The Genomic Variation and Differentially Expressed Genes on the 6P Chromosomes in Wheat–Agropyron cristatum Addition Lines 5113 and II-30-5 Confer Different Desirable Traits
Abstract
1. Introduction
2. Results
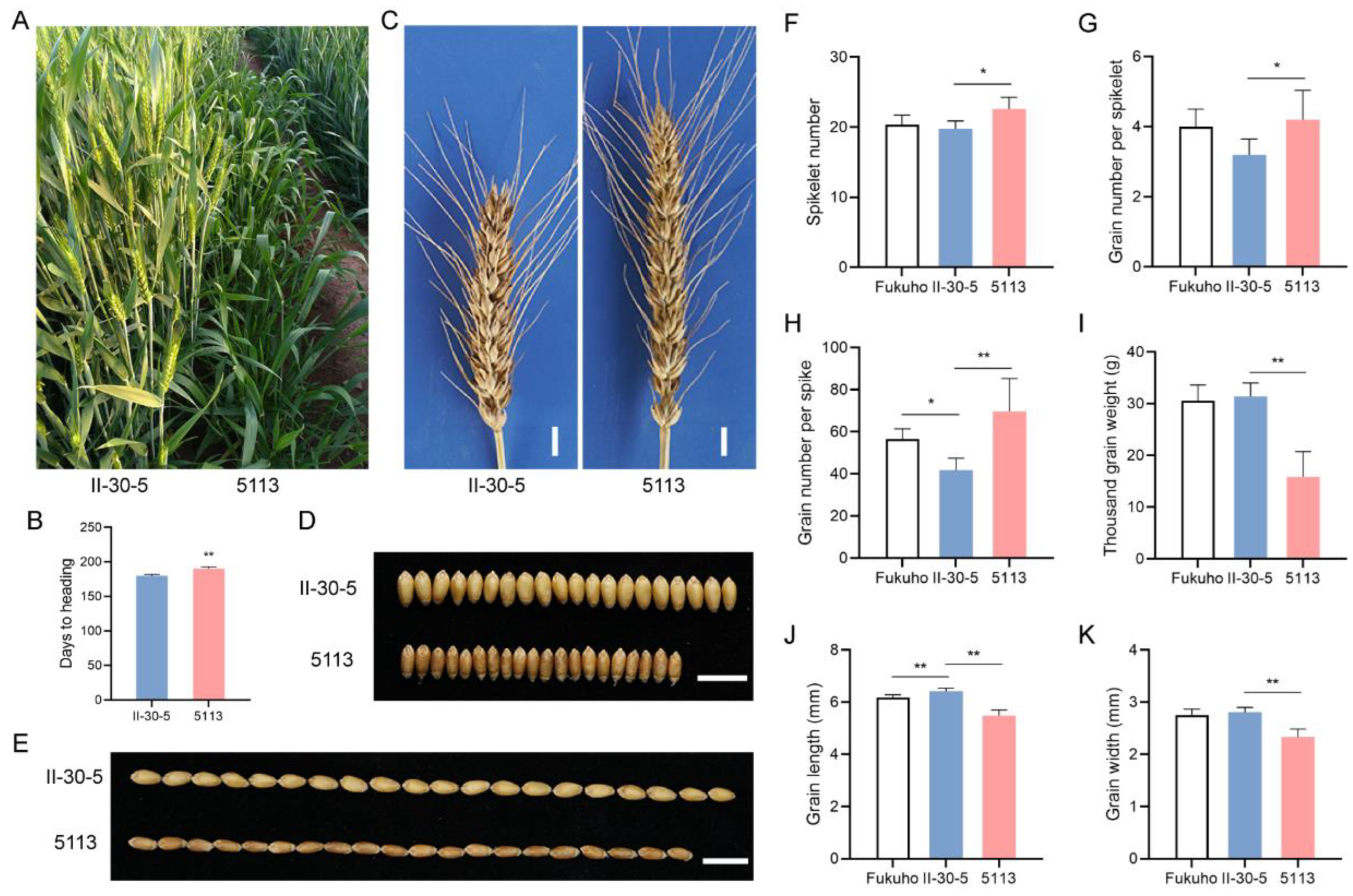
2.1. 5113 and II-30-5 Exhibited Significant Differences in Heading Date, GNS, and Thousand-Grain Weight
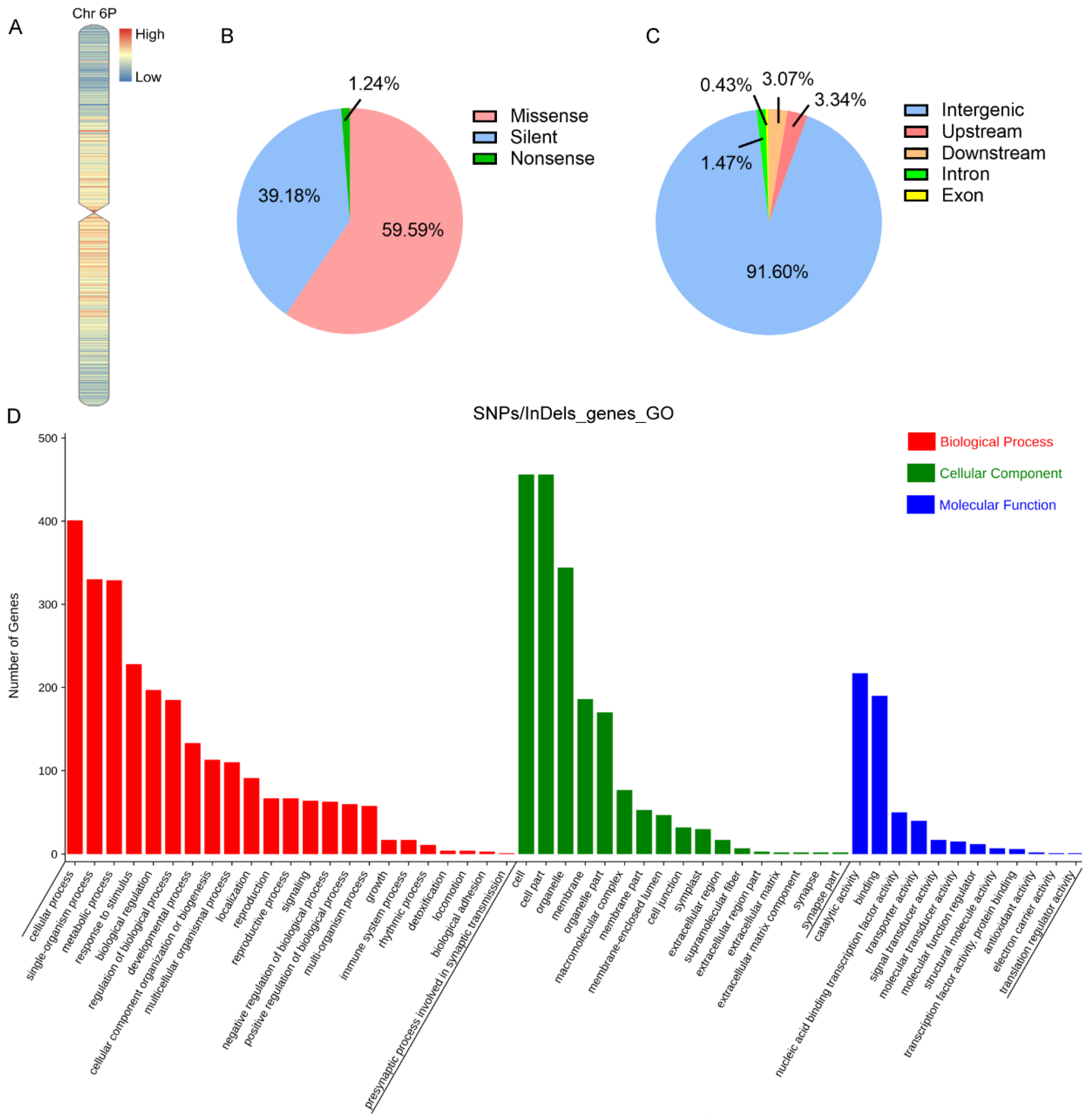
2.2. The Genetic Variation of 5113 and II-30-5 at the Whole Genome Level
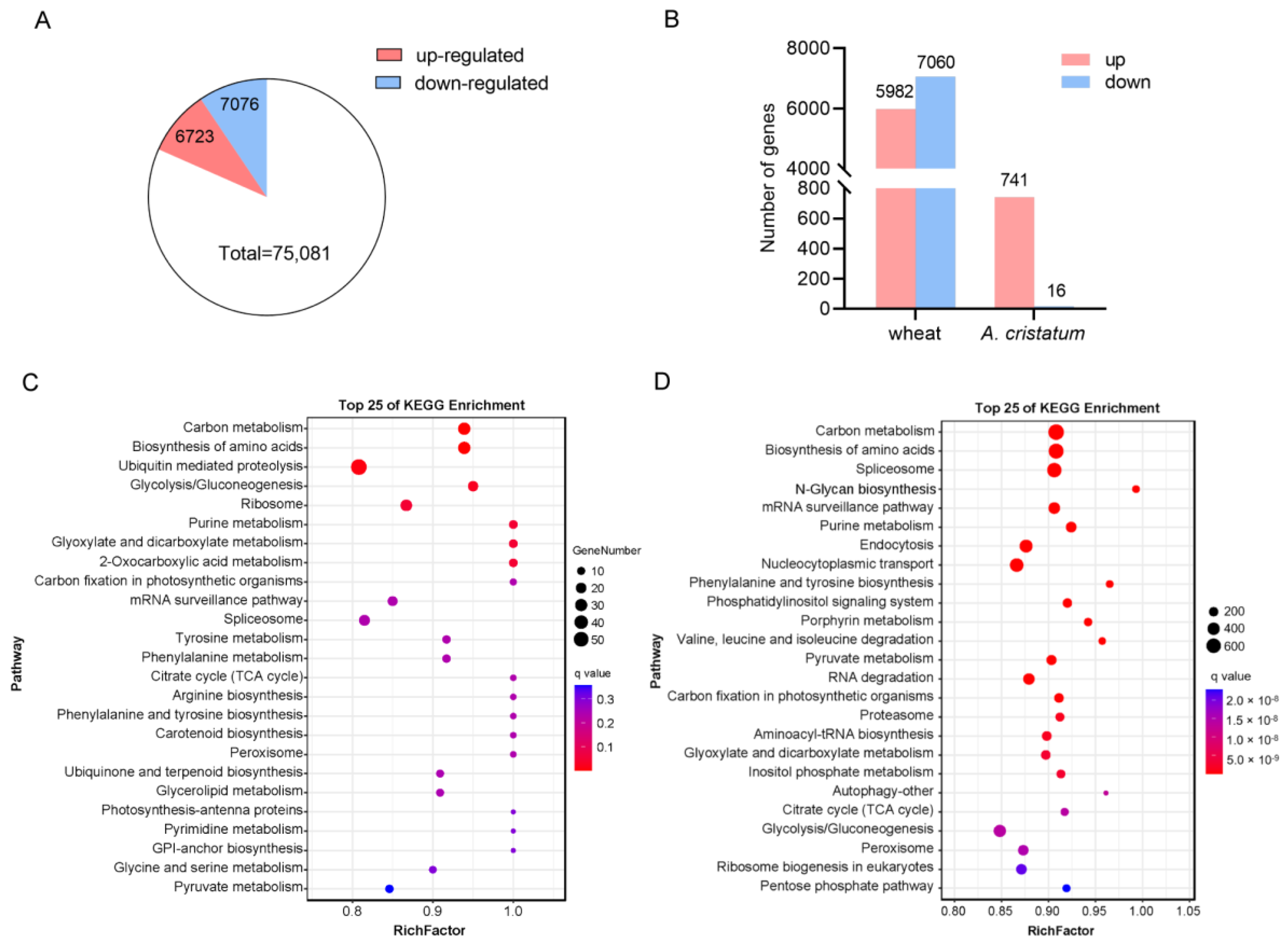
2.3. RNA-Seq Analysis of the Addition Lines 5113 and II-30-5
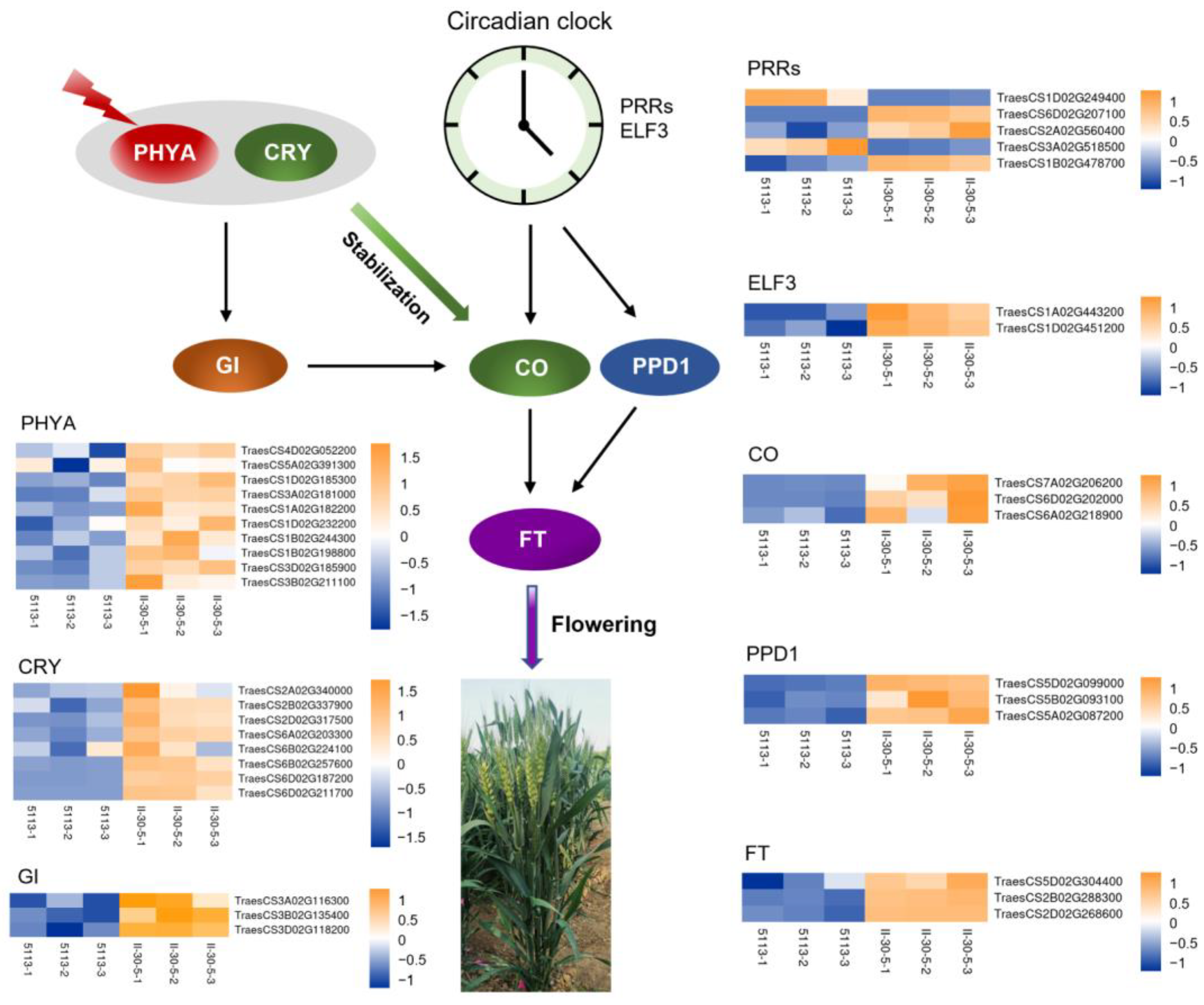
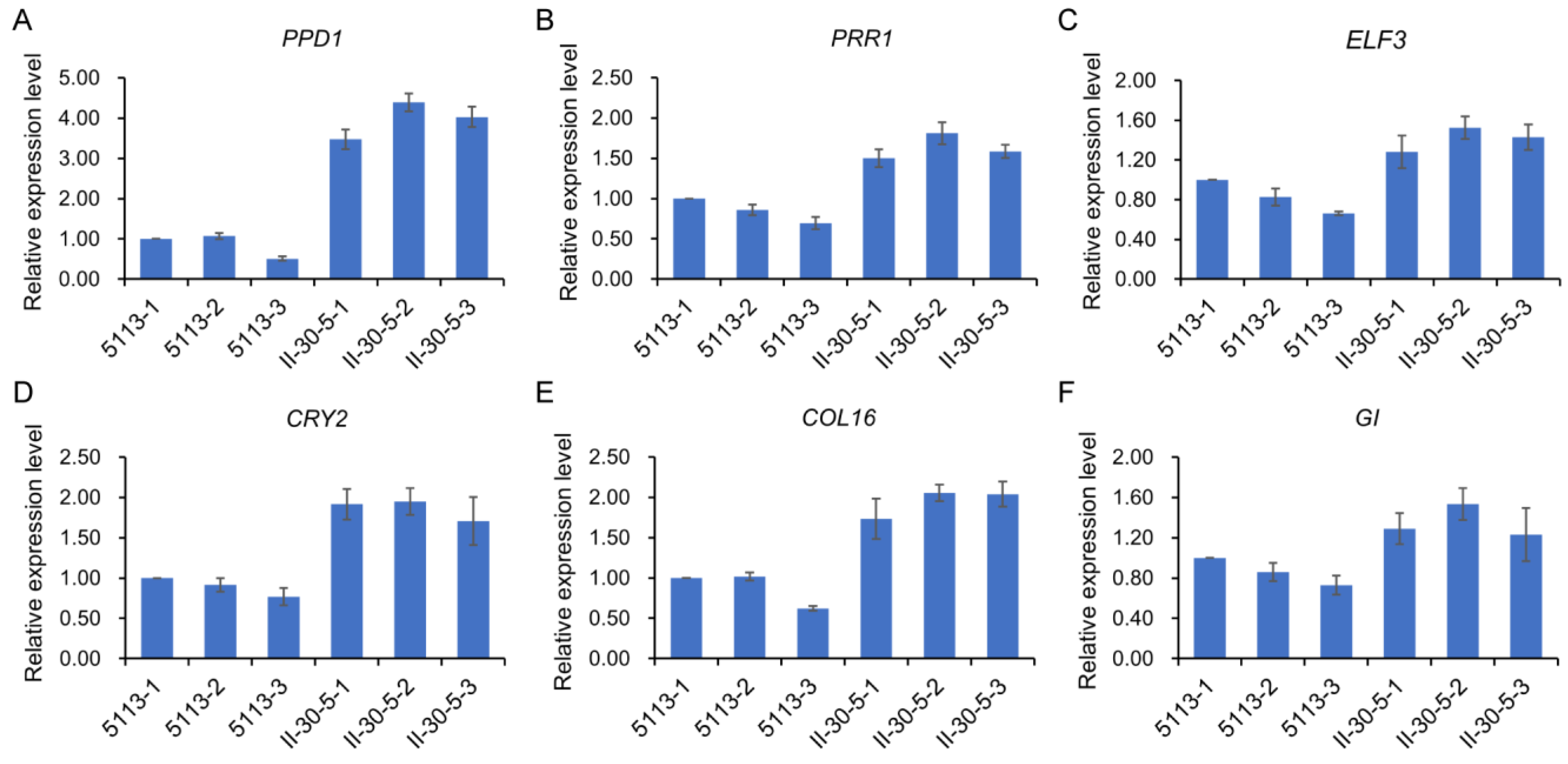
2.4. Changes in Photoperiod Pathway-Related Genes between 5113 and II-30-5
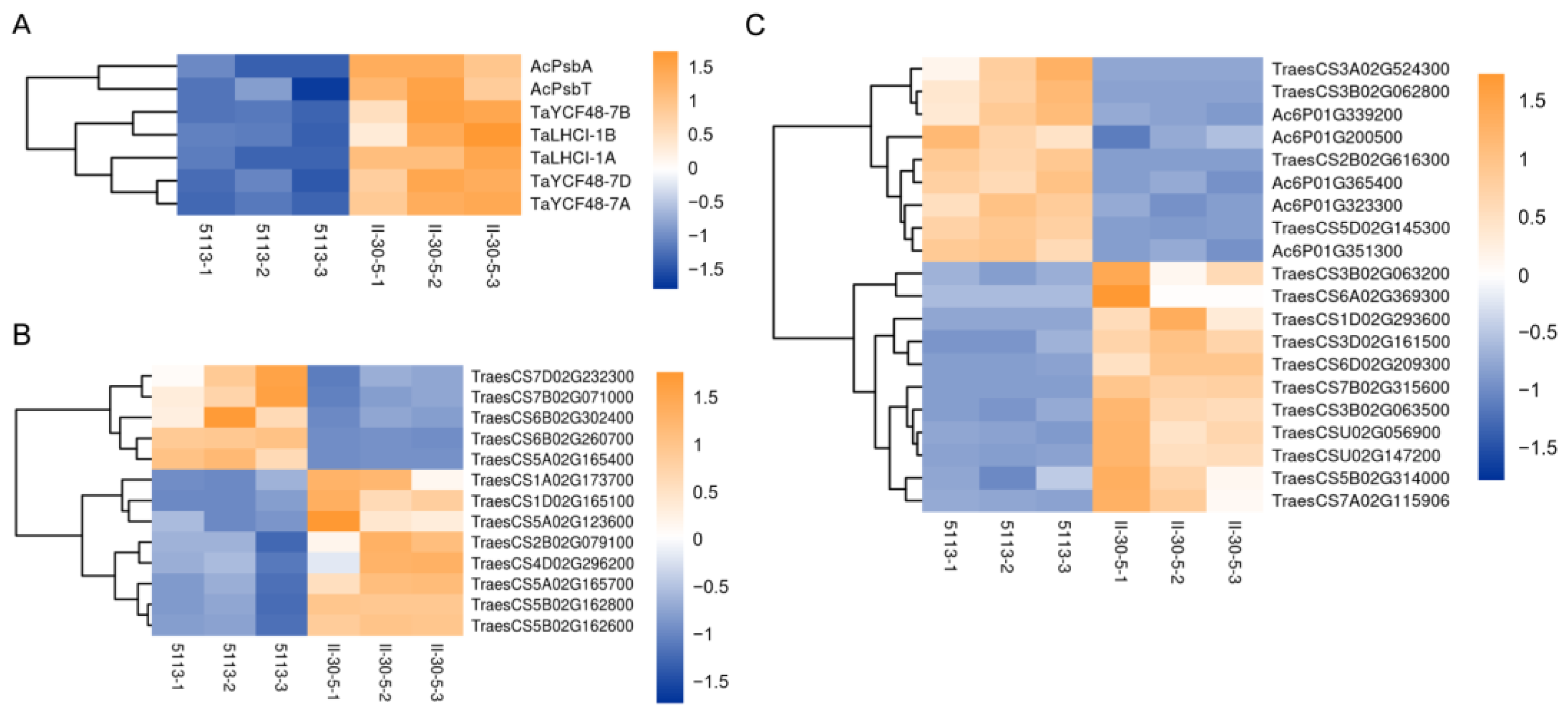
2.5. Changes in Spike-Development-Related Genes between 5113 and II-30-5
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
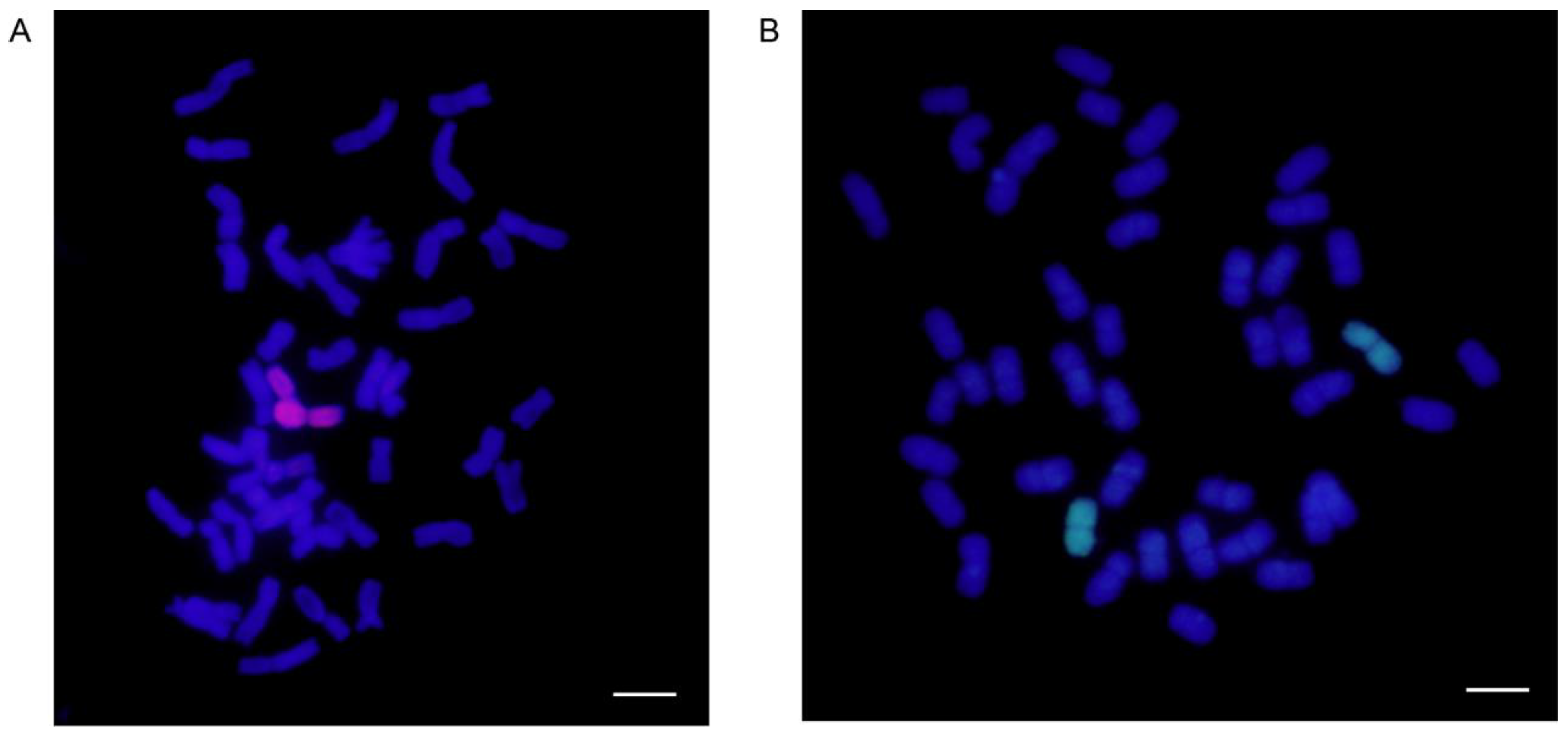
4.2. GISH for Wheat–A. cristatum 6P Addition Lines
4.3. Genome Resequencing of the Two Addition Lines
4.4. RNA Extraction
4.5. RNA Sequencing and Transcriptome Analysis
4.6. qRT–PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Repellin, A.; Baga, M.; Jauhar, P.P.; Chibbar, R.N. Genetic enrichment of cereal crops via alien gene transfer: New challenges. Plant Cell Tissue Organ Cult. 2001, 64, 159–183. [Google Scholar] [CrossRef]
- Mujeeb-Kazi, A.; Kazi, A.G.; Dundas, I.; Rasheed, A.; Ogbonnaya, F.; Kishii, M.; Bonnett, D.; Wang, R.R.C.; Xu, S.; Chen, P.D.; et al. Genetic diversity for wheat improvement as a conduit to food security. Adv. Agron. 2013, 122, 179–257. [Google Scholar] [CrossRef]
- Wieser, H.; Kieffer, R.; Lelley, T. The influence of 1B/1R chromosome translocation on gluten protein composition and technological properties of bread wheat. Czech J. Anim. Sci. 2000, 80, 1640–1647. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Liu, C.; Liu, F.; Xu, N.; Yao, M.; Yu, H.; Wang, Y.; Chen, J.; Bai, S.; et al. Development and identification of an elite wheat-Hordeum californicum T6HcS/6BL translocation line ND646 containing several desirable traits. Genet. Mol. Biol. 2022, 45, e20220117. [Google Scholar] [CrossRef]
- Yuan, W.; Tomita, M. Thinopyrum ponticum chromatin-integrated wheat genome shows salt-tolerance at germination stage. Int. J. Mol. Sci. 2015, 16, 4512–4517. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, Q.; Bao, Y.; Liu, S.; Wang, H.; Li, X. Molecular cytogenetic identification of a novel dwarf wheat line with introgressed Thinopyrum ponticum chromatin. J. Biosci. 2012, 37, 149–155. [Google Scholar] [CrossRef]
- Lutz, J.; Hsam, S.L.K.; Limpert, E.; Zeller, F.J. Chromosomal location of powdery mildew resistance genes in Triticum aestivum L. (common wheat). 2. genes Pm2 and Pm19 from Aegilops-Squarrosa L. Heredity 1995, 74, 152–156. [Google Scholar] [CrossRef]
- Singh, R.P.; Nelson, J.C.; Sorrells, M.E. Mapping Yr28 and other genes for resistance to stripe rust in wheat. Crop Sci. 2000, 40, 1148–1155. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Zeller, F.J. Evidence of allelism between genes Pm8 and Pm17 and chromosomal location of powdery mildew and leaf rust resistance genes in the common wheat cultivar ‘Amigo’. Plant Breed. 1997, 116, 119–122. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Zhang, P.; Cowger, C.; Parks, R.; Lagudah, E.S.; Hoxha, S. Rye-derived powdery mildew resistance gene Pm8 in wheat is suppressed by the Pm3 locus. Theor. Appl. Genet. 2011, 123, 359–367. [Google Scholar] [CrossRef]
- Ren, S.X.; McIntosh, R.A.; Lu, Z.J. Genetic suppression of the cereal rye-derived gene Pm8 in wheat. Euphytica 1997, 93, 353–360. [Google Scholar] [CrossRef]
- Liu, J.; Tao, W.; Liu, D.; Chen, P.; Li, W.; Xiang, Q.; Duan, X. Transmission of 6VS chromosome in wheat-Haynaldia villosa translocation lines and genetic stability of Pm21 carried by 6VS. Acta Bot. Sin. 1999, 41, 1058–1060. [Google Scholar]
- Qi, L.; Cao, M.; Chen, P.; Li, W.; Liu, D. Identification, mapping, and application of polymorphic DNA associated with resistance gene Pm21 of wheat. Genome 1996, 39, 191–197. [Google Scholar] [CrossRef]
- He, H.; Zhu, S.; Zhao, R.; Jiang, Z.; Ji, Y.; Ji, J.; Qiu, D.; Li, H.; Bie, T. Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 2018, 11, 879–882. [Google Scholar] [CrossRef]
- He, H.; Ji, J.; Li, H.; Tong, J.; Feng, Y.; Wang, X.; Han, R.; Bie, T.; Liu, C.; Zhu, S. Genetic diversity and evolutionary analyses reveal the powdery mildew resistance gene Pm21 undergoing diversifying selection. Front. Genet. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Li, P.; Dong, Y.; Zhao, G. Establishment of wheat-Agropyron cristatum alien addition lines. I. Cytology of F3, F2BC1, BC4, and BC3F1 progenies. Acta Genet. Sin. 1997, 24, 154–159. [Google Scholar]
- Han, H.; Liu, W.; Zhang, J.; Zhou, S.; Yang, X.; Li, X.; Li, L. Identification of P genome chromosomes in Agropyron cristatum and wheat-A. cristatum derivative lines by FISH. Sci. Rep. 2019, 9, 9712. [Google Scholar] [CrossRef]
- Wang, X.; Han, B.; Sun, Y.; Kang, X.; Zhang, M.; Han, H.; Zhou, S.; Liu, W.; Lu, Y.; Yang, X.; et al. Introgression of chromosome 1P from Agropyron cristatum reduces leaf size and plant height to improve the plant architecture of common wheat. Theor. Appl. Genet. 2022, 135, 1951–1963. [Google Scholar] [CrossRef]
- Li, H.; Lv, M.; Song, L.; Zhang, J.; Gao, A.; Li, L.; Liu, W. Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS ONE 2016, 11, e0145928. [Google Scholar] [CrossRef]
- Ji, X.; Liu, T.; Xu, S.; Wang, Z.; Han, H.; Zhou, S.; Guo, B.; Zhang, J.; Yang, X.; Li, X.; et al. Comparative transcriptome analysis reveals the gene expression and regulatory characteristics of broad-spectrum immunity to leaf rust in a wheat-Agropyron cristatum 2P addition line. Int. J. Mol. Sci. 2022, 23, 7370. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, X.; Wang, Z.; Qi, K.; Yang, W.; Liu, W.; Zhang, J.; Zhou, S.; Lu, Y.; Yang, X.; et al. Chromosome 5P of Agropyron cristatum induces chromosomal translocation by disturbing homologous chromosome pairing in a common wheat background. Crop J. 2023, 11, 228–237. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; Wang, H.; Li, H.; Li, L.; Li, X.; Liu, W. The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor. Appl. Genet. 2006, 114, 13–20. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Liu, W.; Wu, X.; Yang, X.; Li, X.; Lu, Y.; Li, L. An intercalary translocation from Agropyron cristatum 6P chromosome into common wheat confers enhanced kernel number per spike. Planta 2016, 244, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Liu, W.; Han, H.; Lu, Y.; Yang, X.; Li, X.; Li, L. Introgression of Agropyron cristatum 6P chromosome segment into common wheat for enhanced thousand-grain weight and spike length. Theor. Appl. Genet. 2015, 128, 1827–1837. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Pan, C.; Yao, M.; Zhang, J.; Yang, X.; Liu, W.; Li, X.; Xi, Y.; Li, L. Chromosomal localization of genes conferring desirable agronomic traits from wheat-Agropyron cristatum disomic addition line 5113. PLoS ONE 2016, 11, e0165957. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, H.; Liu, W.; Song, L.; Zhang, J.; Zhou, S.; Yang, X.; Li, X.; Li, L. Deletion mapping and verification of an enhanced-grain number per spike locus from the 6PL chromosome arm of Agropyron cristatum in common wheat. Theor. Appl. Genet. 2019, 132, 2815–2827. [Google Scholar] [CrossRef]
- Sun, Y.; Lyu, M.; Han, H.; Zhou, S.; Lu, Y.; Liu, W.; Yang, X.; Li, X.; Zhang, J.; Liu, X.; et al. Identification and fine mapping of alien fragments associated with enhanced grain weight from Agropyron cristatum chromosome 7P in common wheat backgrounds. Theor. Appl. Genet. 2021, 134, 3759–3772. [Google Scholar] [CrossRef]
- Szakacs, E.; Molnar-Lang, M. Molecular cytogenetic evaluation of chromosome instability in Triticum aestivum-Secale cereale disomic addition lines. J. Appl. Genet. 2010, 51, 149–152. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wu, J.; Gao, A.; Li, L. Analysis of genetic diversity in natural populations of Psathyrostachys Huashanica Keng using microsatellite (SSR) markers. Agric. Sci. China 2010, 9, 463–471. [Google Scholar] [CrossRef]
- Wang, Q.; Xiang, J.; Gao, A.; Yang, X.; Liu, W.; Li, X.; Li, L. Analysis of chromosomal structural polymorphisms in the St, P, and Y genomes of Triticeae (Poaceae). Genome 2010, 53, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ren, Z.; Tan, F.; Tang, Z.; Fu, S.; Yan, B.; Ren, T. Molecular cytogenetic characterization of new wheat-rye 1R(1B) substitution and translocation lines from a Chinese Secale cereal L. aigan with resistance to stripe rust. PLoS ONE 2016, 11, e0163642. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, C.; Zeng, Z.; Li, G.; Song, X.; Yang, Z. Genomic rearrangement between wheat and Thinopyrum elongatum revealed by mapped functional molecular markers. Genes Genom. 2012, 34, 67–75. [Google Scholar] [CrossRef]
- Kishii, M.; Yamada, T.; Sasakuma, T.; Tsujimoto, H. Production of wheat-Leymus racemosus chromosome addition lines. Theor. Appl. Genet. 2004, 109, 255–260. [Google Scholar] [CrossRef]
- Han, H.; Bai, L.; Su, J.; Zhang, J.; Song, L.; Gao, A.; Yang, X.; Li, X.; Liu, W.; Li, L. Genetic rearrangements of six wheat-Agropyron cristatum 6P addition lines revealed by molecular markers. PLoS ONE 2014, 9, e91066. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Pan, C.; Wang, Z.; Liu, F.; Zhang, J.; Yang, X.; Li, X.; Liu, W.; Li, L. Characterization, identification and evaluation of a novel wheat-Agropyron cristatum (L.) Gaertn. disomic addition line II-30-5. Genet. Resour. Crop Evol. 2020, 67, 2213–2223. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Mishra, S.; Khurana, P.; Khurana, J.P. CRY2 gene of rice (Oryza sativa subsp. indica) encodes a blue light sensory receptor involved in regulating flowering, plant height and partial photomorphogenesis in dark. Plant Cell Rep. 2023, 42, 73–89. [Google Scholar] [CrossRef]
- Suarez-Lopez, P.; Wheatley, K.; Robson, F.; Onouchi, H.; Valverde, F.; Coupland, G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef]
- Lv, B.; Nitcher, R.; Han, X.; Wang, S.; Ni, F.; Li, K.; Pearce, S.; Wu, J.; Dubcovsky, J.; Fu, D. Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS ONE 2014, 9, e94171. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kudo, T.; Makita, N.; Kiba, T.; Kinoshita, T.; Sakakibara, H. Flowering time control in rice by introducing Arabidopsis clock-associated PSEUDO-RESPONSE REGULATOR 5. Biosci. Biotechnol. Biochem. 2020, 84, 970–979. [Google Scholar] [CrossRef]
- Yoshida, R.; Fekih, R.; Fujiwara, S.; Oda, A.; Miyata, K.; Tomozoe, Y.; Nakagawa, M.; Niinuma, K.; Hayashi, K.; Ezura, H.; et al. Possible role of EARLY FLOWERING 3 (ELF3) in clock-dependent floral regulation by SHORT VEGETATIVE PHASE (SVP) in Arabidopsis thaliana. New Phytol. 2009, 182, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shim, J.; Kinmonth-Schultz, H.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Creux, N.; Harmer, S. Circadian rhythms in plants. Sci. Prog. 2019, 11, a034611. [Google Scholar] [CrossRef] [PubMed]
- Schwemmle, B. Thermoperiodic effects and circadian rhythms in flowering of plants. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 239–243. [Google Scholar] [CrossRef]
- Kong, X.; Wang, F.; Geng, S.; Guan, J.; Tao, S.; Jia, M.; Sun, G.; Wang, Z.; Wang, K.; Ye, X.; et al. The wheat AGL6-like MADS-box gene is a master regulator for floral organ identity and a target for spikelet meristem development manipulation. Plant Biotechnol. J. 2022, 20, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, Y.; Tanaka, W.; Kojima, M.; Sakakibara, H.; Hirano, H.Y. WUSCHEL-RELATED HOMEOBOX4 is involved in meristem maintenance and is negatively regulated by the CLE gene FCP1 in rice. Plant Cell 2013, 25, 229–241. [Google Scholar] [CrossRef]
- Yang, T.; Guo, L.; Ji, C.; Wang, H.; Wang, J.; Zheng, X.; Xiao, Q.; Wu, Y. The B3 domain-containing transcription factor ZmABI19 coordinates expression of key factors required for maize seed development and grain filling. Plant Cell 2021, 33, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, B.; He, Y. Improving grain yield via promotion of kernel weight in high yielding winter wheat genotypes. Biology 2022, 11, 42. [Google Scholar] [CrossRef]
- Mabbitt, P.D.; Wilbanks, S.M.; Eaton-Rye, J.J. Structure and function of the hydrophilic Photosystem II assembly proteins: Psb27, Psb28 and Ycf48. Plant Physiol. Biochem. 2014, 81, 96–107. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef]
- Fagerlund, R.D.; Forsman, J.A.; Biswas, S.; Vass, I.; Davies, F.K.; Summerfield, T.C.; Eaton-Rye, J.J. Stabilization of Photosystem II by the PsbT protein impacts photodamage, repair and biogenesis. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148234. [Google Scholar] [CrossRef]
- Jackson, S.A.; Hervey, J.R.; Dale, A.J.; Eaton-Rye, J.J. Removal of both Ycf48 and Psb27 in Synechocystis sp. PCC 6803 disrupts Photosystem II assembly and alters QA oxidation in the mature complex. FEBS Lett. 2014, 588, 3751–3760. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Behal, R.H.; Back, S.L.; Nikolau, B.J.; Wurtele, E.S.; Oliver, D.J. The role of pyruvate dehydrogenase and acetyl-coenzyme A synthetase in fatty acid synthesis in developing Arabidopsis seeds. Plant Physiol. 2000, 123, 497–508. [Google Scholar] [CrossRef]
- Behal, R.H.; Lin, M.; Back, S.; Oliver, D.J. Role of acetyl-coenzyme A synthetase in leaves of Arabidopsis thaliana. Arch. Biochem. Biophys. 2002, 402, 259–267. [Google Scholar] [CrossRef]
- Lin, M.; Oliver, D.J. The role of acetyl-coenzyme a synthetase in Arabidopsis. Plant Physiol. 2008, 147, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rock, C.O. Evaluation of epigallocatechin gallate and related plant polyphenols as inhibitors of the FabG and FabI reductases of bacterial type II fatty-acid synthase. J. Biol. Chem. 2004, 279, 30994–31001. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Contreras, S.C.; Chou, A.; Siegel, J.B.; Gonzalez, R. Combination of type II fatty acid biosynthesis enzymes and thiolases supports a functional beta-oxidation reversal. Metab. Eng. 2018, 45, 11–19. [Google Scholar] [CrossRef]
- Baack, E.J.; Whitney, K.D.; Rieseberg, L.H. Hybridization and genome size evolution: Timing and magnitude of nuclear DNA content increases in Helianthus homoploid hybrid species. New Phytol. 2005, 167, 623–630. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Li, G.; Yang, Z. Diversified chromosome rearrangements detected in a wheat-Dasypyrum breviaristatum substitution line induced by Gamma-Ray irradiation. Plants 2019, 8, 175. [Google Scholar] [CrossRef]
- Endo, T.R. The gametocidal chromosome as a tool for chromosome manipulation in wheat. Chromosome Res. 2007, 15, 67–75. [Google Scholar] [CrossRef]
- Louis, E.J. The chromosome ends of Saccharomyces cerevisiae. Yeast 1995, 11, 1553–1573. [Google Scholar] [CrossRef]
- Rocchi, M.; Capozzi, O.; Archidiacono, N. Centromere repositioning during evolution. Chromosome Res. 2014, 22, 405. [Google Scholar]
- Zhao, J.; Xie, Y.; Kong, C.; Lu, Z.; Jia, H.; Ma, Z.; Zhang, Y.; Cui, D.; Ru, Z.; Wang, Y.; et al. Centromere repositioning and shifts in wheat evolution. Plant Commun. 2023, 100556. [Google Scholar] [CrossRef] [PubMed]
- Alkhimova, A.G.; Heslop-Harrison, J.S.; Shchapova, A.I.; Vershinin, A.V. Rye chromosome variability in wheat-rye addition and substitution lines. Chromosome Res. 1999, 7, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bariah, I.; Keidar-Friedman, D.; Kashkush, K. Identification and characterization of large-scale genomic rearrangements during wheat evolution. PLoS ONE 2020, 15, e0231323. [Google Scholar] [CrossRef]
- Mirzaghaderi, G.; Mason, A.S. Revisiting pivotal-differential genome evolution in wheat. Trends Plant Sci. 2017, 22, 674–684. [Google Scholar] [CrossRef]
- Hao, C.Y.; Jiao, C.Z.; Hou, J.; Li, T.; Liu, H.X.; Wang, Y.Q.; Zheng, J.; Liu, H.; Bi, Z.H.; Xu, F.F.; et al. Resequencing of 145 landmark cultivars reveals asymmetric sub-genome selection and strong founder genotype effects on wheat breeding in China. Mol. Plant. 2020, 13, 1733–1751. [Google Scholar] [CrossRef]
- Miedaner, T.; Vasquez, A.; Castiblanco, V.; Castillo, H.E.; Foroud, N.; Wurschum, T.; Leiser, W. Genome-wide association study for deoxynivalenol production and aggressiveness in wheat and rye head blight by resequencing 92 isolates of Fusarium culmorum. BMC Genom. 2021, 22, 630. [Google Scholar] [CrossRef]
- Wang, J.R.; Fu, W.W.; Wang, R.; Hu, D.X.; Cheng, H.; Zhao, J.; Jiang, Y.; Kang, Z.S. WGVD: An integrated web-database for wheat genome variation and selective signatures. Database 2020, 2020, baaa090. [Google Scholar] [CrossRef]
- Zhang, H.; He, L.; Cai, L. Transcriptome sequencing: RNA-seq. Comput. Syst. Biol. Methods Protoc. 2018, 1754, 15–27. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Han, H.; Zhang, Y.; Liu, W.; Hu, Z.; Li, L. Degenerate oligonucleotide primed-polymerase chain reaction-based chromosome painting of P genome chromosomes in Agropyron cristatum and wheat-A. cristatum addition Lines. Crop Sci. 2015, 55, 2798–2805. [Google Scholar] [CrossRef]
- Jung, Y.; Han, D. BWA-MEME: BWA-MEM emulated with a machine learning approach. Bioinformatics 2022, 38, 2404–2413. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.D.; Lv, D.K.; Ge, Y.; Shi, J.S.; Weijers, D.; Yu, G.C.; Chen, J.H. RIdeogram: Drawing SVG graphics to visualize and map genome-wide data on the idiograms. PeerJ Comput. Sci. 2020, 6, e251. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Schlapbach, R.; Rehrauer, H. RNA-Seq data analysis: From raw data quality control to differential expression analysis. Methods Mol. Biol. 2017, 1669, 295–307. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Zheng, W.; Wang, L.; Cai, X.; Yang, J.; Song, R.; Yang, S.; Wang, Y.; Xiao, J.; et al. Recognition of glycoside hydrolase 12 proteins by the immune receptor RXEG1 confers Fusarium head blight resistance in wheat. Plant Biotechnol. J. 2023, 21, 769–781. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Han, H.; Guo, B.; Qi, K.; Zhang, J.; Zhou, S.; Yang, X.; Li, X.; Lu, Y.; Liu, W.; et al. The Genomic Variation and Differentially Expressed Genes on the 6P Chromosomes in Wheat–Agropyron cristatum Addition Lines 5113 and II-30-5 Confer Different Desirable Traits. Int. J. Mol. Sci. 2023, 24, 7056. https://doi.org/10.3390/ijms24087056
Yang W, Han H, Guo B, Qi K, Zhang J, Zhou S, Yang X, Li X, Lu Y, Liu W, et al. The Genomic Variation and Differentially Expressed Genes on the 6P Chromosomes in Wheat–Agropyron cristatum Addition Lines 5113 and II-30-5 Confer Different Desirable Traits. International Journal of Molecular Sciences. 2023; 24(8):7056. https://doi.org/10.3390/ijms24087056
Chicago/Turabian StyleYang, Wenjing, Haiming Han, Baojin Guo, Kai Qi, Jinpeng Zhang, Shenghui Zhou, Xinming Yang, Xiuquan Li, Yuqing Lu, Weihua Liu, and et al. 2023. "The Genomic Variation and Differentially Expressed Genes on the 6P Chromosomes in Wheat–Agropyron cristatum Addition Lines 5113 and II-30-5 Confer Different Desirable Traits" International Journal of Molecular Sciences 24, no. 8: 7056. https://doi.org/10.3390/ijms24087056
APA StyleYang, W., Han, H., Guo, B., Qi, K., Zhang, J., Zhou, S., Yang, X., Li, X., Lu, Y., Liu, W., Liu, X., & Li, L. (2023). The Genomic Variation and Differentially Expressed Genes on the 6P Chromosomes in Wheat–Agropyron cristatum Addition Lines 5113 and II-30-5 Confer Different Desirable Traits. International Journal of Molecular Sciences, 24(8), 7056. https://doi.org/10.3390/ijms24087056






