Deciphering the Prognostic and Therapeutic Significance of Cell Cycle Regulator CENPF: A Potential Biomarker of Prognosis and Immune Microenvironment for Patients with Liposarcoma
Abstract
:1. Introduction
2. Results
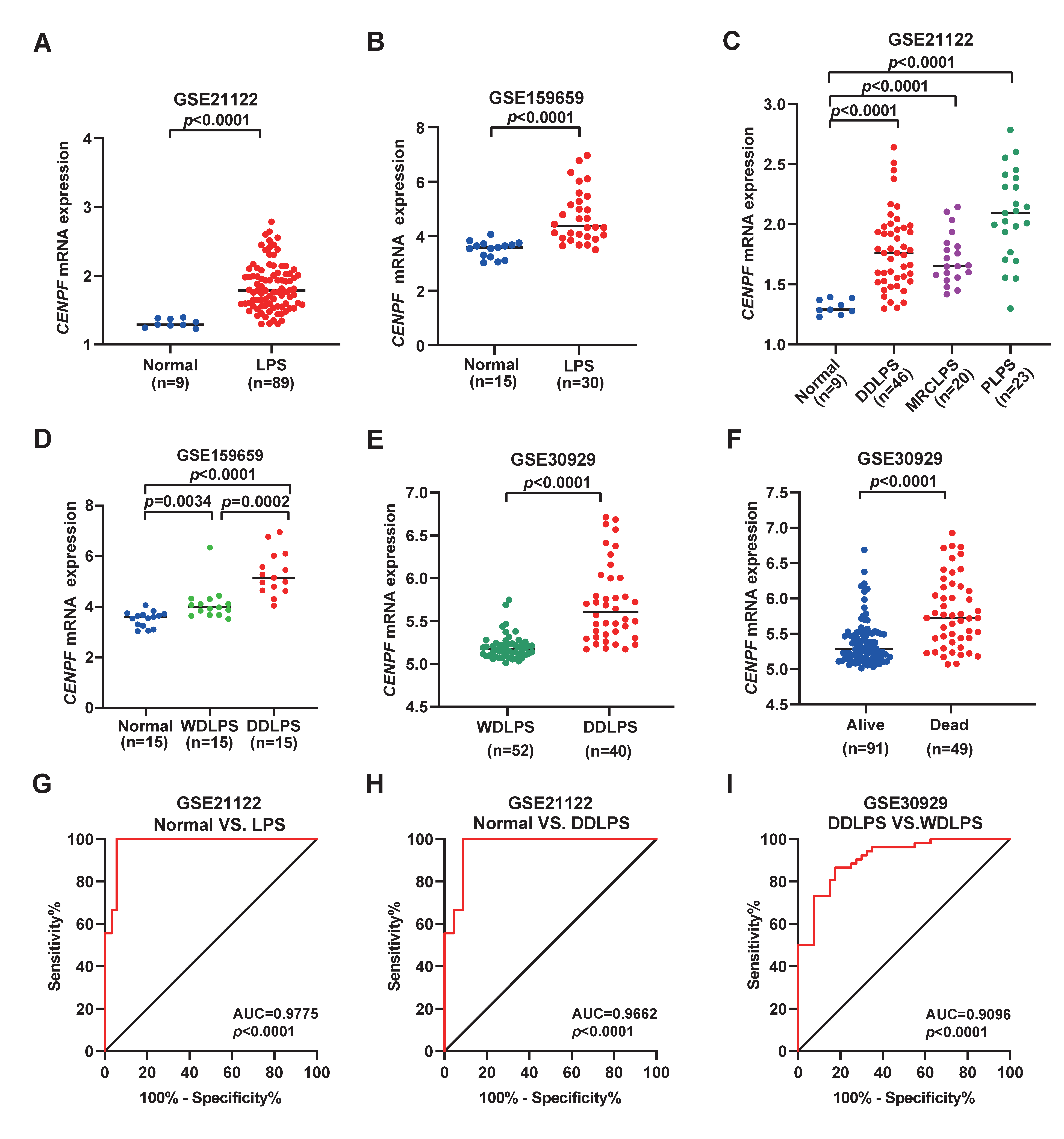
2.1. Cell Cycle-Related Gene CENPF Is upregulated in Liposarcoma
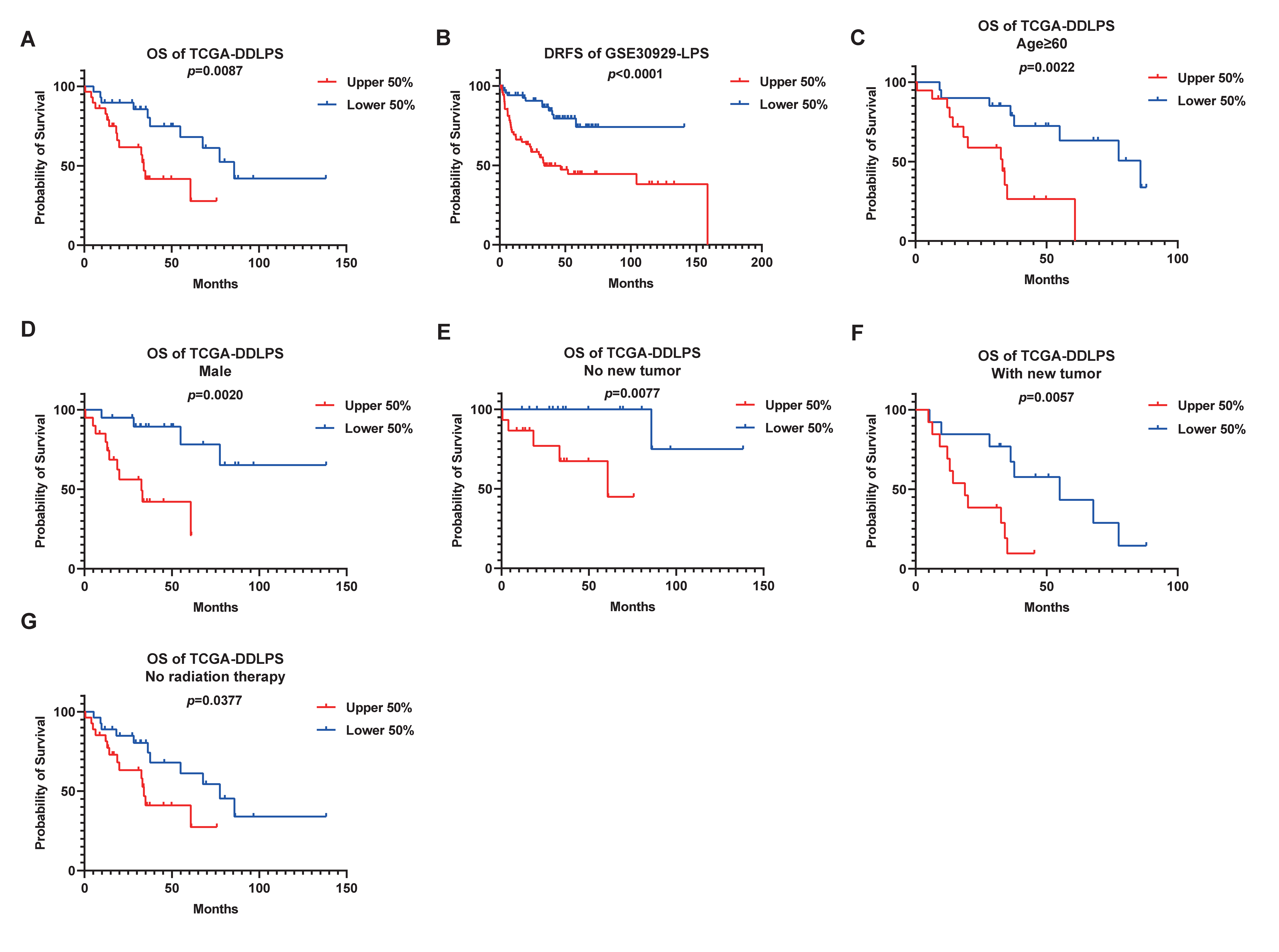
2.2. Overexpression of CENPF Predicts Poor Prognosis of LPS Patients
2.3. Verification of the Prognostic Value of CENPF for LPS Patients by Statistical Analysis
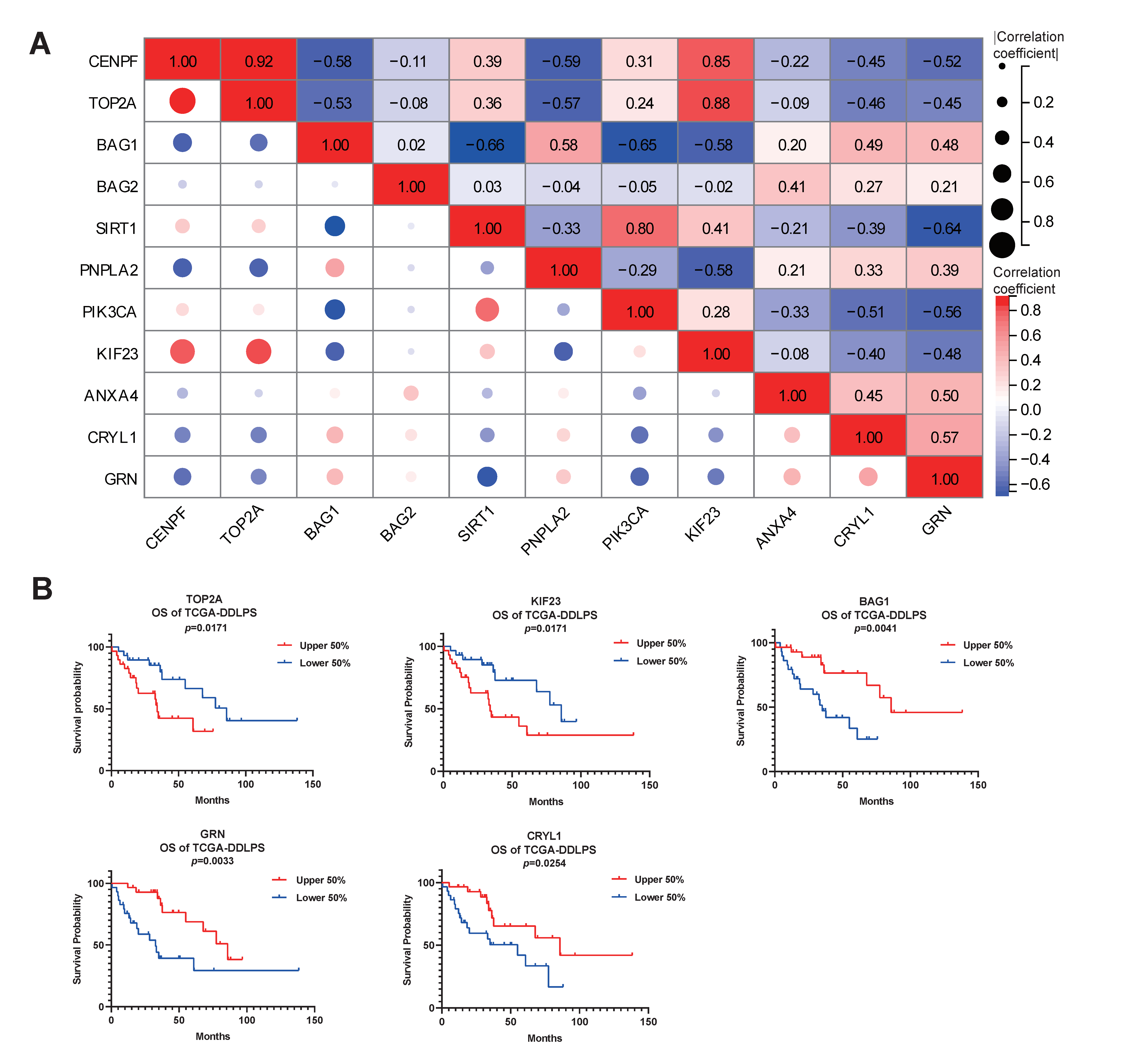
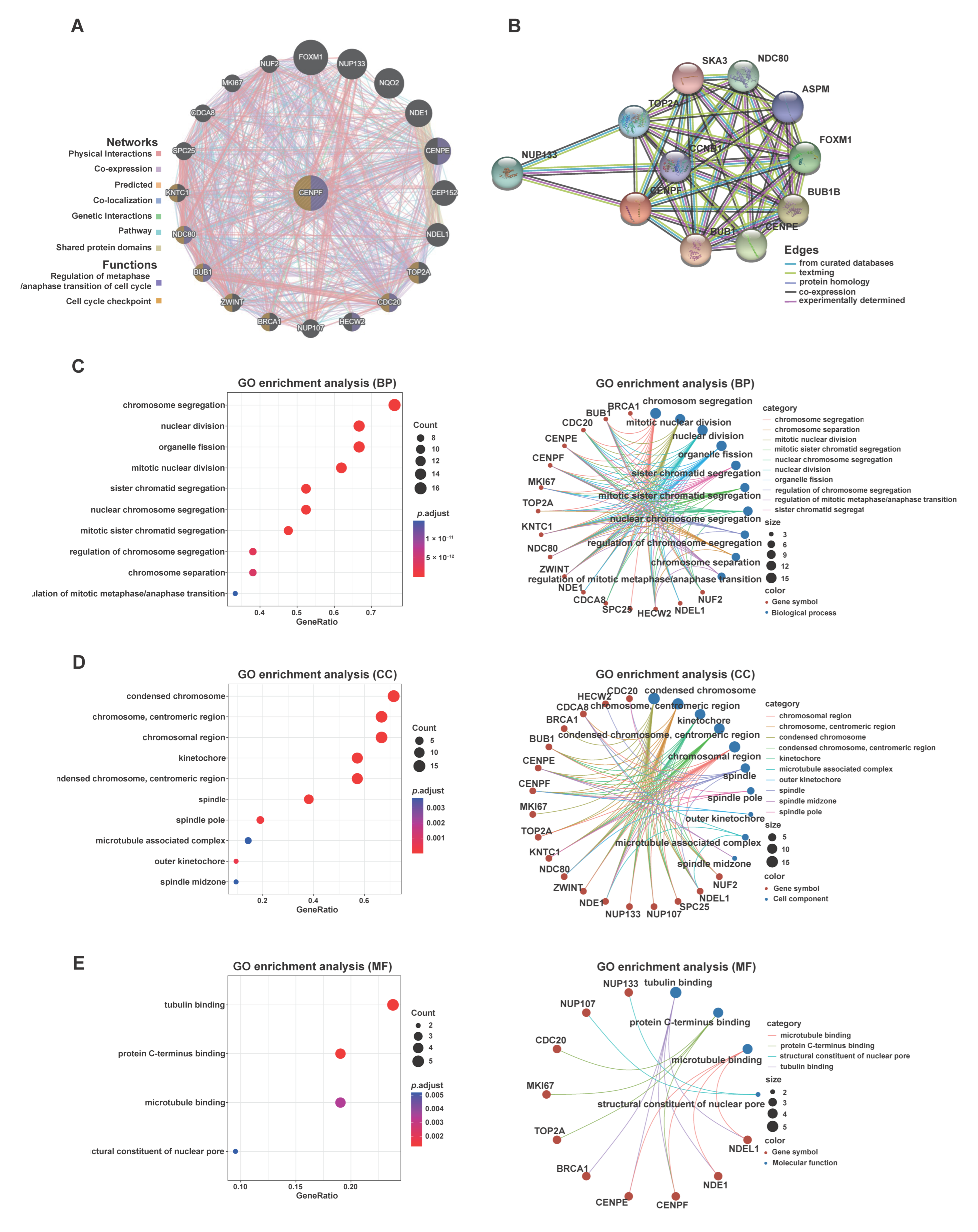
2.4. Elucidation of the Potential Functions and Molecular Mechanisms of CENPF in LPS Development
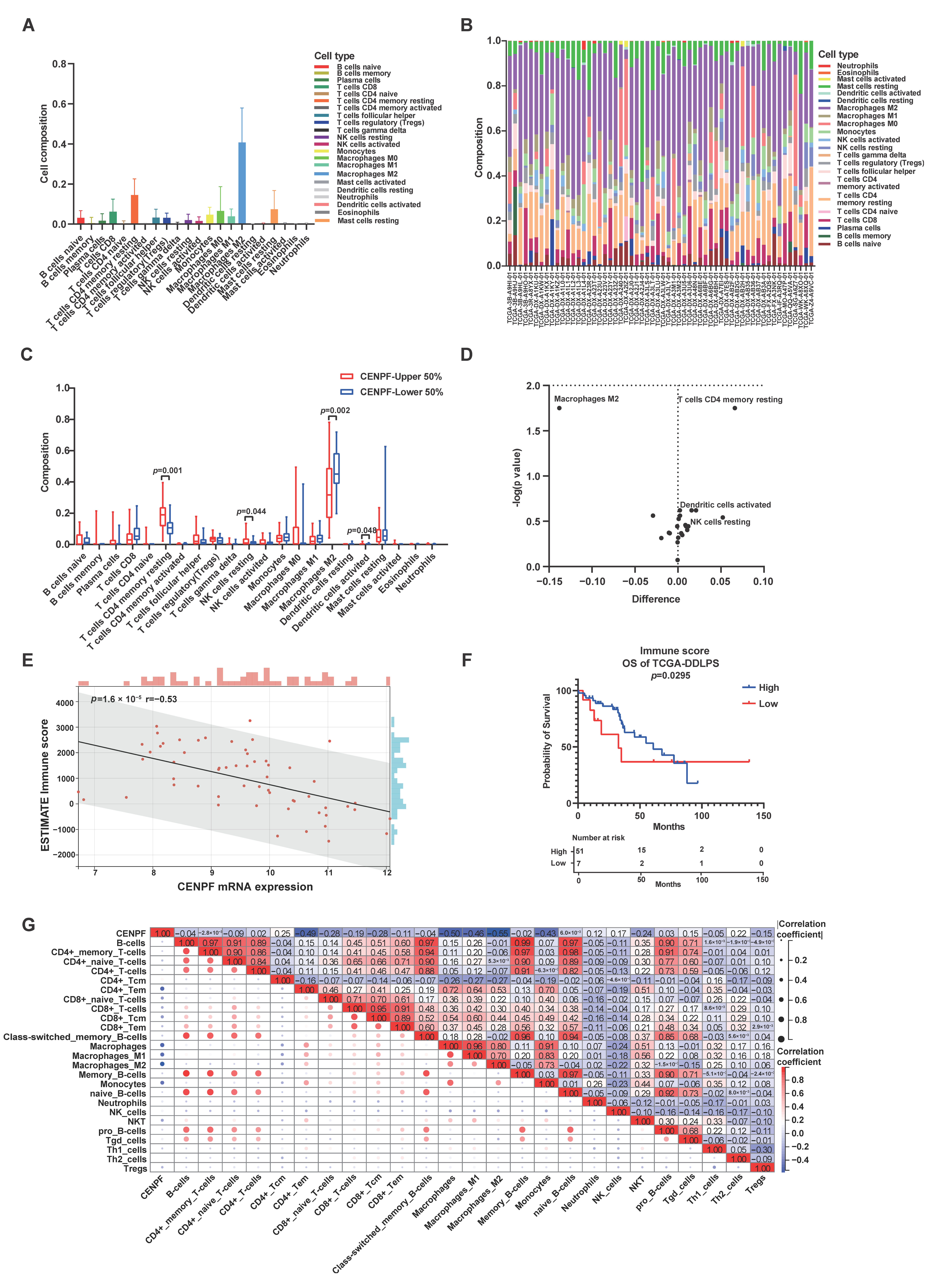
2.5. The Correlation between CENPF Expression and Immune Infiltration Level in LPS
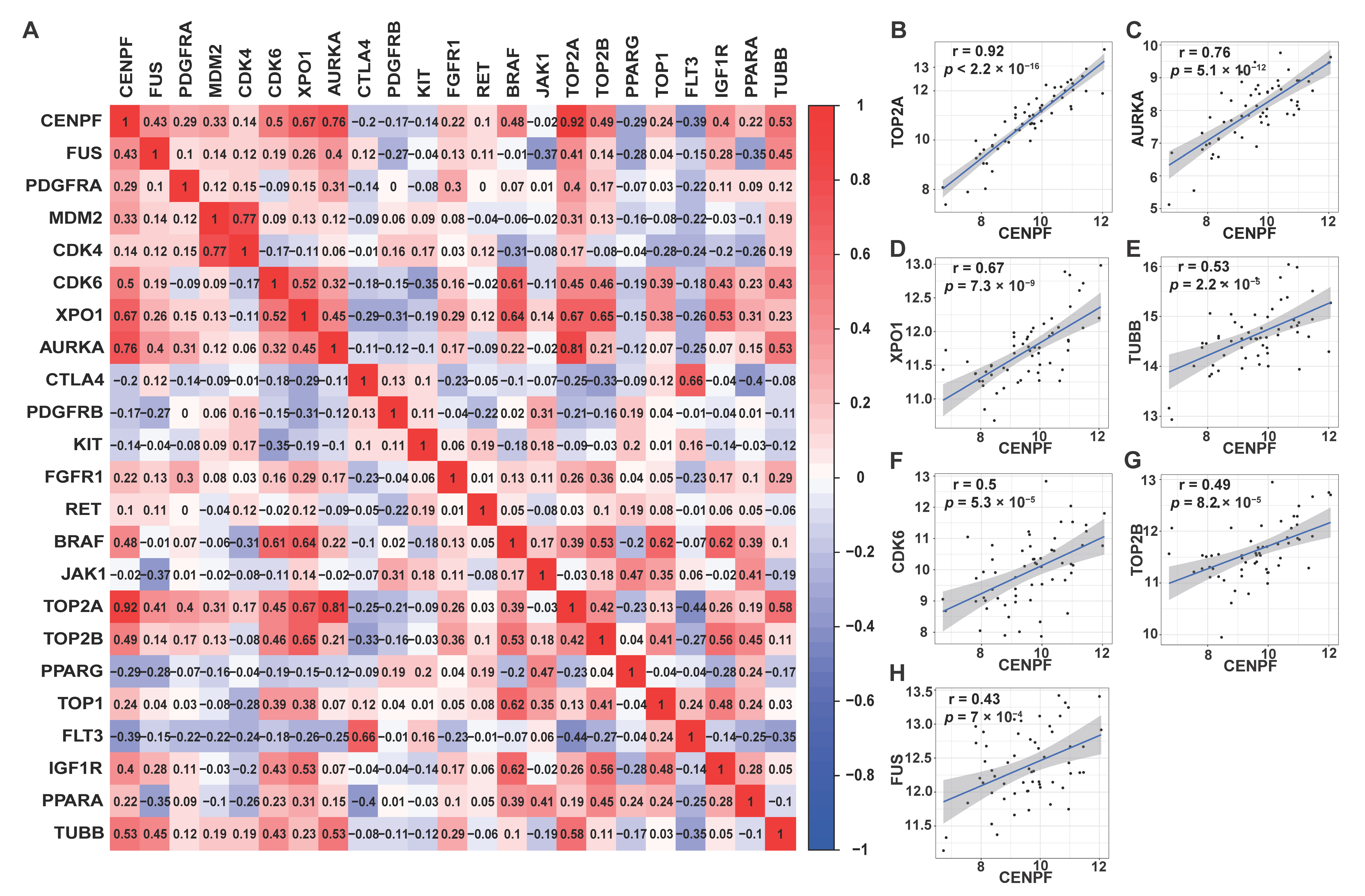
2.6. Therapeutic Drugs for Liposarcoma Patients
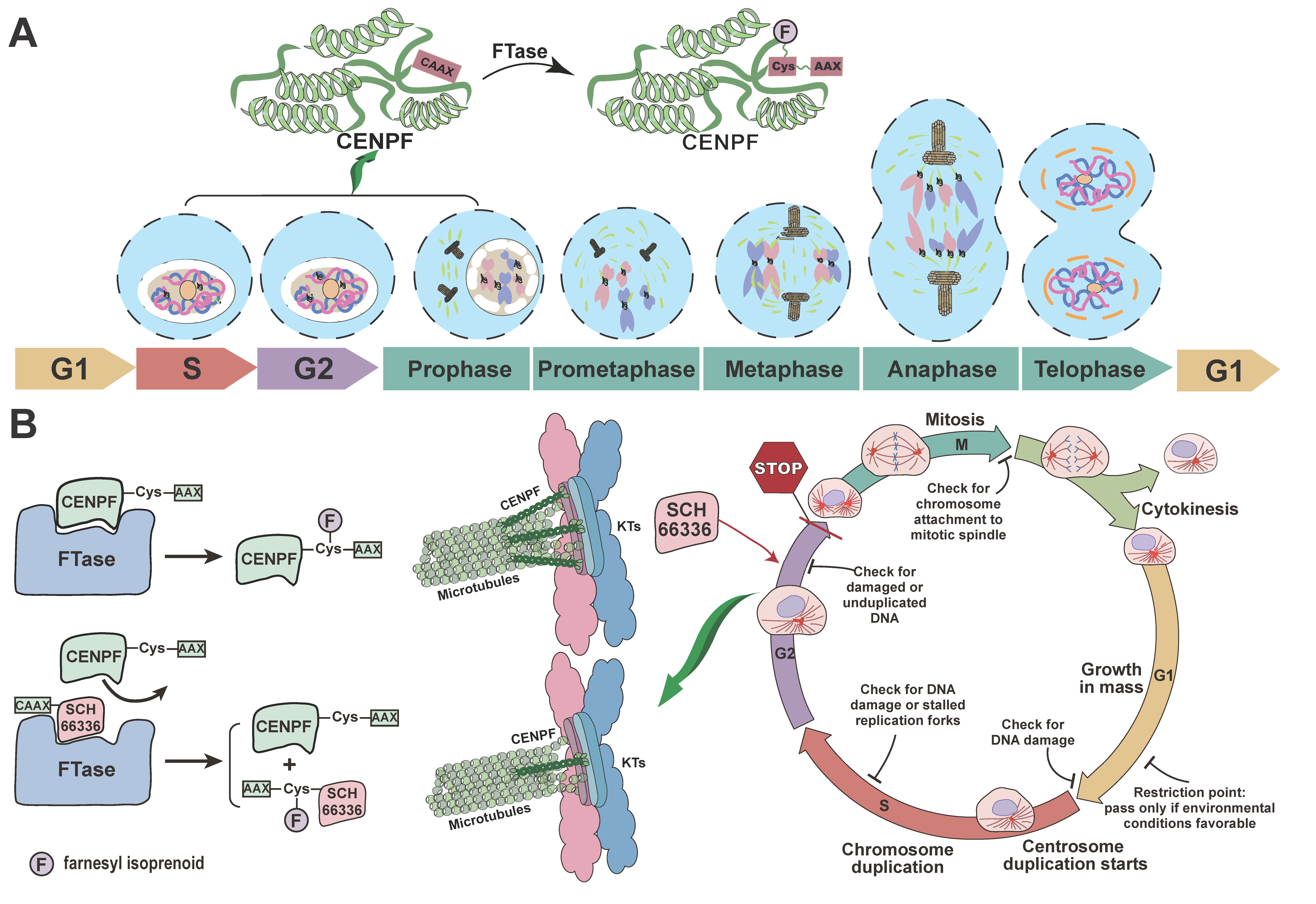
3. Discussion
4. Materials and Methods
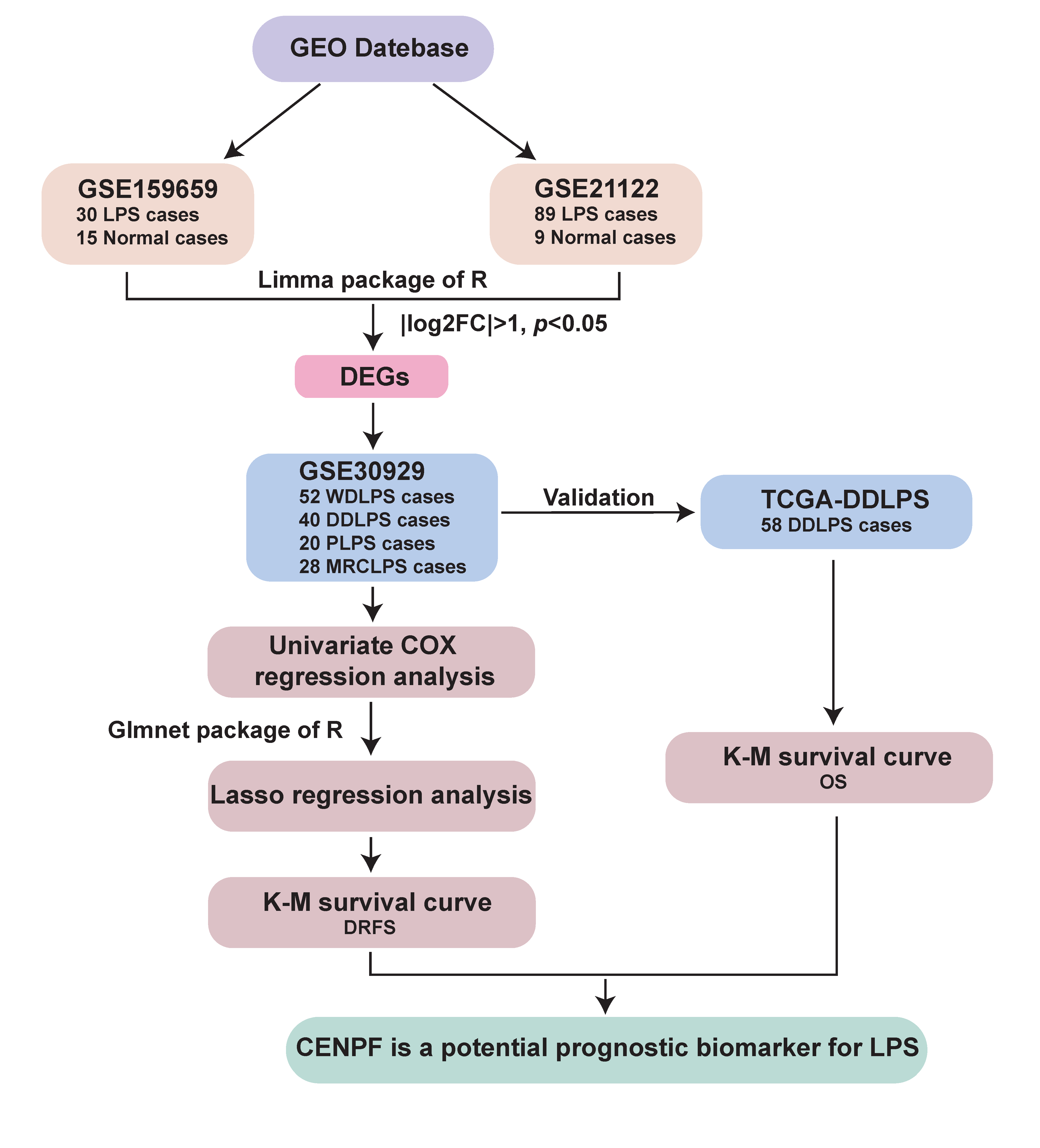
4.1. Data Collection and Interpretation
4.2. Screening of Gene with Potential Prognostic Value for LPS
4.3. Comparison of the Relationship between CENPF Expression and Clinical Features
4.4. Validation of the Prognostic Value of CENPF for LPS
4.5. Construction of co-Expressed Gene and Protein–Protein Interaction (PPI) Network
4.6. Functional Enrichment Analysis of CENPF
4.7. Comprehensive Analysis of Immune Infiltration
4.8. Drug Sensitivity Analysis of CENPF in Soft Tissue Sarcoma
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas Manji, G.; Singer, S.; Koff, A.; Schwartz, G.K. Application of molecular biology to individualize therapy for patients with liposarcoma. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2015, 35, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Ng, V.Y.; Scharschmidt, T.J.; Mayerson, J.L.; Fisher, J.L. Incidence and survival in sarcoma in the United States: A focus on musculoskeletal lesions. Anticancer Res. 2013, 33, 2597–2604. [Google Scholar] [PubMed]
- Haddox, C.L.; Riedel, R.F. Recent advances in the understanding and management of liposarcoma. Fac. Rev. 2021, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qin, Y.; Ouyang, S.; Qian, J.; Tu, S.; Yao, J. Challenging surgical treatment of giant retroperitoneal liposarcoma: A case report. Oncol. Lett. 2022, 24, 314. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Petersen, I.A. Preoperative Radiation for Soft Tissue Sarcomas: How Much Is Needed? Curr. Treat. Options Oncol. 2022, 23, 68–77. [Google Scholar] [CrossRef]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of sarcoma alliance for research through collaboration study 002 [corrected]. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Abdul Razak, A.R.; Bauer, S.; Suarez, C.; Lin, C.C.; Quek, R.; Hütter-Krönke, M.L.; Cubedo, R.; Ferretti, S.; Guerreiro, N.; Jullion, A.; et al. Co-Targeting of MDM2 and CDK4/6 with Siremadlin and Ribociclib for the Treatment of Patients with Well-Differentiated or Dedifferentiated Liposarcoma: Results from a Proof-of-Concept, Phase Ib Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Gits, C.M.; van Kuijk, P.F.; Jonkers, M.B.; Boersma, A.W.; Smid, M.; van Ijcken, W.F.; Coindre, J.-M.; Chibon, F.; Verhoef, C.; Mathijssen, R.H.; et al. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. Int. J. Cancer. 2014, 135, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.; Downs-Kelly, E.; Goldblum, J.R.; Turner, S.; Kulkarni, S.; Tubbs, R.R.; Rubin, B.P.; Skacel, M. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod. Pathol. 2008, 21, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Park, H.; Ha, S.Y.; Paik, K.Y.; Lee, S.E.; Kim, J.M.; Park, J.B.; Kwon, C.D.; Joh, J.-W.; Choi, Y.-L.; et al. CDK4 amplification predicts recurrence of well-differentiated liposarcoma of the abdomen. PLoS ONE 2014, 9, e99452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, M.; Lee, M.Y.; Piplani, H.; Andres, A.M.; Zhou, B.; Yeon, A.; Kim, M.; Kim, H.L.; Kim, J. Centromere protein F (CENPF), a microtubule binding protein, modulates cancer metabolism by regulating pyruvate kinase M2 phosphorylation signaling. Cell Cycle 2018, 17, 2802–2818. [Google Scholar] [CrossRef] [Green Version]
- Musinipally, V.; Howes, S.; Alushin, G.M.; Nogales, E. The microtubule binding properties of CENP-E’s C-terminus and CENP-F. J. Mol. Biol. 2013, 425, 4427–4441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.; Winkfein, R.J.; Mack, G.; Rattner, J.B.; Yen, T.J. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 1995, 130, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.G.; Li, D.; Wang, L.; Su, X.M.; Tang, X.B. CENPF/CDK1 signaling pathway enhances the progression of adrenocortical carcinoma by regulating the G2/M-phase cell cycle. J. Transl. Med. 2022, 20, 78. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Wang, L.; Wang, T.; Tang, X.; Su, X. Centromere Protein F (CENPF) Serves as a Potential Prognostic Biomarker and Target for Human Hepatocellular Carcinoma. J. Cancer. 2021, 12, 2933–2951. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, J.; Lan, J.; Zhou, K.; Gao, Y.; Song, Z.; Deng, Y.; Liu, L.; Dong, Y.; Liu, X. Overexpression of CENPF correlates with poor prognosis and tumor bone metastasis in breast cancer. Cancer Cell Int. 2019, 19, 264. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, K.; Geng, L.; Sun, J.; Xu, W.; Liu, D.; Gong, S.; Zhu, Y. Identification of candidate diagnostic and prognostic biomarkers for pancreatic carcinoma. EBioMedicine 2019, 40, 382–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wang, X.; Zhao, X.; Zhang, X.; Chen, H.; Ma, Y.; Liu, Y. Centromere protein F and Forkhead box M1 correlation with prognosis of non-small cell lung cancer. Oncol. Lett. 2020, 19, 1368–1374. [Google Scholar] [CrossRef]
- Han, Y.; Xu, S.; Cheng, K.; Diao, C.; Liu, S.; Zou, W.; Bi, Y. CENPF promotes papillary thyroid cancer progression by mediating cell proliferation and apoptosis. Exp. Ther. Med. 2021, 21, 401. [Google Scholar] [CrossRef]
- Gobble, R.M.; Qin, L.X.; Brill, E.R.; Angeles, C.V.; Ugras, S.; O’Connor, R.B.; Moraco, N.H.; DeCarolis, P.L.; Antonescu, C.; Singer, S. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res. 2011, 71, 2697–2705. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhang, Q.; Zhao, Y.; Xu, F.; Zhang, J.; He, H.; Wang, X.; Feng, H. Association of sirtuin-1 and vascular endothelial growth factor expression with tumor progression and poor prognosis in liposarcoma. J. Int. Med. Res. 2020, 48, 300060520926355. [Google Scholar] [CrossRef] [PubMed]
- Serguienko, A.; Braadland, P.; Meza-Zepeda, L.A.; Bjerkehagen, B.; Myklebost, O. Accurate 3-gene-signature for early diagnosis of liposarcoma progression. Clin. Sarcoma Res. 2020, 10, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.S.; Kim, E.J.; Park, K.H.; Kim, K.H.; Yi, S.Y.; Kim, H.S.; Cho, Y.J.; Shin, K.-H.; Ahn, J.B.; et al. Prognostic implications of PIK3CA amplification in curatively resected liposarcoma. Oncotarget 2016, 7, 24549–24558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Kaufman, P.D. Ki-67, more than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Wu, T.; Tian, Q.; Carlson, L.A.; Wang, W.; Wu, G. Expression and prognosis analyses of BUB1, BUB1B and BUB3 in human sarcoma. Aging 2021, 13, 12395–12409. [Google Scholar] [CrossRef]
- Shi, M.; Guo, H.; Bai, Y.; Niu, J.; Niu, X.; Sun, K.; Chen, Y. Upregulated mitosis-associated genes CENPE, CENPF, and DLGAP5 predict poor prognosis and chemotherapy resistance of Acute Myeloid Leukemia. Cancer Biomark. Sect. A Dis. Markers 2022, 35, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Kao, C.Y.; Lee, H.J.; Creighton, C.J.; Ittmann, M.M.; Tsai, S.J.; Tsai, S.Y.; Tsai, M.J. Dysregulation of miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of prostate cancer. Nat. Commun. 2016, 7, 11418. [Google Scholar] [CrossRef] [Green Version]
- Laoukili, J.; Kooistra, M.R.; Brás, A.; Kauw, J.; Kerkhoven, R.M.; Morrison, A.; Clevers, H.; Medema, R. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005, 7, 126–136. [Google Scholar] [CrossRef]
- Perpelescu, M.; Fukagawa, T. The ABCs of CENPs. Chromosoma 2011, 120, 425–446. [Google Scholar] [CrossRef]
- Peterka, M.; Kornmann, B. Miro-dependent mitochondrial pool of CENP-F and its farnesylated C-terminal domain are dispensable for normal development in mice. PLoS Genet. 2019, 15, e1008050. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.T.; Hittle, J.C.; Jablonski, S.A.; Campbell, M.S.; Yoda, K.; Yen, T.J. Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat. Cell Biol. 2003, 5, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Liu, X.; Liang, C.; Hua, J.; Xu, J.; Wang, W.; Meng, Q.; Liu, J.; Zhang, B.; Yu, X.; et al. Deciphering the Prognostic Implications of the Components and Signatures in the Immune Microenvironment of Pancreatic Ductal Adenocarcinoma. Front. Immunol. 2021, 12, 648917. [Google Scholar] [CrossRef]
- Petitprez, F.; Vano, Y.A.; Becht, E.; Giraldo, N.A.; de Reyniès, A.; Sautès-Fridman, C.; Fridman, W.H. Transcriptomic analysis of the tumor microenvironment to guide prognosis and immunotherapies. Cancer Immunol. Immunother. CII 2018, 67, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Glabbeke, M.; Verweij, J.; Judson, I.; Nielsen, O.S. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur. J. Cancer 2002, 38, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.J.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Schöffski, P. Established and Experimental Systemic Treatment Options for Advanced Liposarcoma. Oncol. Res. Treat. 2022, 45, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Van Tine, B.A.; Krarup-Hansen, A.; Hess, L.M.; Abdul Razak, A.R.; Soldatenkova, V.; Wright, J.; Park, S.H. Quality of life of patients with soft tissue sarcoma treated with doxorubicin in the ANNOUNCE phase III clinical trial. Rare Tumors 2022, 14, 20363613221100033. [Google Scholar] [CrossRef]
- Schafer-Hales, K.; Iaconelli, J.; Snyder, J.P.; Prussia, A.; Nettles, J.H.; El-Naggar, A.; Khuri, F.R.; Giannakakou, P.; Marcus, A.I. Farnesyl transferase inhibitors impair chromosomal maintenance in cell lines and human tumors by compromising CENP-E and CENP-F function. Mol. Cancer Ther. 2007, 6, 1317–1328. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, S. Lonafarnib: First Approval. Drugs 2021, 81, 283–289. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Massaro, J.; D’Agostino, R.B., Sr.; Shappell, H.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.H.; Nazarian, A.; et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome. Circulation 2016, 134, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Ashar, H.R.; James, L.; Gray, K.; Carr, D.; Black, S.; Armstrong, L.; Bishop, W.R.; Kirschmeier, P. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J. Biol. Chem. 2000, 275, 30451–30457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bono, J.S.; Tolcher, A.W.; Rowinsky, E.K. Farnesyltransferase inhibitors and their potential in the treatment of breast carcinoma. Semin. Oncol. 2003, 30 (Suppl. 16), 79–92. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Sensini, M.; Corvino, A.; Garzaro, M.; Pecorari, G. Liposarcoma of Hypopharynx and Esophagus: A Unique Entity? J. Gastrointest Cancer 2016, 47, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Rita Gonçalves, A.; Lopes, J.; Moura Santos, P.; Lopes da Silva, H.; Crujo, C.; Velosa, J. Primary liposarcoma of the sigmoid presenting as colonic intussusception—A case report. Rev. Esp. Enferm. Dig. 2016, 108, 591–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhou, Y.; Wang, H.; Ling, J. Survival analysis and treatment strategies for limb liposarcoma patients with metastasis at presentation. Medicine 2021, 100, e25296. [Google Scholar] [CrossRef] [PubMed]
- Eilber, F.C.; Eilber, F.R.; Eckardt, J.; Rosen, G.; Riedel, E.; Maki, R.G.; Brennan, M.; Singer, S. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann. Surg. 2004, 240, 686–695; discussion 695–697. [Google Scholar] [CrossRef]
- Patel, R.B.; Li, T.; Liao, Z.; Jaldeepbhai, J.A.; Perera, H.; Muthukuda, S.K.; Dhirubhai, D.H.; Singh, V.; Du, X.; Yang, J. Recent translational research into targeted therapy for liposarcoma. Stem Cell Investig 2017, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Hussein, D.; Taylor, S.S. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J. Cell Sci. 2002, 115 Pt 17, 3403–3414. [Google Scholar] [CrossRef]
- Aytes, A.; Mitrofanova, A.; Lefebvre, C.; Alvarez, M.J.; Castillo-Martin, M.; Zheng, T.; Eastham, J.A.; Gopalan, A.; Pienta, K.J.; Shen, M.M.; et al. Cross-species regulatory network analysis identifies a synergistic interaction between FOXM1 and CENPF that drives prostate cancer malignancy. Cancer Cell 2014, 25, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.Y.; Liu, L.; Chen, S.P.; Zhang, X.; Mi, Y.J.; Liu, Z.G.; Li, M.Z.; Zhang, H.; Qian, C.N.; Shao, J.Y.; et al. Prognostic significance and therapeutic implications of centromere protein F expression in human nasopharyngeal carcinoma. Mol. Cancer 2010, 9, 237. [Google Scholar]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Namløs, H.M.; Müller, C.; Edén, P.; Fernebro, J.; Berner, J.M.; Bjerkehagen, B.; Åkerman, M.; Bendahl, P.-O.; Isinger, A.; et al. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: Hypoxia-induced transcription profile signifies metastatic potential. BMC Genom. 2007, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Li, W.; Zhang, X.; Wu, R.; Zheng, K.; Zhou, T.; Dong, Y.; He, Y.; Wang, D. Integrated bioinformatics analysis for the screening of hub genes and therapeutic drugs in Hepatocellular carcinoma. Curr. Pharm. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Cui, F.; Ning, S.; Xu, Z.; Hu, J. Spindle pole body component 25 in the androgen-induced regression of castration-resistant prostate cancer. Transl. Androl. Urol. 2022, 11, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ozmen Yaylaci, A.; Canbek, M. The role of ubiquitin signaling pathway on liver regeneration in rats. Mol Cell Biochem. 2022, 478, 131–147. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Zhang, T.; Barisic, M.; Kalitsis, P.; Hudson, D.F. Topoisomerase IIα is essential for maintenance of mitotic chromosome structure. Proc. Natl. Acad. Sci. United States Am. 2020, 117, 12131–12142. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.L.; Scott, M.I.; Holt, S.V.; Hussein, D.; Taylor, S.S. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 2004, 117 Pt 8, 1577–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciossani, G.; Overlack, K.; Petrovic, A.; Huis In ‘t Veld, P.J.; Koerner, C.; Wohlgemuth, S.; Maffini, S.; Musacchio, A. The kinetochore proteins CENP-E and CENP-F directly and specifically interact with distinct BUB mitotic checkpoint Ser/Thr kinases. J. Biol. Chem. 2018, 293, 10084–10101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C.; Lin, S.; Yao, D.; Li, M.; Chen, W.; Zheng, S.; Zhao, Z. Identification of promising prognostic genes for relapsed acute lymphoblastic leukemia. Blood Cells Mol. Dis. 2019, 77, 113–119. [Google Scholar] [CrossRef]
- Jen, J.; Wang, Y.C. Zinc finger proteins in cancer progression. J. Biomed. Sci. 2016, 23, 53. [Google Scholar] [CrossRef] [Green Version]
- Haldrup, C.; Pedersen, A.L.; Øgaard, N.; Strand, S.H.; Høyer, S.; Borre, M.; Ørntoft, T.F.; Sørensen, K.D. Biomarker potential of ST6GALNAC3 and ZNF660 promoter hypermethylation in prostate cancer tissue and liquid biopsies. Mol. Oncol. 2018, 12, 545–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Yin, C.; Yang, X.; Shi, H.; Zhang, Z.; Zhou, J.; Zhang, P. Six Genes Associated with Lymphatic Metastasis in Colon Adenocarcinoma Linked to Prognostic Value and Tumor Immune Cell Infiltration. Evid. -Based Complement. Altern. Med. eCAM 2022, 2022, 4304361. [Google Scholar] [CrossRef]
- Liu, J.; Dong, C.; Jiang, G.; Lu, X.; Liu, Y.; Wu, H. Transcription factor expression as a predictor of colon cancer prognosis: A machine learning practice. BMC Med. Genom. 2020, 13 (Suppl. 9), 135. [Google Scholar] [CrossRef]
- Pu, W.; Qian, F.; Liu, J.; Shao, K.; Xiao, F.; Jin, Q.; Liu, Q.; Jiang, S.; Zhang, R.; Zhang, J.; et al. Targeted Bisulfite Sequencing Reveals DNA Methylation Changes in Zinc Finger Family Genes Associated With KRAS Mutated Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 759813. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, X.; Chu, H.J.; Li, N.; Huang, L.Y.; Chen, J. Centromere Protein I (CENP-I) Is Upregulated in Gastric Cancer, Predicts Poor Prognosis, and Promotes Tumor Cell Proliferation and Migration. Technol. Cancer Res. Treat. 2021, 20, 15330338211045510. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, W.; Chen, Y.; Zhu, L.; Mo, A.; Xie, Q. Identification of potential druggable targets of cell cycle with small-molecule inhibitors in oral squamous cell carcinoma. Pharm. Genom. 2022, 32, 125–137. [Google Scholar] [CrossRef]
- Yu, P.; Dai, Y.; Zhuang, T.; Yue, X.; Chen, Y.; Wang, X.; Duan, X.; Ping, Y.; Xie, Y.; Cao, Y.; et al. Identification and Validation of Three Hub Genes Involved in Cell Proliferation and Prognosis of Castration-Resistant Prostate Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 8761112. [Google Scholar] [CrossRef]
- Yang, P.; Pei, X.; Deng, J.; Li, X. Comprehensive Analysis of Centromere Protein Family Member Genes in Lung Adenocarcinoma. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 57–72. [Google Scholar] [CrossRef]
- Thangavelu, P.U.; Lin, C.Y.; Vaidyanathan, S.; Nguyen, T.H.M.; Dray, E.; Duijf, P.H.G. Overexpression of the E2F target gene CENPI promotes chromosome instability and predicts poor prognosis in estrogen receptor-positive breast cancer. Oncotarget 2017, 8, 62167–62182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Peng, Y.; Inuzuka, H.; Wei, W. Targeting micro-environmental pathways by PROTACs as a therapeutic strategy. Semin. Cancer Biol. 2022, 86, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; Meylan, M.; de Reyniès, A.; Sautès-Fridman, C.; Fridman, W.H. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front. Immunol. 2020, 11, 784. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Roulleaux Dugage, M.; Nassif, E.F.; Italiano, A.; Bahleda, R. Improving Immunotherapy Efficacy in Soft-Tissue Sarcomas: A Biomarker Driven and Histotype Tailored Review. Front. Immunol. 2021, 12, 775761. [Google Scholar] [CrossRef]
- Zhu, N.; Hou, J. Assessing immune infiltration and the tumor microenvironment for the diagnosis and prognosis of sarcoma. Cancer Cell Int. 2020, 20, 577. [Google Scholar] [CrossRef]
- Han, K.; Qian, K.; Zhao, T.; Liu, X.S.; Zhang, Y. Prediction of prognosis of patients with lung cancer in combination with the immune score. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef] [PubMed]
- Öjlert, Å.K.; Halvorsen, A.R.; Nebdal, D.; Lund-Iversen, M.; Solberg, S.; Brustugun, O.T.; Lingjaerde, O.C.; Helland, Å. The immune microenvironment in non-small cell lung cancer is predictive of prognosis after surgery. Mol. Oncol. 2019, 13, 1166–1179. [Google Scholar] [CrossRef] [Green Version]
- Duurland, C.L.; Santegoets, S.J.; Abdulrahman, Z.; Loof, N.M.; Sturm, G.; Wesselink, T.H.; Arens, R.; Boekestijn, S.; Ehsan, I.; van Poelgeest, M.I.E.; et al. CD161 expression and regulation defines rapidly responding effector CD4+ T cells associated with improved survival in HPV16-associated tumors. J. Immunother. Cancer 2022, 10, e003995. [Google Scholar] [CrossRef]
- Varis, A.; Salmela, A.L.; Kallio, M.J. Cenp-F (mitosin) is more than a mitotic marker. Chromosoma 2006, 115, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef] [Green Version]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.J.; Hu, F.F.; Xia, M.X.; Han, L.; Zhang, Q.; Guo, A.Y. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef] [PubMed]

| Subgroup | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| All patients (n = 59) | ||||
| CENPF expression: high vs. low (n = 58) | 3.112 (1.280–7.565) | 0.012 | 3.752 (1.410–9.984) | 0.008 |
| New tumor after treatment: yes vs. no (n = 58) | 4.331 (1.800–10.421) | 0.001 | 4.886 (1.249–19.120) | 0.023 |
| Cancer status: with tumor vs. tumor free (n = 56) | 4.987 (1.479–16.814) | 0.010 | 3.861 (0.763–19.533) | 0.102 |
| Gender: female vs. male (n = 59) | 1.997 (0.919–4.338) | 0.081 | ||
| Age: ≥60 vs. < 60 (n = 59) | 1.860 (0.700–4.944) | 0.213 | ||
| Metastasis: yes vs. no (n = 25) | 2.218 (0.569–8.646) | 0.251 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Lian, Y.; Zhao, B.; Han, J.; Li, X.; Wu, J.; Hou, M.; Yue, M.; Zhang, K.; Liu, G.; et al. Deciphering the Prognostic and Therapeutic Significance of Cell Cycle Regulator CENPF: A Potential Biomarker of Prognosis and Immune Microenvironment for Patients with Liposarcoma. Int. J. Mol. Sci. 2023, 24, 7010. https://doi.org/10.3390/ijms24087010
Chen J, Lian Y, Zhao B, Han J, Li X, Wu J, Hou M, Yue M, Zhang K, Liu G, et al. Deciphering the Prognostic and Therapeutic Significance of Cell Cycle Regulator CENPF: A Potential Biomarker of Prognosis and Immune Microenvironment for Patients with Liposarcoma. International Journal of Molecular Sciences. 2023; 24(8):7010. https://doi.org/10.3390/ijms24087010
Chicago/Turabian StyleChen, Jiahao, Yingying Lian, Binbin Zhao, Jiayang Han, Xinyu Li, Jialin Wu, Mengwen Hou, Man Yue, Kaifeng Zhang, Guangchao Liu, and et al. 2023. "Deciphering the Prognostic and Therapeutic Significance of Cell Cycle Regulator CENPF: A Potential Biomarker of Prognosis and Immune Microenvironment for Patients with Liposarcoma" International Journal of Molecular Sciences 24, no. 8: 7010. https://doi.org/10.3390/ijms24087010
APA StyleChen, J., Lian, Y., Zhao, B., Han, J., Li, X., Wu, J., Hou, M., Yue, M., Zhang, K., Liu, G., Tu, M., Ruan, W., Ji, S., & An, Y. (2023). Deciphering the Prognostic and Therapeutic Significance of Cell Cycle Regulator CENPF: A Potential Biomarker of Prognosis and Immune Microenvironment for Patients with Liposarcoma. International Journal of Molecular Sciences, 24(8), 7010. https://doi.org/10.3390/ijms24087010






