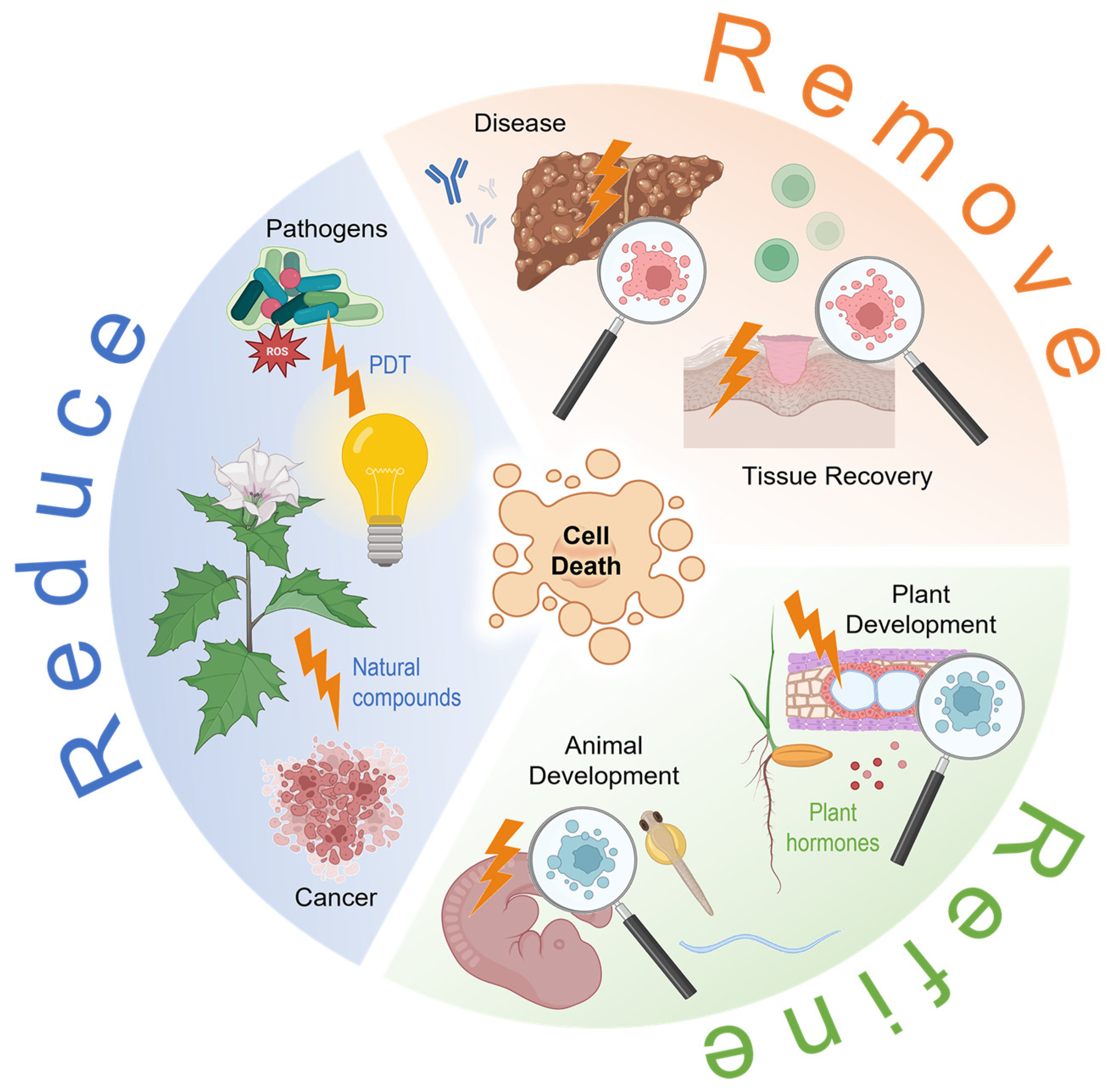
Remove, Refine, Reduce: Cell Death in Biological Systems
1. Cell Death as a Component of Development and Homeostasis
2. Remove
2.1. Cell Death in Disease
2.2. Cell Death Caused by Exogenous Effects
3. Refine
3.1. Cell Death during Development
3.2. Cell Death in Tissue Recovery
4. Reduce
4.1. Induced Cell Death to Fight Cancer
4.2. Induced Cell Death to Combat Pathogens
5. Conclusions
Conflicts of Interest
References
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Yuan, J.; Kroemer, G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010, 24, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Krüger, M.; Richter, P. To die or not to die: Cell death in biology and disease. Int. J. Mol. Sci. 2022, 23, 6374. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, V. Cell Death, a Life-Giving Event, Can Also Trigger Severe Disease. Horizon—The EU Research & Innovation Magazine. 20 March 2023. Available online: https://ec.europa.eu/research-and-innovation/en/horizon-magazine/cell-death-life-giving-event-can-also-trigger-severe-disease (accessed on 30 March 2023).
- Shojaie, L.; Iorga, A.; Dara, L. Cell death in liver diseases: A review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef]
- Yin, S.; Shi, Q.; Shao, W.; Zhang, C.; Zhang, Y.; Qiu, X.; Huang, J. Hepatocyte-derived Igκ exerts a protective effect against cona-induced acute liver injury. Int. J. Mol. Sci. 2020, 21, 9379. [Google Scholar] [CrossRef] [PubMed]
- Oulé, M.K.; Azinwi, R.; Bernier, A.-M.; Kablan, T.; Maupertuis, A.-M.; Mauler, S.; Nevry, R.K.; Dembélé, K.; Forbes, L.; Diop, L. Polyhexamethylene guanidine hydrochloride-based disinfectant: A novel tool to fight meticillin-resistant Staphylococcus aureus and nosocomial infections. J. Med. Microbiol. 2008, 57, 1523–1528. [Google Scholar] [CrossRef]
- Müller, G.; Kramer, A. Effect of selected wound antiseptics on adult articular cartilage (bovine sesamoid bone) in the presence of Escherichia coli and Staphylococcus aureus. J. Orthop. Res. 2005, 23, 127–133. [Google Scholar] [CrossRef]
- Park, D.U.; Park, J.; Yang, K.W.; Park, J.H.; Kwon, J.H.; Oh, H.B. Properties of polyhexamethylene guanidine (PHMG) associated with fatal lung injury in korea. Molecules 2020, 25, 3301. [Google Scholar] [CrossRef]
- Jeong, M.H.; Jeon, M.S.; Kim, G.E.; Kim, H.R. Polyhexamethylene guanidine phosphate induces apoptosis through endoplasmic reticulum stress in lung epithelial cells. Int. J. Mol. Sci. 2021, 22, 1215. [Google Scholar] [CrossRef]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Krüger, M. Influence of microgravity on apoptosis in cells, tissues, and other systems in vivo and in vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Wu, Y.H.; Yen, W.C.; Liu, H.Y.; Hwang, T.L.; Stern, A.; Chiu, D.T. The redox role of G6PD in cell growth, cell death, and cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Vanegas, O.C.; Patel, M.; Rosti, V.; Li, H.; Waka, J.; Merghoub, T.; Pandolfi, P.P.; Notaro, R.; Manova, K.; et al. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO J. 2002, 21, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Yang, H.C.; Hung, C.Y.; Ou, M.H.; Pan, Y.Y.; Cheng, M.L.; Stern, A.; Lo, S.J.; Chiu, D.T. Impaired embryonic development in glucose-6-phosphate dehydrogenase-deficient Caenorhabditis elegans due to abnormal redox homeostasis induced activation of calcium-independent phospholipase and alteration of glycerophospholipid metabolism. Cell Death Dis. 2017, 8, e2545. [Google Scholar] [CrossRef]
- Yang, H.C.; Yu, H.; Ma, T.H.; Tjong, W.Y.; Stern, A.; Chiu, D.T. Tert-butyl hydroperoxide (TBHP)-induced lipid peroxidation and embryonic defects resemble glucose-6-phosphate dehydrogenase (G6PD) deficiency in C. elegans. Int. J. Mol. Sci. 2020, 21, 8688. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; Woltering, E.J. Multiple mediators of plant programmed cell death: Interplay of conserved cell death mechanisms and plant-specific regulators. Bioessays 2003, 25, 47–57. [Google Scholar] [CrossRef]
- Major, I.T.; Yoshida, Y.; Campos, M.L.; Kapali, G.; Xin, X.F.; Sugimoto, K.; de Oliveira Ferreira, D.; He, S.Y.; Howe, G.A. Regulation of growth-defense balance by the jasmonate ZIM-domain (JAZ)-myc transcriptional module. New Phytol. 2017, 215, 1533–1547. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Wei, X.; Zhou, Y.; Dai, Y.; Zhu, Z. OsJAZ13 negatively regulates jasmonate signaling and activates hypersensitive cell death response in rice. Int. J. Mol. Sci. 2020, 21, 4379. [Google Scholar] [CrossRef]
- Anderton, H.; Alqudah, S. Cell death in skin function, inflammation, and disease. Biochem. J. 2022, 479, 1621–1651. [Google Scholar] [CrossRef]
- Annibaldi, A.; Meier, P. Checkpoints in TNF-induced cell death: Implications in inflammation and cancer. Trends Mol. Med. 2018, 24, 49–65. [Google Scholar] [CrossRef]
- Feoktistova, M.; Makarov, R.; Leverkus, M.; Yazdi, A.S.; Panayotova-Dimitrova, D. TNF is partially required for cell-death-triggered skin inflammation upon acute loss of cflip. Int. J. Mol. Sci. 2020, 21, 8859. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.; Stoesz, S.P.; Buckley, P.; Gould, M.N. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem. J. 1999, 31, 433–441. [Google Scholar] [CrossRef]
- Santiago-Sánchez, G.S.; Pita-Grisanti, V.; Quiñones-Díaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejía, P.E. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Baile, M.; Bello-Gil, D.; Pérez-Valenciano, E.; Sanz, J.M.; García-Morales, P.; Maestro, B.; Ventero, M.P.; Alenda, C.; Barberá, V.M.; Saceda, M. Clyta-daao, free and immobilized in magnetic nanoparticles, induces cell death in human cancer cells. Biomolecules 2020, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Baile, M.; García-Morales, P.; Pérez-Valenciano, E.; Ventero, M.P.; Sanz, J.M.; de Juan Romero, C.; Barberá, V.M.; Alenda, C.; Saceda, M. Cell death mechanisms induced by clyta-daao chimeric enzyme in human tumor cell lines. Int. J. Mol. Sci. 2020, 21, 8522. [Google Scholar] [CrossRef]
- Greco, G.; Catanzaro, E.; Fimognari, C. Natural products as inducers of non-canonical cell death: A weapon against cancer. Cancers 2021, 13, 304. [Google Scholar] [CrossRef]
- Mbemi, A.T.; Sims, J.N.; Yedjou, C.G.; Noubissi, F.K.; Gomez, C.R.; Tchounwou, P.B. Vernonia calvoana shows promise towards the treatment of ovarian cancer. Int. J. Mol. Sci. 2020, 21, 4429. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.E.; Sung, J.Y.; Yu, S.; Kim, Y.; Shim, J.; Kim, H.J.; Cho, M.L.; Lee, J.S.; Kim, Y.N. Pygenic acid a (PA) sensitizes metastatic breast cancer cells to anoikis and inhibits metastasis in vivo. Int. J. Mol. Sci. 2020, 21, 8444. [Google Scholar] [CrossRef]
- Johnson, A.G.; Kranzusch, P.J. What bacterial cell death teaches us about life. PLoS Pathog. 2022, 18, e1010879. [Google Scholar] [CrossRef]
- Pierson, D.L. Microbial contamination of spacecraft. Gravit. Space Biol. Bull. 2001, 14, 1–6. [Google Scholar]
- Urbaniak, C.; Morrison, M.D.; Thissen, J.B.; Karouia, F.; Smith, D.J.; Mehta, S.; Jaing, C.; Venkateswaran, K. Microbial tracking-2, a metagenomics analysis of bacteria and fungi onboard the international space station. Microbiome 2022, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Curtis, P.D. The impacts of microgravity on bacterial metabolism. Life 2022, 12, 774. [Google Scholar] [CrossRef]
- Tirumalai, M.R.; Karouia, F.; Tran, Q.; Stepanov, V.G.; Bruce, R.J.; Ott, C.M.; Pierson, D.L.; Fox, G.E. Evaluation of acquired antibiotic resistance in Escherichia coli exposed to long-term low-shear modeled microgravity and background antibiotic exposure. mBio 2019, 10, e02637-18. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Gricajeva, A.; Kalėdienė, L.; Vitta, P. Antimicrobial photoinactivation approach based on natural agents for control of bacteria biofilms in spacecraft. Int. J. Mol. Sci. 2020, 21, 6932. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, M. Remove, Refine, Reduce: Cell Death in Biological Systems. Int. J. Mol. Sci. 2023, 24, 7028. https://doi.org/10.3390/ijms24087028
Krüger M. Remove, Refine, Reduce: Cell Death in Biological Systems. International Journal of Molecular Sciences. 2023; 24(8):7028. https://doi.org/10.3390/ijms24087028
Chicago/Turabian StyleKrüger, Marcus. 2023. "Remove, Refine, Reduce: Cell Death in Biological Systems" International Journal of Molecular Sciences 24, no. 8: 7028. https://doi.org/10.3390/ijms24087028
APA StyleKrüger, M. (2023). Remove, Refine, Reduce: Cell Death in Biological Systems. International Journal of Molecular Sciences, 24(8), 7028. https://doi.org/10.3390/ijms24087028




