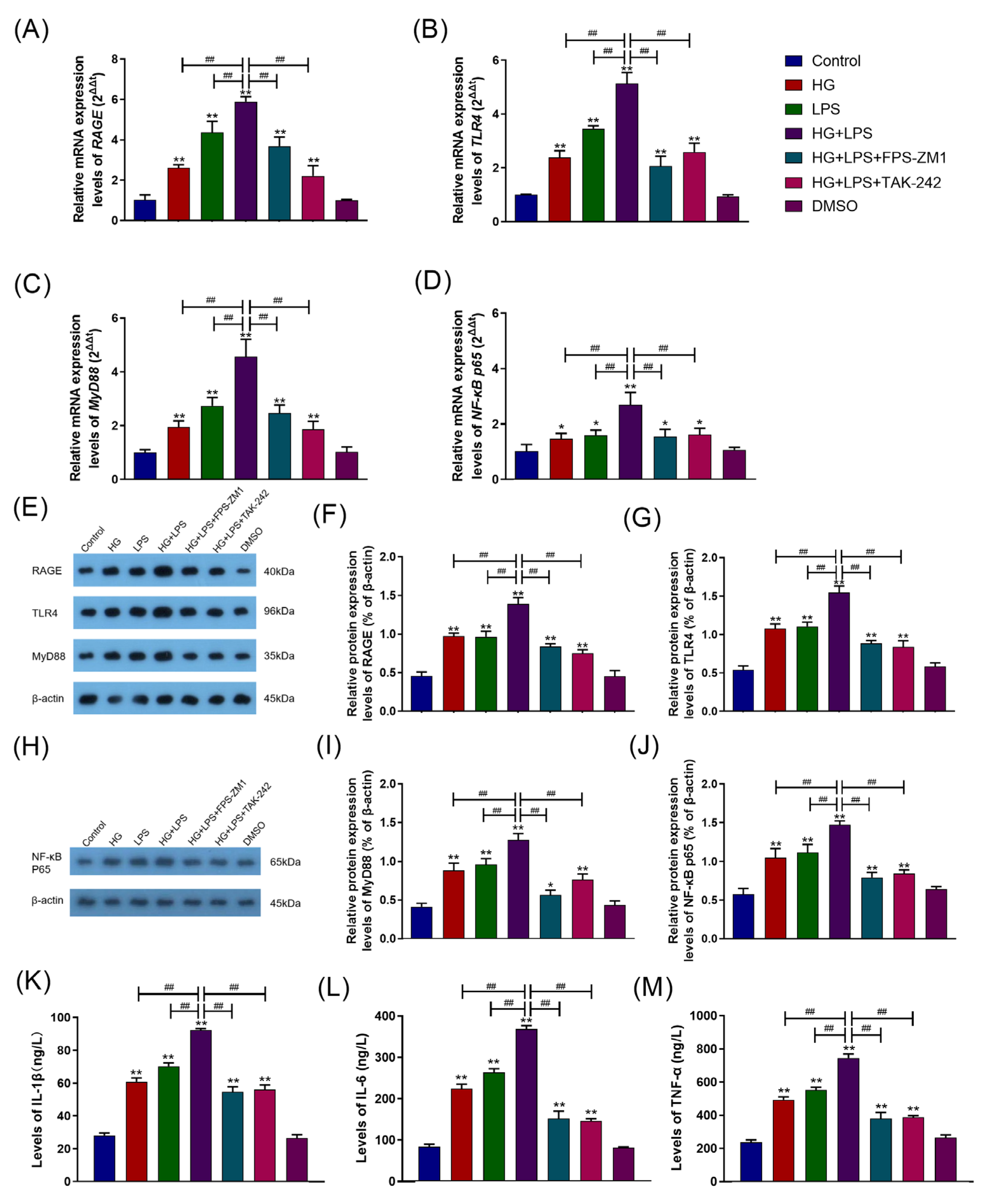
2.4. HG Enhanced LPS-Induced Pro-Inflammatory Cytokine Secretion and Upregulated the RAGE/TLR4/MyD88/NF-κB p65 Pathway in BAMs
2.5. RAGE–TLR4 Crosstalk Regulated the Synergism between HG and LPS on the Inflammatory Response in BAMs
3. Discussions
4. Materials and Methods
4.1. Isolation and Treatment of BAMs
4.2. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.3. Immunoblot Assay
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Earley, B.; Buckham Sporer, K.; Gupta, S. Invited review: Relationship between cattle transport, immunity and respiratory disease. Animal 2017, 11, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Takizawa, K.; Matsuura, T.; Yokosawa, N.; Tosaki, K.; Katsuda, K.; Tanimura, N.; Shibahara, T. Bovine peritonitis associated with Mannheimia haemolytica serotype 2 in a three-day-old Japanese Black calf. J. Vet. Med. Sci. 2019, 81, 143–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, D.B.; Timsit, E.; Amat, S.; Abbott, D.W.; Buret, A.G.; Alexander, T.W. The nasopharyngeal microbiota of beef cattle before and after transport to a feedlot. BMC Microbiol. 2017, 17, 70. [Google Scholar]
- Baker, E.H.; Archer, J.R.H.; Srivastava, S.A. Hyperglycemia, Lung Infection, and Inflammation. Clin. Pulm. Med. 2009, 16, 258–264. [Google Scholar] [CrossRef]
- Yu, J.; Shi, J.; Wang, D.; Dong, S.; Zhang, Y.; Wang, M.; Gong, L.; Fu, Q.; Liu, D. Heme Oxygenase-1/Carbon Monoxide-regulated Mitochondrial Dynamic Equilibrium Contributes to the Attenuation of Endotoxin-induced Acute Lung Injury in Rats and in Lipopolysaccharide-activated Macrophages. Anesthesiology 2016, 125, 1190–1201. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Liu, W.; Du, S.; Zhang, Y.; Gong, L.; Dong, S.; Li, X.; Gao, Q.; Yang, J.; et al. Heme oxygenase-1 protects against endotoxin-induced acute lung injury depends on NAD+-mediated mitonuclear communication through PGC1α/PPARγ signaling pathway. Inflamm. Res. 2022, 71, 1095–1108. [Google Scholar] [CrossRef]
- Mitchell, G.; Hattingh, J.; Ganhao, M. Stress in cattle assessed after handling, after transport and after slaughter. Vet. Rec. 1988, 123, 201–205. [Google Scholar] [CrossRef]
- Hagenmaier, J.A.; Reinhardt, C.D.; Bartle, S.J.; Henningson, J.N.; Ritter, M.J.; Calvo-Lorenzo, M.S.; Vogel, G.J.; Guthrie, C.A.; Siemens, M.G.; Thomson, D.U. Effect of handling intensity at the time of transport for slaughter on physiological response and carcass characteristics in beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 2017, 95, 1963–1976. [Google Scholar]
- Wu, C.-P.; Huang, K.-L.; Peng, C.-K.; Lan, C.-C. Acute Hyperglycemia Aggravates Lung Injury via Activation of the SGK1-NKCC1 Pathway. Int. J. Mol. Sci. 2020, 21, 4803. [Google Scholar] [CrossRef]
- Aljada, A.; Friedman, J.; Ghanim, H.; Mohanty, P.; Hofmeyer, D.; Chaudhuri, A.; Dandona, P. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism 2006, 55, 1177–1185. [Google Scholar] [CrossRef]
- Lapar, D.J.; Hajzus, V.A.; Zhao, Y.; Lau, C.L.; French, B.A.; Kron, I.L.; Sharma, A.K.; Laubach, V.E. Acute hyperglycemic exacerbation of lung ischemia-reperfusion injury is mediated by receptor for advanced glycation end-products signaling. Am. J. Respir. Cell Mol. Biol. 2012, 46, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viurcos-Sanabria, R.; Escobedo, G. Immunometabolic bases of type 2 diabetes in the severity of COVID-19. World J. Diabetes 2021, 12, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, Y.; Zhang, X.; Zhao, X.; Li, R. Protective effects of thalidomide on high-glucose-induced podocyte injury through in vitro modulation of macrophage M1/M2 differentiation. J. Immunol. Res. 2020, 8263598. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tang, J.; Chen, Q.; Tang, D.; Liu, M.; Luo, M.; Wang, Y.; Wang, J.; Zhao, Z.; Tang, C.; et al. miR-429 regulates alveolar macrophage inflammatory cytokine production and is involved in LPS-induced acute lung injury. Biochem. J. 2015, 471, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Fang, J.; Zhou, H.; Liu, X.; Su, S.B. High glucose induces and activates Toll-like receptor 4 in endothelial cells of diabetic retinopathy. Diabetol. Metab. Syndr. 2015, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Kanneganti, T.-D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003, 198, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Yin, C.; Shou, S.; Wang, J.; Yu, L.; Li, X.; Chai, Y. Ulinastatin protects against LPS-induced acute lung injury by attenuating TLR4/NF-κB pathway activation and reducing inflammatory mediators. Shock 2018, 50, 595–605. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Ling, Z.; Hwang, D.H.; Jialal, I. High Glucose Induces Toll-Like Receptor Expression in Human Monocytes: Mechanism of Activation. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef] [Green Version]
- Mudaliar, H.; Pollock, C.; Jin, M.; Wu, H.; Chadban, S.; Panchapakesan, U. The Role of TLR2 and 4-Mediated Inflammatory Pathways in Endothelial Cells Exposed to High Glucose. PLoS ONE 2014, 9, e108844. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, A.; Shibutani, Y.; Seno, K.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Advanced glycation end products and lipopolysaccharides stimulate interleukin-6 secretion via the RAGE/TLR4-NF-κB-ROS pathways and resveratrol attenuates these inflammatory responses in mouse macrophages. Exp. Ther. Med. 2017, 14, 4363–4370. [Google Scholar] [PubMed] [Green Version]
- Luan, Z.-G.; Zhang, H.; Yang, P.-T.; Ma, X.-C.; Zhang, C.; Guo, R.-X. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology 2010, 215, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, P.; Zhang, J.-C.; Zhang, Q.; Yao, S.-L. Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int. J. Mol. Med. 2017, 40, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam VS, R.R.; Faiz, A.; Lam, M.; Rathnayake, S.N.H.; Ditz, B.; Pouwels, S.D.; Brandsma, C.-A.; Timens, W.; Hiemstra, P.S.; Tew, G.W.; et al. RAGE and TLR4 differentially regulate airway hyperresponsiveness: Implications for COPD. Allergy 2021, 76, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-Induced Reactive Oxygen Species Increase Expression of the Receptor for Advanced Glycation End Products (RAGE) and RAGE Ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wu, J.; Guo, X.; Huang, X.; Huang, Q. RAGE Plays a Role in LPS-Induced NF-κB Activation and Endothelial Hyperpermeability. Sensors 2017, 17, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, Z.A.; Armour, C.L.; Phipps, S.; Sukkar, M.B. RAGE and TLRs: Relatives, friends or neighbours? Mol. Immunol. 2013, 56, 739–744. [Google Scholar] [CrossRef]
- Lin, L. RAGE on the Toll Road? Cell Mol. Immunol. 2006, 3, 351–358. [Google Scholar]
- Gąsiorowski, K.; Brokos, B.; Echeverria, V.; Barreto, G.E.; Leszek, J. RAGE-TLR Crosstalk Sustains Chronic Inflammation in Neurodegeneration. Mol. Neurobiol. 2018, 55, 1463–1476. [Google Scholar] [CrossRef]
- Van Engen, N.K.; Coetzee, J.F. Effects of transportation on cattle health and production: A review. Anim. Health Res. Rev. 2018, 19, 142–154. [Google Scholar] [CrossRef]
- Talwar, H.; Bauerfeld, C.; Bouhamdan, M.; Farshi, P.; Liu, Y.; Samavati, L. MKP-1 negatively regulates LPS-mediated IL-1β production through p38 activation and HIF-1α expression. Cell Signal. 2017, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Shin, J.-S.; Jang, D.S.; Lee, K.-T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Li, X.; Zhao, E.; Li, L.; Ma, X. Unfractionated Heparin Modulates Lipopolysaccharide-Induced Cytokine Production by Different Signaling Pathways in THP-1 Cells. J. Interferon Cytokine Res. 2018, 38, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lee, S.-M. Necrostatin-1 protects against D-Galactosamine and lipopolysaccharide-induced hepatic injury by preventing TLR4 and RAGE signaling. Inflammation 2017, 40, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Ramirez-Carrozzi, V.R.; Yamamoto, M.; Mizutani, T.; Kuwata, H.; Iba, H.; Matsumoto, M.; Honda, K.; Smale, S.T.; Takeda, K. Class-specific regulation of pro-inflammatory genes by MyD88 pathways and IkappaBzeta. J. Biol. Chem. 2008, 283, 12468–12477. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, M.; Murata, H.; Yamamoto, K.-I.; Ono, T.; Sakaguchi, Y.; Motoyama, A.; Hibino, T.; Kataoka, K.; Huh, N.-h. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS ONE 2011, 6, e23132. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, Y.; Liu, X.; Wang, N.; Cao, H.; Lu, Y.; Zhou, H.; Zheng, J. Inhibition of clathrin/dynamin-dependent internalization interferes with LPS-mediated TRAM-TRIF-dependent signaling pathway. Cell Immunol. 2012, 274, 121–129. [Google Scholar] [CrossRef]
- Niederberger, E.; Geisslinger, G. Proteomics and NF-κB: An update. Expert Rev. Proteom. 2013, 10, 189–204. [Google Scholar] [CrossRef]
- Byrd-Leifer, C.A.; Block, E.F.; Takeda, K.; Akira, S.; Ding, A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur. J. Immunol. 2001, 31, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Y.; Herrera, M.T.; Soldevila, G.; Garcia-Garcia, L.; Fabián, G.; Pérez-Armendariz, E.M.; Bobadilla, K.; Guzmán-Beltrán, S.; Sada, E.; Torres, M. High glucose concentrations induce TNF-α production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012, 13, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Zhang, N.; Ma, D.; Ma, X.; Wang, Q. Effect of the Rho kinase inhibitor Y-27632 and fasudil on inflammation and fibrosis in human mesangial cells (HMCs) under high glucose via the Rho/ROCK signaling pathway. Int. J. Clin. Exp. Med. 2017, 10, 13224–13234. [Google Scholar]
- Nielsen, T.B.; Pantapalangkoor, P.; Yan, J.; Luna, B.M.; Dekitani, K.; Bruhn, K.; Tan, B.; Junus, J.; Bonomo, R.A.; Schmidt, A.M.; et al. Diabetes Exacerbates Infection via Hyperinflammation by Signaling through TLR4 and RAGE. mBio 2017, 8, e00818-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef] [Green Version]
- Ayala, T.S.; Tessaro, F.H.G.; Jannuzzi, G.P.; Bella, L.M.; Ferreira, K.S.; Martins, J.O. High Glucose Environments Interfere with Bone Marrow-Derived Macrophage Inflammatory Mediator Release, the TLR4 Pathway and Glucose Metabolism. Sci. Rep. 2019, 9, 11447. [Google Scholar] [CrossRef] [Green Version]
- Nareika, A.; Im, Y.-B.; Game, B.A.; Slate, E.H.; Sanders, J.J.; London, S.D.; Lopes-Virella, M.F.; Huang, Y. High glucose enhances lipopolysaccharide-stimulated CD14 expression in U937 mononuclear cells by increasing nuclear factor kappaB and AP-1 activities. J. Endocrinol. 2008, 196, 45–55. [Google Scholar] [CrossRef]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef]
- Prantner, D.; Nallar, S.; Vogel, S.N. The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways. FASEB J. 2020, 34, 15659–15674. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Jia, H.; Li, J.; Liu, X.; Engelhardt, J.F.; Wang, Y. Cloning and identification of microRNAs in bovine alveolar macrophages. Mol. Cell Biochem. 2009, 332, 9–16. [Google Scholar] [CrossRef] [PubMed]



| Group | Treatment |
|---|---|
| Control | 0 μg/mL LPS + 5.5 mM glucose |
| HG | 0 μg/mL LPS + 25.5 mM glucose |
| LPS | 5 μg/mL LPS + 5.5 mM glucose |
| HG +LPS | 5 μg/mL LPS + 25.5 mM glucose |
| HG + LPS + FPS-ZM1 | 5 μg/mL LPS + 25.5 mM glucose +1 μM FPS-ZM1 |
| HG + LPS + TAK-242 | 5 μg/mL LPS + 25.5 mM glucose +10 μM TAK-242 |
| DMSO | 0 μg/mL LPS + 5.5 mM glucose + DMSO |
| Gene Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (3′–5′) |
|---|---|---|
| β-actin | GCCCATCTATGAGGGGTACG | TCACGGACGATTTCCGCT |
| RAGE | GACAGTCGCCCTGCTCATT | CCTCTGGCTGGTTCAGTTCC |
| TLR4 | TGCCTTCACTACAGGGACTTT | TGGGACACCACGACAATAAC |
| NF-κB p65 | GAGATCATCGAGCAGCCCAA | ATAGTGGGGTGGGTCTTGGT |
| MyD88 | AGAAGAGGTGCCGTCGGATGG | TTGGTGTAGTCACAGACAGTGATGAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Li, Y.; Tan, T.; Qi, J.; Fang, J.; Guo, H.; Ren, Z.; Gou, L.; Geng, Y.; Cui, H.; et al. RAGE–TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages. Int. J. Mol. Sci. 2023, 24, 7007. https://doi.org/10.3390/ijms24087007
Yan L, Li Y, Tan T, Qi J, Fang J, Guo H, Ren Z, Gou L, Geng Y, Cui H, et al. RAGE–TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages. International Journal of Molecular Sciences. 2023; 24(8):7007. https://doi.org/10.3390/ijms24087007
Chicago/Turabian StyleYan, Longfei, Yanran Li, Tianyu Tan, Jiancheng Qi, Jing Fang, Hongrui Guo, Zhihua Ren, Liping Gou, Yi Geng, Hengmin Cui, and et al. 2023. "RAGE–TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages" International Journal of Molecular Sciences 24, no. 8: 7007. https://doi.org/10.3390/ijms24087007
APA StyleYan, L., Li, Y., Tan, T., Qi, J., Fang, J., Guo, H., Ren, Z., Gou, L., Geng, Y., Cui, H., Shen, L., Yu, S., Wang, Z., & Zuo, Z. (2023). RAGE–TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages. International Journal of Molecular Sciences, 24(8), 7007. https://doi.org/10.3390/ijms24087007







