The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions
Abstract
:1. Introduction
2. Results
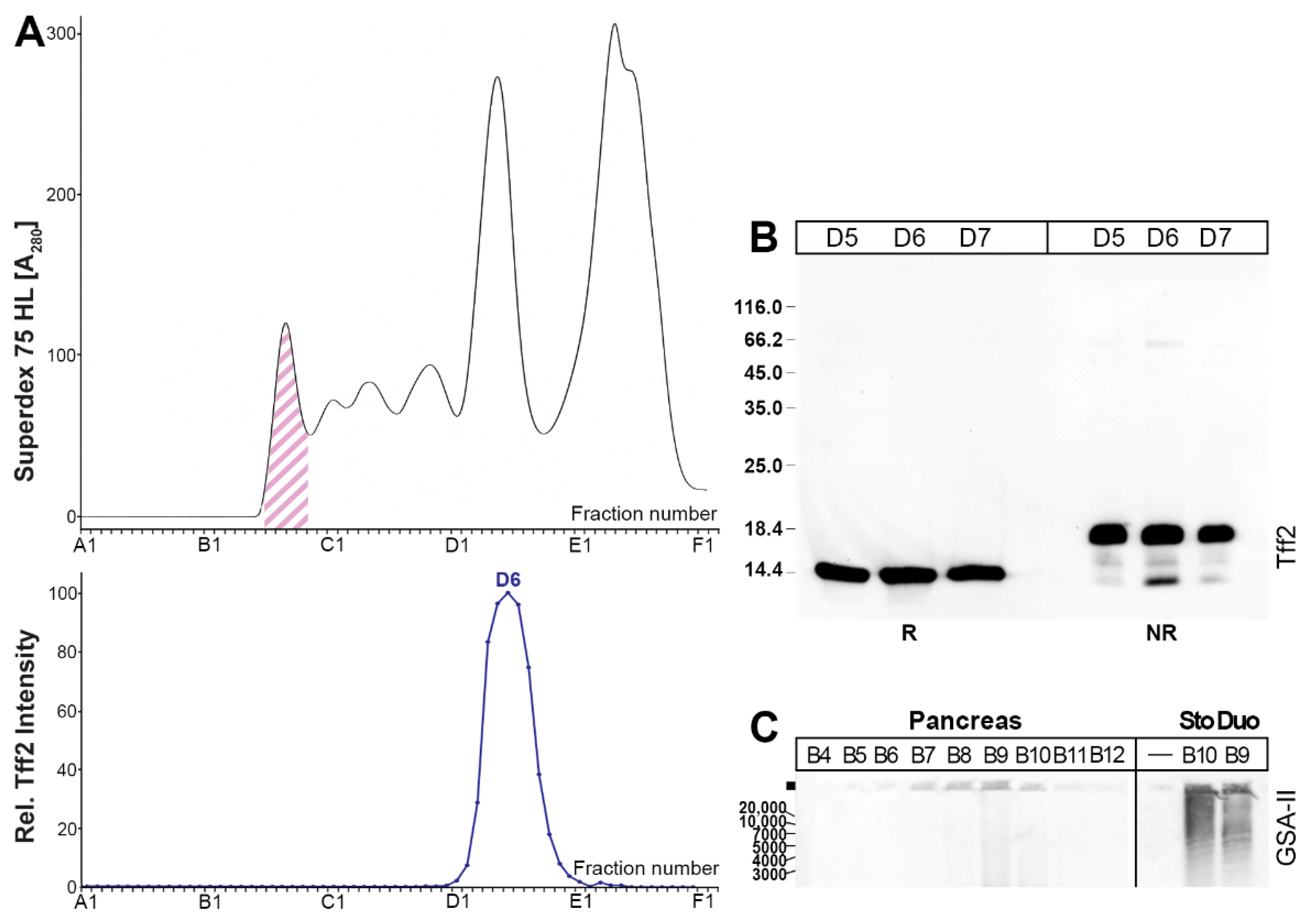
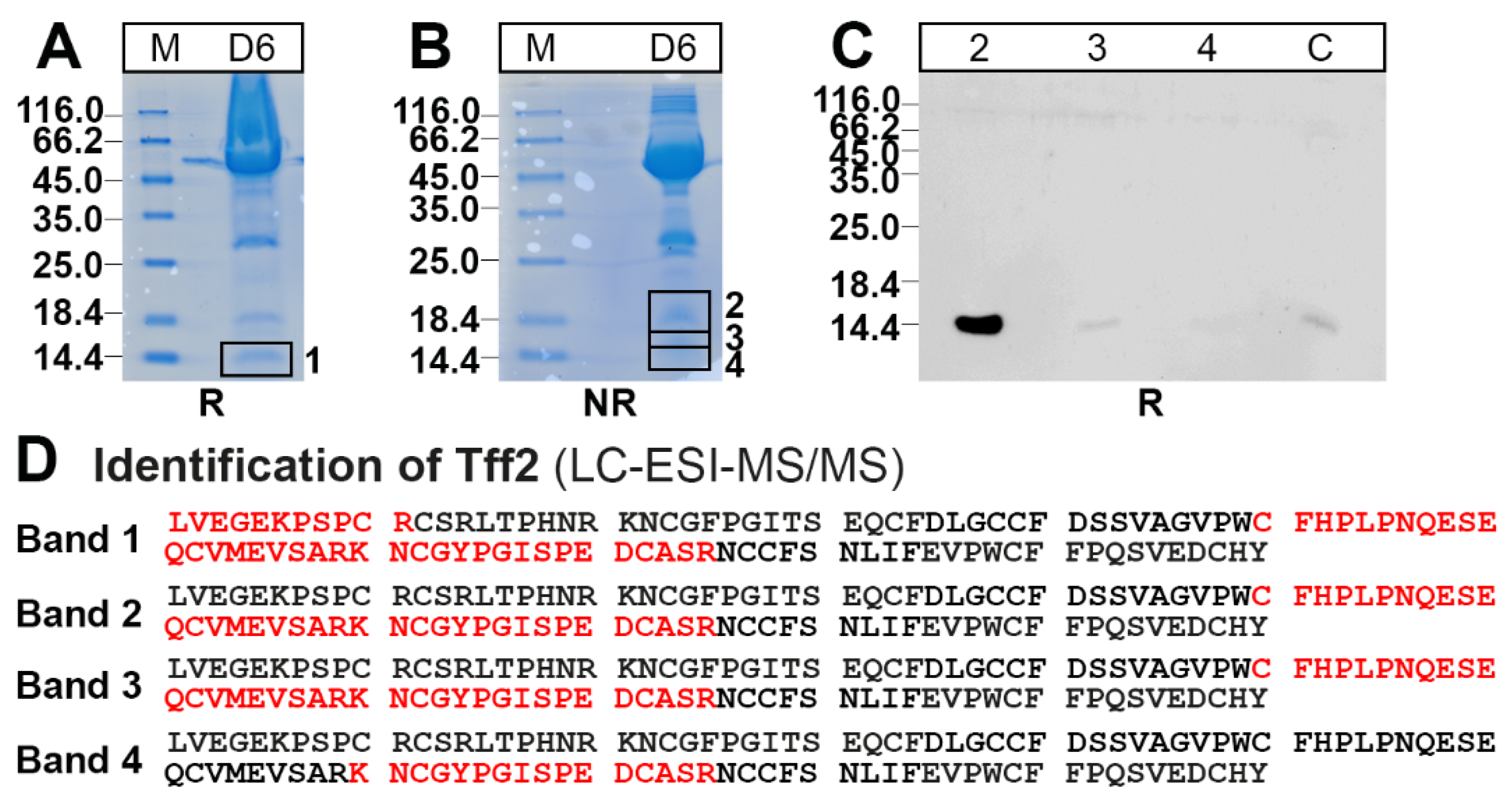
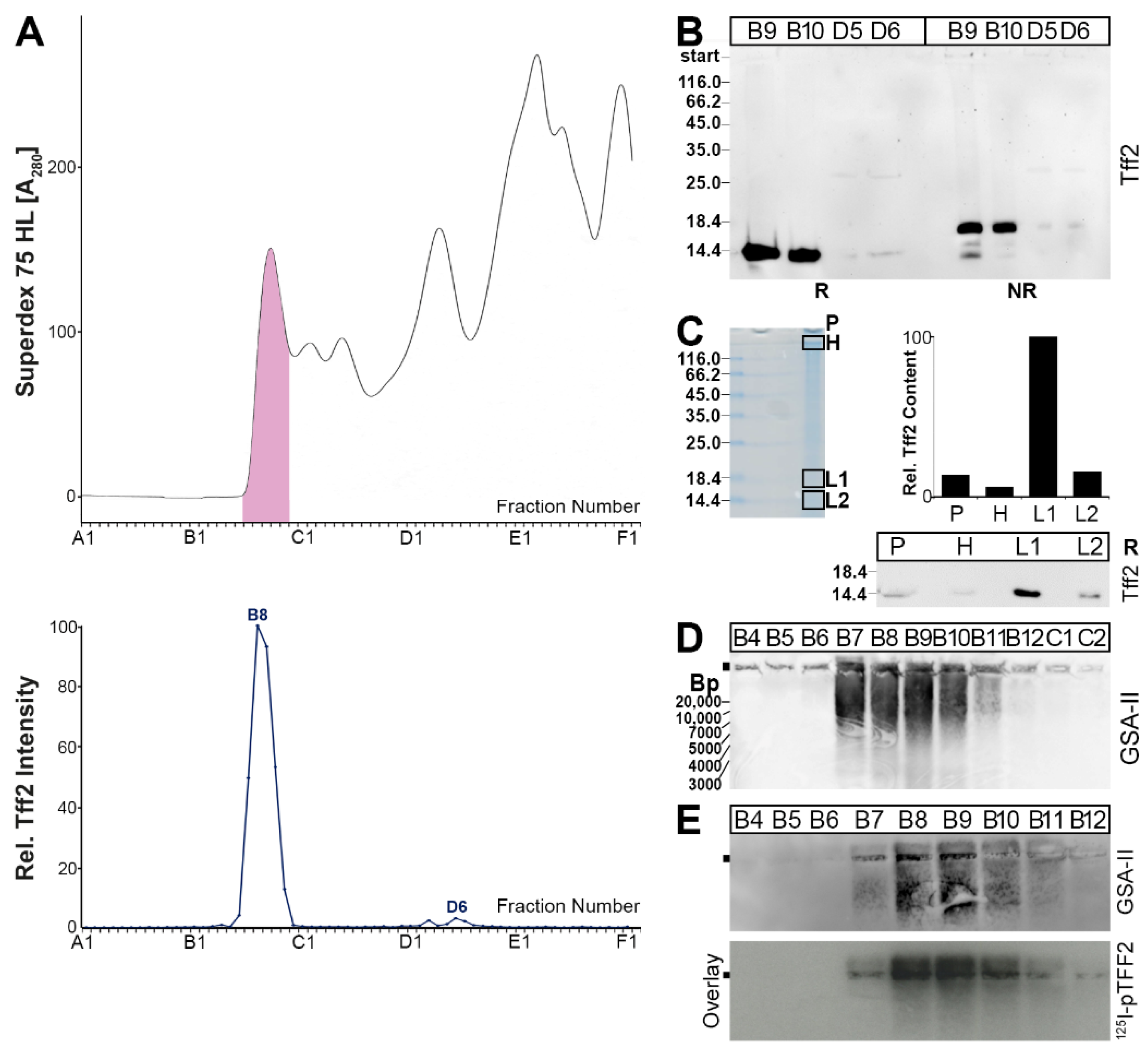
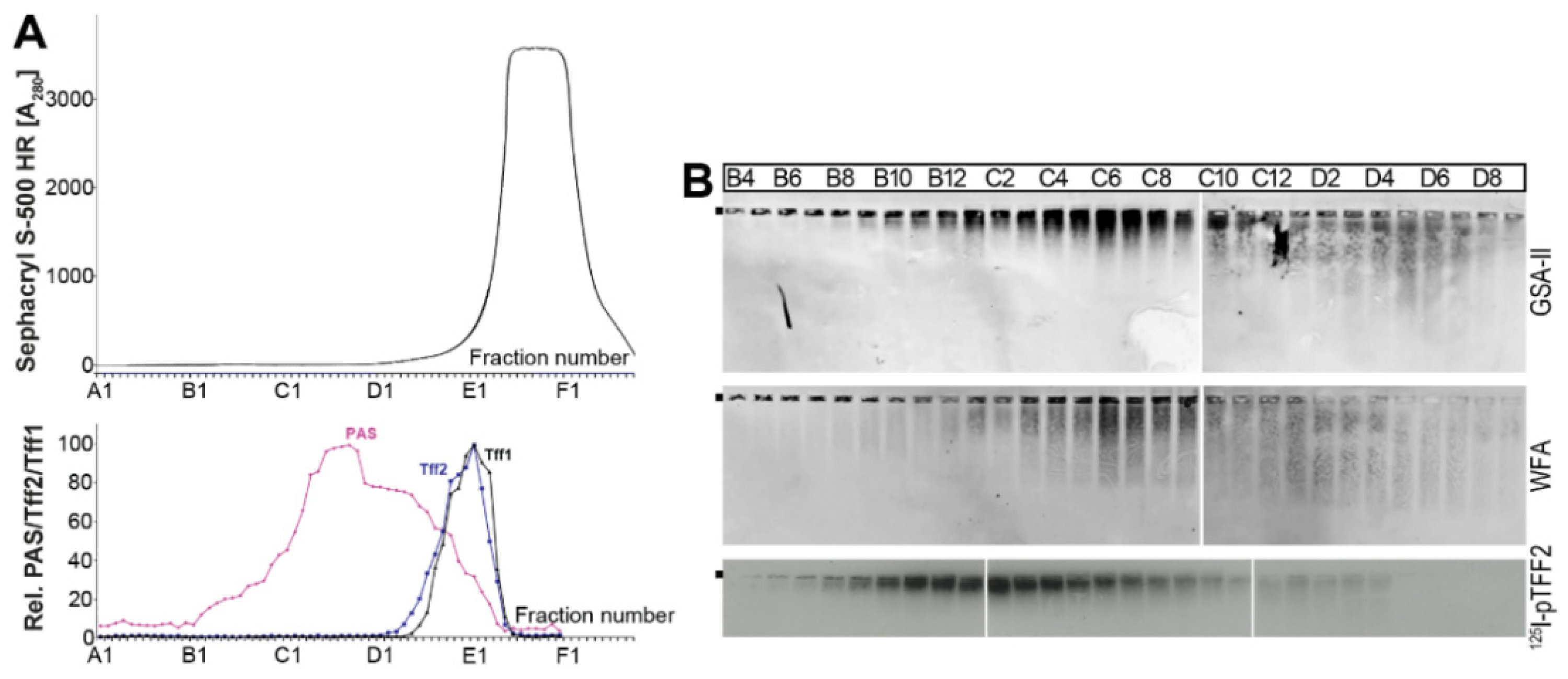
2.1. Characterization of Tff2 Forms in the Murine Stomach, Pancreas, and Duodenum by SEC
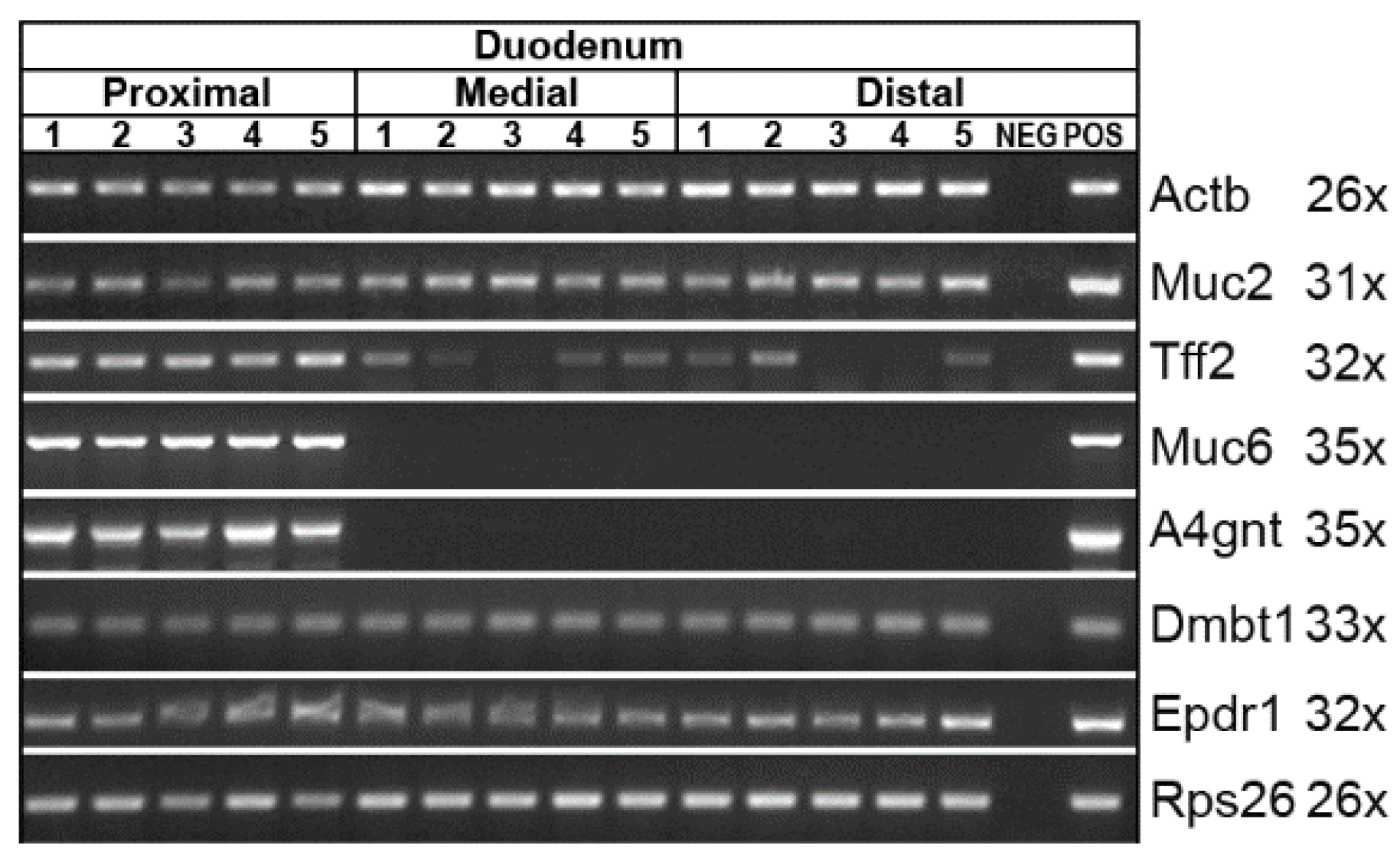
2.2. Expression Analysis of the Murine Stomach, Pancreas, and Duodenum (RT-PCR Analysis)
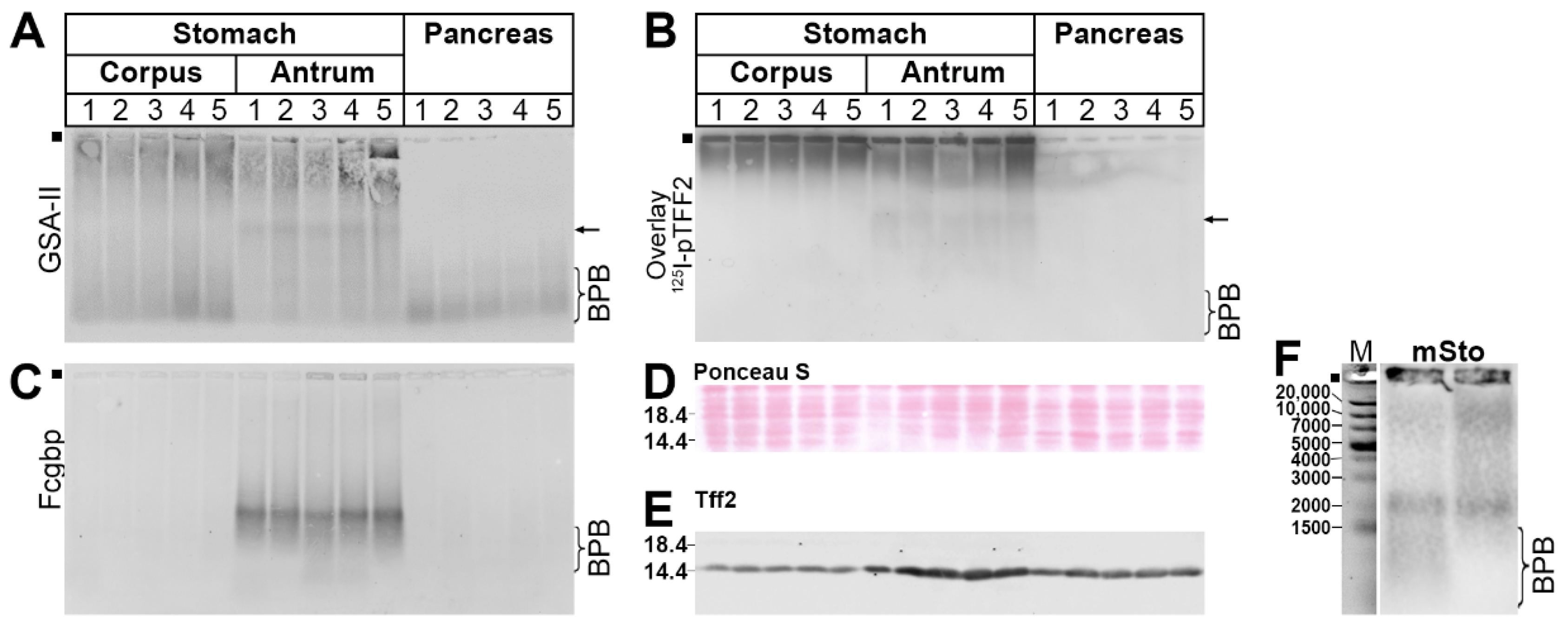
2.3. Protein Analysis of the Murine Gastric Corpus, Gastric Antrum, and Pancreas
3. Discussion
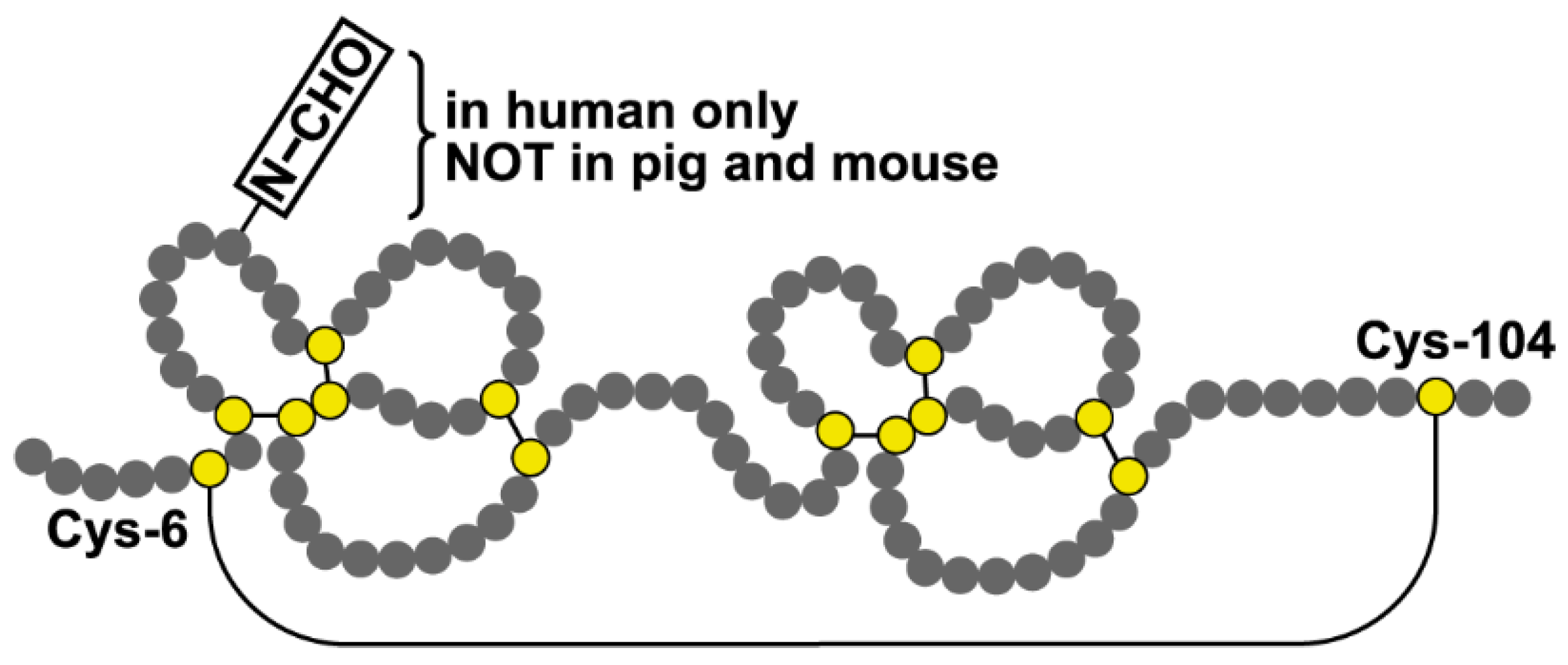
3.1. The Predominant Gastric Tff2 Form Is Different When Compared with the Pancreatic Form
3.2. Tff2 Forms in the Duodenum
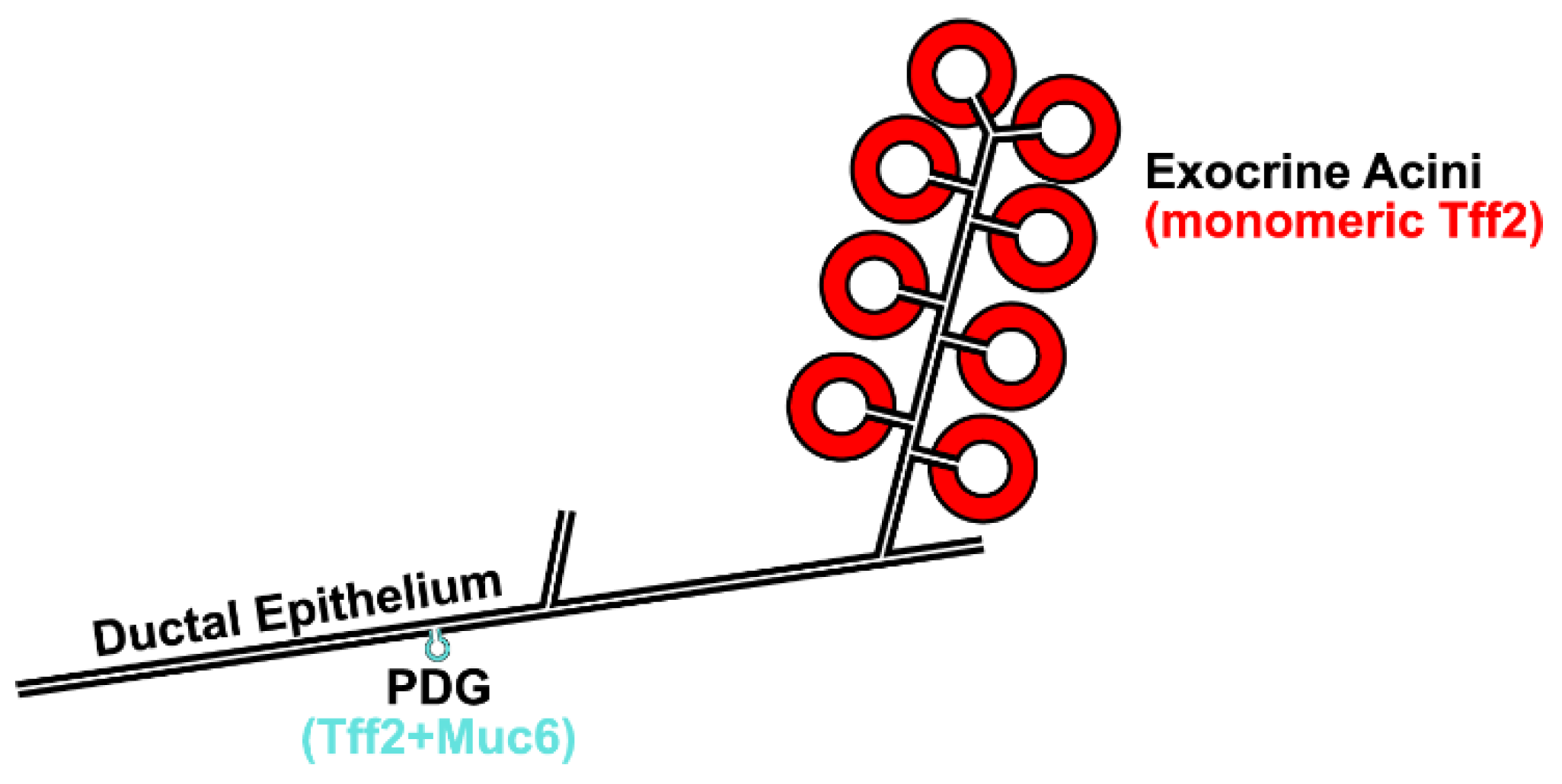
3.3. Possible Receptor-Mediated Protective Function of Pancreatic Tff2 in the Mouse
4. Materials and Methods
4.1. Animals
4.2. Extraction of Proteins, Protein Purification by SEC
4.3. SDS-PAGE, AgGE, and Western Blot Analysis
4.4. Identification of Proteins by Bottom-Up Proteomics
4.5. Tff2 Binding Studies
4.6. RNA Extraction, PCR Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgGE | Agarose gel electrophoresis |
| FCGBP | IgG Fc binding protein |
| Gkn | Gastrokine |
| IL | Interleukin |
| PDG | Pancreatic duct gland |
| SEC | Size exclusion chromatography |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TFF | Trefoil factor family |
References
- Jorgensen, K.H.; Thim, L.; Jacobsen, H.E. Pancreatic spasmolytic polypeptide (PSP): I. preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul. Pept. 1982, 3, 207–219. [Google Scholar] [CrossRef]
- Thim, L. Trefoil peptides: From structure to function. Cell. Mol. Life Sci. 1997, 53, 888–903. [Google Scholar] [CrossRef]
- Kjellev, S. The trefoil factor family—Small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Lefebvre, O.; Wolf, C.; Kédinger, M.; Chenard, M.P.; Tomasetto, C.; Chambon, P.; Rio, M.C. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J. Cell Biol. 1993, 122, 191–198. [Google Scholar] [CrossRef]
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–188. [Google Scholar]
- Paulsen, F.; Varoga, D.; Paulsen, A.; Tsokos, M. Trefoil factor family (TFF) peptides of normal human Vater’s ampulla. Cell Tissue Res. 2005, 321, 67–74. [Google Scholar] [CrossRef]
- Ota, H.; Hayama, M.; Momose, M.; El-Zimaity, H.M.; Matsuda, K.; Sano, K.; Maruta, F.; Okumura, N.; Katsuyama, T. Co-localization of TFF2 with gland mucous cell mucin in gastric mucous cells and in extracellular mucous gel adherent to normal and damaged gastric mucosa. Histochem. Cell Biol. 2006, 126, 617–625. [Google Scholar] [CrossRef]
- Hertel, S.C.; Chwieralski, C.E.; Hinz, M.; Riom, M.-C.; Tomasetto, C.; Hoffmann, W. Profiling trefoil factor family (TFF) expression in the mouse: Identification of an antisense TFF1-related transcript in the kidney and liver. Peptides 2004, 25, 755–762. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Chwieralski, C.E.; Bälder, R.; Hinz, M.; Braun, A.; Krug, N.; Hoffmann, W. Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 2007, 36, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Tomasetto, C.; Rio, M.C.; Gautier, C.; Wolf, C.; Hareuveni, M.; Chambon, P.; Lathe, R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990, 9, 407–414. [Google Scholar] [CrossRef]
- Lefebvre, O.; Chenard, M.P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996, 274, 259–262. [Google Scholar] [CrossRef]
- Ebert, M.P.A.; Hoffmann, J.; Haeckel, C.; Rutkowski, K.; Schmid, R.M.; Wagner, M.; Adler, G.; Schulz, H.U.; Roessner, A.; Hoffmann, W.; et al. Induction of TFF1 gene expression in pancreas overexpressing transforming growth factor alpha. Gut 1999, 45, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Hirata, K.; Kodama, S.; Nakano, Y.; Minaki-Nakagawa, Y.; Aoyama, Y.; Sakikubo, M.; Goto, T.; Yoshida, M.; Masui, T.; Yamamoto, T.; et al. Exocrine tissue-driven TFF2 prevents apoptotic cell death of endocrine lineage during pancreas organogenesis. Sci. Rep. 2019, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.N.; Raaberg, L.; Poulsen, S.S.; Thim, L.; Holst, J.J. Immunohistochemical localization of pancreatic spasmolytic polypeptide (PSP) in the pig. Histochemistry 1992, 98, 113–119. [Google Scholar] [CrossRef]
- Rasmussen, T.N.; Harling, H.; Thim, L.; Pierzynowski, S.; Weström, B.R.; Holst, J.H. Regulation of secretion of pancreatic spasmolytic polypeptide from porcine pancreas. Am. J. Physiol. 1993, 264, G22–G29. [Google Scholar] [CrossRef]
- Ohshio, G.; Suwa, H.; Kawaguchi, Y.; Imamura, M.; Yamaoka, Y.; Yamabe, H.; Matsumoto, M.; Yoshioka, H.; Hashimoto, Y.; Takeda, H. Differential expression of human spasmolytic polypeptide (trefoil factor family-2) in pancreatic carcinomas, ampullary carcinomas, and mucin-producing tumors of the pancreas. Dig. Dis. Sci. 2000, 45, 659–664. [Google Scholar] [CrossRef]
- Jackerott, M.; Lee, Y.C.; Mollgard, K.; Kofod, H.; Jensen, J.; Rohleder, S.; Neubauer, N.; Gaarn, L.W.; Lykke, J.; Dodge, R.; et al. Trefoil factors are expressed in human and rat endocrine pancreas: Differential regulation by growth hormone. Endocrinology 2006, 147, 5752–5759. [Google Scholar] [CrossRef] [Green Version]
- Madsen, J.; Nielsen, O.; Tornøe, I.; Thim, L.; Holmskov, U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 2007, 55, 505–513. [Google Scholar] [CrossRef]
- Guppy, N.J.; El-Bahrawy, M.E.; Kocher, H.M.; Fritsch, K.; Qureshi, Y.A.; Poulsom, R.; Jeffery, R.E.; Wright, N.A.; Otto, W.R.; Alison, M.R. Trefoil factor family peptides in normal and diseased human pancreas. Pancreas 2012, 41, 888–896. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Liss, A.S.; Sontheimer, A.; Mino-Kenudson, M.; Castillo, C.F.; Warshaw, A.L.; Thayer, S.P. Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair. Stem Cell Res. 2015, 15, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Baus-Loncar, M.; Kayademir, T.; Takaishi, S.; Wang, T. Trefoil factor family 2 deficiency and immune response. Cell. Mol. Life Sci. 2005, 62, 2947–2955. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Cao, L.; Sandor, F.; Rogers, A.B.; Whary, M.T.; Nambiar, P.R.; Cerny, A.; Bowen, G.; Yan, J.; Takaishi, S.; et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 2007, 75, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Hinz, M.; Schwegler, H.; Chwieralski, C.E.; Laube, G.; Linke, R.; Pohle, W.; Hoffmann, W. Trefoil factor family (TFF) expression in the mouse brain and pituitary: Changes in the developing cerebellum. Peptides 2004, 25, 827–832. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int. J. Mol. Sci. 2021, 22, 4909. [Google Scholar] [CrossRef]
- Goldenring, J.R.; Mills, J.C. Cellular plasticity, reprogramming, and regeneration: Metaplasia in the stomach and beyond. Gastroenterology 2022, 162, 415–430. [Google Scholar] [CrossRef]
- Welter, C.; Theisinger, B.; Seitz, G.; Tomasetto, C.; Rio, M.C.; Chambon, P.; Blin, N. Association of the human spasmolytic polypeptide and an estrogen-induced breast cancer protein (pS2) with human pancreatic carcinoma. Lab. Investig. 1992, 66, 187–192. [Google Scholar]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides. Encyclopedia 2021, 1, 974–987. [Google Scholar] [CrossRef]
- Hanisch, F.G.; Ragge, H.; Kalinski, T.; Meyer, F.; Kalbacher, H.; Hoffmann, W. Human gastric TFF2 peptide contains an N-linked fucosylated N,N′-diacetyllactosediamine (LacdiNAc) oligosaccharide. Glycobiology 2013, 23, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Semple, J.I.; Newton, J.L.; Westley, B.R.; May, F.E.B. Dramatic diurnal variation in the concentration of the human trefoil peptide TFF2 in gastric juice. Gut 2001, 48, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Hauser, F.; Hoffmann, W. xP1 and xP4. P-domain peptides expressed in Xenopus laevis stomach mucosa. J. Biol. Chem. 1991, 266, 21306–21309. [Google Scholar] [CrossRef]
- Jagla, W.; Wiede, A.; Kölle, S.; Hoffmann, W. Differential expression of the TFF-peptides xP1 and xP4 in the gastrointestinal tract of Xenopus laevis. Cell Tissue Res. 1998, 291, 13–18. [Google Scholar] [CrossRef]
- Hauser, F.; Roeben, C.; Hoffmann, W. xP2, a new member of the P-domain peptide family of potential growth factors, is synthesized in Xenopus laevis skin. J. Biol. Chem. 1992, 267, 14451–14455. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more. Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Oinuma, T.; Kawano, J.; Suganuma, T. Glycoconjugate histochemistry of Xenopus laevis fundic gland with special reference to mucous neck cells during development. Anat. Rec. 1991, 230, 502–512. [Google Scholar] [CrossRef]
- Stürmer, R.; Reising, J.; Hoffmann, W. The TFF Peptides xP1 and xP4 Appear in Distinctive Forms in the Xenopus laevis Gastric Mucosa: Indications for Different Protective Functions. Int. J. Mol. Sci. 2019, 20, 6052. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, J. Dual Roles of Gastric Gland Mucin-specific O-glycans in Prevention of Gastric Cancer. Acta Histochem. Cytochem. 2014, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, U.; Krause, W.J. Molecular anatomy of an endodermal gland: Investigations on mucus glycoproteins and cell turnover in Brunner’s glands of Didelphis virginiana using lectins and PCNA immunoreactivity. J. Cell. Biochem. 1995, 58, 56–64. [Google Scholar] [CrossRef]
- Schumacher, U.; Duku, M.; Katoh, M.; Jörns, J.; Krause, W.J. Histochemical similarities of mucins produced by Brunner’s glands and pyloric glands: A comparative study. Anat. Rec. Part A 2004, 278A, 540–550. [Google Scholar] [CrossRef]
- Thim, L.; Madsen, F.; Poulsen, S.S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Investig. 2002, 32, 519–527. [Google Scholar] [CrossRef]
- Schwarz, H.; Hoffmann, W. Subcellular Localization of the TFF Peptides xP1 and xP4 in the Xenopus laevis Gastric/Esophageal Mucosa: Different Secretion Modes Reflecting Diverse Protective Functions. Int. J. Mol. Sci. 2020, 21, 761. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Katsuyama, T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem. J. 1992, 24, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different Forms of TFF2, A Lectin of the Human Gastric Mucus Barrier: In Vitro Binding Studies. Int. J. Mol. Sci. 2019, 20, 5871. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and their Different Roles in the Mucosal Innate Immune Defense and More: An Update. Curr. Med. Chem. 2021, 28, 7387–7399. [Google Scholar] [CrossRef]
- Oertel, M.; Graness, A.; Thim, L.; Bühling, F.; Kalbacher, H.; Hoffmann, W. Trefoil factor family-peptides promote migration of human bronchial epithelial cells: Synergistic effect with epidermal growth factor. Am. J. Respir. Cell Mol. Biol. 2001, 25, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Graness, A.; Chwieralski, C.E.; Reinhold, D.; Thim, L.; Hoffmann, W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-α-induced interleukin-6 (IL-6) and IL-8 secretion. J. Biol. Chem. 2002, 277, 18440–18446. [Google Scholar] [CrossRef] [Green Version]
- Chwieralski, C.E.; Schnurra, I.; Thim, L.; Hoffmann, W. Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am. J. Respir. Cell Mol. Biol. 2004, 31, 528–537. [Google Scholar] [CrossRef]
- Lalani, E.N.; Williams, R.; Jayaram, Y.; Gilbert, C.; Chaudhary, K.S.; Siu, L.S.; Koumarianou, A.; Playford, R.; Stamp, G.W. Trefoil factor-2, human spasmolytic polypeptide, promotes branching morphogenesis in MCF-7 cells. Lab. Investig. 1999, 79, 537–546. [Google Scholar]
- Siu, L.-S.; Romanska, H.; Abel, P.D.; Baus-Loncar, M.; Kayademir, T.; Stamp, G.W.H.; Lalani, E.-N. TFF2 (trefoil factor family factor2) inhibits apoptosis in breast and colorectal cancer cell lines. Peptides 2004, 25, 855–863. [Google Scholar] [CrossRef]
- Hoffmann, W. TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 2005, 62, 2932–2938. [Google Scholar] [CrossRef]
- Cho, S.Y.; Klemke, R.L. Extracellular-regulated Kinase Activation and CAS/Crk Coupling Regulate Cell Migration and Suppress Apoptosis during Invasion of the Extracellular Matrix. J. Cell Biol. 2000, 149, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Self-Renewal and Cancers of the Gastric Epithelium: An Update and the Role of the Lectin TFF1 as an Antral Tumor Suppressor. Int. J. Mol. Sci. 2022, 23, 5377. [Google Scholar] [CrossRef]
- Porębska, N.; Poźniak, M.; Matynia, A.; Żukowska, D.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Galectins as modulators of receptor tyrosine kinases signaling in health and disease. Cytokine Growth Factor Rev. 2021, 60, 89–106. [Google Scholar] [CrossRef]
- Dubeykovskaya, Z.; Dubeykovskiy, A.; Solal-Cohen, J.; Wang, T.C. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J. Biol. Chem. 2009, 284, 3650–3662. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W. Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510. [Google Scholar] [CrossRef]
- Iber, D.; Menshykau, D. The control of branching morphogenesis. Open Biol. 2013, 3, 130088. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Diet impact on obesity beyond calories and trefoil factor 2 (TFF2) as an illustration: Metabolic implications and potential applications. Biomolecules 2021, 11, 1830. [Google Scholar] [CrossRef]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.-M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef]
- Fox, J.G.; Rogers, A.B.; Whary, M.T.; Ge, Z.; Ohtani, M.; Jones, E.K.; Wang, T.C. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 -/- C57BL6 x Sv129 Helicobacter pylori-infected mice. Am. J. Pathol. 2007, 171, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, J.; Mino-Kenudson, M.; Liss, A.S.; Chowdhury, S.; Wang, T.C.; Fernández-Del Castillo, C.; Lillemoe, K.D.; Warshaw, A.L.; Thayer, S.P. Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice. Gastroenterology 2016, 151, 1232–1244.e10. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, R.; Müller, S.; Hanisch, F.-G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial Porcine Gastric Mucin Preparations, also Used as Artificial Saliva, are a Rich Source for the Lectin TFF2: In Vitro Binding Studies. Chembiochem. 2018, 19, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Falk, P.; Roth, K.A.; Gordon, J.I. Lectins are sensitive tools for defining the differention programs of mouse gut epithelial cell lineages. Am. J. Physiol.-Gastrointest. Liver Physiol. 1994, 266, G987–G1003. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiong, Z.J.; Li, J.; Zou, C.; Cairo, C.W.; Klassen, J.S.; Privé, G.G. Crystal structures of human lysosomal EPDR1 reveal homology with the superfamily of bacterial lipoprotein transporters. Commun. Biol. 2019, 2, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmrich, I.; Erdmann, S.; Melchers, U.; Chtarbova, S.; Finke, U.; Hentsch, S.; Hoffmann, I.; Oertel, M.; Hoffmann, W.; Müller, O. The novel ependymin related gene UCC1 is highly expressed in colorectal tumor cells. Cancer Lett. 2001, 165, 71–79. [Google Scholar] [CrossRef]
- Znalesniak, E.B.; Salm, F.; Hoffmann, W. Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 644. [Google Scholar] [CrossRef] [Green Version]
- Otto, W.R.; Rao, J.; Cox, H.M.; Kotzian, E.; Lee, C.Y.; Goodlad, R.A.; Lane, A.; Gorman, M.; Freemont, P.A.; Hansen, H.F.; et al. Effects of pancreatic spasmolytic Polypeptide (PSP) on epithelial cell function. Eur. J. Biochem. 1996, 235, 64–72. [Google Scholar] [CrossRef]
- Von Heijne, G. The signal peptide. J. Membr. Biol. 1990, 115, 195–201. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Kalinski, T.; Meyer, F.; Hoffmann, W. Self-renewal of the human gastric epithelium: New insights from expression profiling using laser microdissection. Mol. Biosyst. 2011, 7, 1105–1112. [Google Scholar] [CrossRef]
- Carleton, A. 25. The distribution of Brunner’s glands in the duodenum of mammals. Proc. Zool. Soc. London 1935, 105, 385–390. [Google Scholar] [CrossRef]
- Paulsen, F.P.; Varoga, D.; Paulsen, A.R.; Corfield, A.; Tsokos, M. Prognostic value of mucins in the classification of ampullary carcinomas. Hum. Pathol. 2006, 37, 160–167. [Google Scholar] [CrossRef]
- Strobel, O.; Rosow, D.E.; Rakhlin, E.Y.; Lauwers, G.Y.; Trainor, A.G.; Alsina, J.; Fernández-Del Castillo, C.; Warshaw, A.L.; Thayer, S.P. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010, 138, 1166–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga Emidio, N.; Hoffmann, W.; Brierley, S.M.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Thim, L.; Mortz, E. Isolation and characterization of putative trefoil peptide receptors. Regul. Pept. 2000, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Pan, S.; Cooke, K.; Moyes, K.W.; Bronner, M.P.; Goodlett, D.R.; Aebershold, R.; Brentnall, T.A. Comparison of pancreas juice proteins from cancer versus pancreatitis using quantitative proteomic analysis. Pancreas 2007, 34, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Randriamanantsoa, S.; Papargyriou, A.; Maurer, H.C.; Peschke, K.; Schuster, M.; Zecchin, G.; Steiger, K.; Öllinger, R.; Saur, D.; Scheel, C.; et al. Spatiotemporal dynamics of self-organized branching in pancreas-derived organoids. Nat. Commun. 2022, 13, 5219. [Google Scholar] [CrossRef]
- Vilchez-Vargas, R.; Salm, F.; Znalesniak, E.B.; Haupenthal, K.; Schanze, D.; Zenker, M.; Link, A.; Hoffmann, W. Profiling of the bacterial microbiota along the murine alimentary tract. Int. J. Mol. Sci. 2022, 23, 1783. [Google Scholar] [CrossRef]
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117. [Google Scholar] [CrossRef]
- Kouznetsova, I.; Laubinger, W.; Kalbacher, H.; Kalinski, T.; Meyer, F.; Roessner, A.; Hoffmann, W. Biosynthesis of gastrokine-2 in the human gastric mucosa: Restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell. Physiol. Biochem. 2007, 20, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Weste, J.; Houben, T.; Harder, S.; Schlüter, H.; Lücke, E.; Schreiber, J.; Hoffmann, W. Different molecular forms of TFF3 in the human respiratory tract: Heterodimerization with IgG Fc binding protein (FCGBP) and proteolytic cleavage in bronchial secretions. Int. J. Mol. Sci. 2022, 23, 15359. [Google Scholar] [CrossRef]
- Fu, T.; Kalbacher, H.; Hoffmann, W. TFF1 is differentially expressed in stationary and migratory rat gastric epithelial cells (RGM-1) after in vitro wounding: Influence of TFF1 RNA interference on cell migration. Cell. Physiol. Biochem. 2013, 32, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.; Keppler, C.; Henkeler, A.; Schilli-Westermann, M.; Linder, D.; Aumüller, G.; Seitz, J. Identification and Characterization of an IgG Binding Protein in the Secretion of the Rat Coagulating Gland. Biol. Chem. 2002, 383, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Znalesniak, E.B.; Kalinski, T.; Möhle, L.; Biswas, A.; Salm, F.; Dunay, I.R.; Hoffmann, W. TFF Peptides Play a Role in the Immune Response Following Oral Infection of Mice with Toxoplasma gondii. Eur. J. Microbiol. Immunol. 2015, 5, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Znalesniak, E.B.; Fu, T.; Salm, F.; Händel, U.; Hoffmann, W. Transcriptional responses in the murine spleen after Toxoplasma gondii infection: Inflammasome and mucus-associated genes. Int. J. Mol. Sci. 2017, 18, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Znalesniak, E.B.; Fu, T.; Guttek, K.; Händel, U.; Reinhold, D.; Hoffmann, W. Increased cerebral Tff1 expression in two murine models of neuroinflammation. Cell. Physiol. Biochem. 2016, 39, 2287–2296. [Google Scholar] [CrossRef]


| Genes Accession No. | Primer No. | Primer Pairs | Nucleotide Positions | Annealing T Size (bp) |
| A4gnt NM_001077424.2 | MB2430 MB2431 | GAAGATTAGGCAGTGAGTTACCA TTAAGACGACACCACACCCG | 2–24 897–878 | 60 °C 896 |
| Dmbt1 NM_001347632.2 | MB2869 MB2870 | GAACCGGCACAATGGGGATCT ATAGGACACTTCATCTGTGGGAAC | 6–26 115–92 | 60 °C 110 |
| Gkn2 NM_025467.1 | MB2732 MB2733 | TTCTGGTGGTGCTGTCCATC TAGGCGACCCAAACAGGAAC | 51–70 446–427 | 60 °C 396 |
| Fcgbp NM_001122603.1 | MB2448 MB2449 | ATTCTGTGTCGCTGGTTCGT CAGTTGGCCATCCCAGTCAT | 384–403 556–537 | 60 °C 173 |
| Muc5ac NM_010844.3 | MB2318 MB2319 | TACCATGAACACCGCTCTGA GTTGGAGAGGAACTCGTTGG | 146–165 718–699 | 58 °C 573 |
| Muc6 NM_001330001.2 | MB2320 MB2321 | CCCTCATGGCTGTGTATGAC TTGTGGTTCAAGTAGGTGCC | 1389–1408 2223–2204 | 58 °C 835 |
| Rps26 NM_013765.2 | MB1494 MB1495 | CCAAAACCTGGAGATGAGGA CAGGCTACGGCAGAGATAGG | 120–139 382–363 | 57 °C 263 |
| Tff3 NM_011575.2 | MB2470 MB2471 | GCTACCCCTCTGTCACATCG ATCAGCCTTGTGTTGGCTG | 166–185 440–421 | 60 °C 275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Znalesniak, E.B.; Laskou, A.; Salm, F.; Haupenthal, K.; Harder, S.; Schlüter, H.; Hoffmann, W. The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions. Int. J. Mol. Sci. 2023, 24, 7059. https://doi.org/10.3390/ijms24087059
Znalesniak EB, Laskou A, Salm F, Haupenthal K, Harder S, Schlüter H, Hoffmann W. The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions. International Journal of Molecular Sciences. 2023; 24(8):7059. https://doi.org/10.3390/ijms24087059
Chicago/Turabian StyleZnalesniak, Eva B., Aikaterini Laskou, Franz Salm, Katharina Haupenthal, Sönke Harder, Hartmut Schlüter, and Werner Hoffmann. 2023. "The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions" International Journal of Molecular Sciences 24, no. 8: 7059. https://doi.org/10.3390/ijms24087059







