Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation
Abstract
:1. Introduction
2. Definition of MC-Related Disorders
2.1. Mastocytosis
2.2. Mast Cell Activation Syndromes
2.3. Hereditary Alpha-Tryptasemia
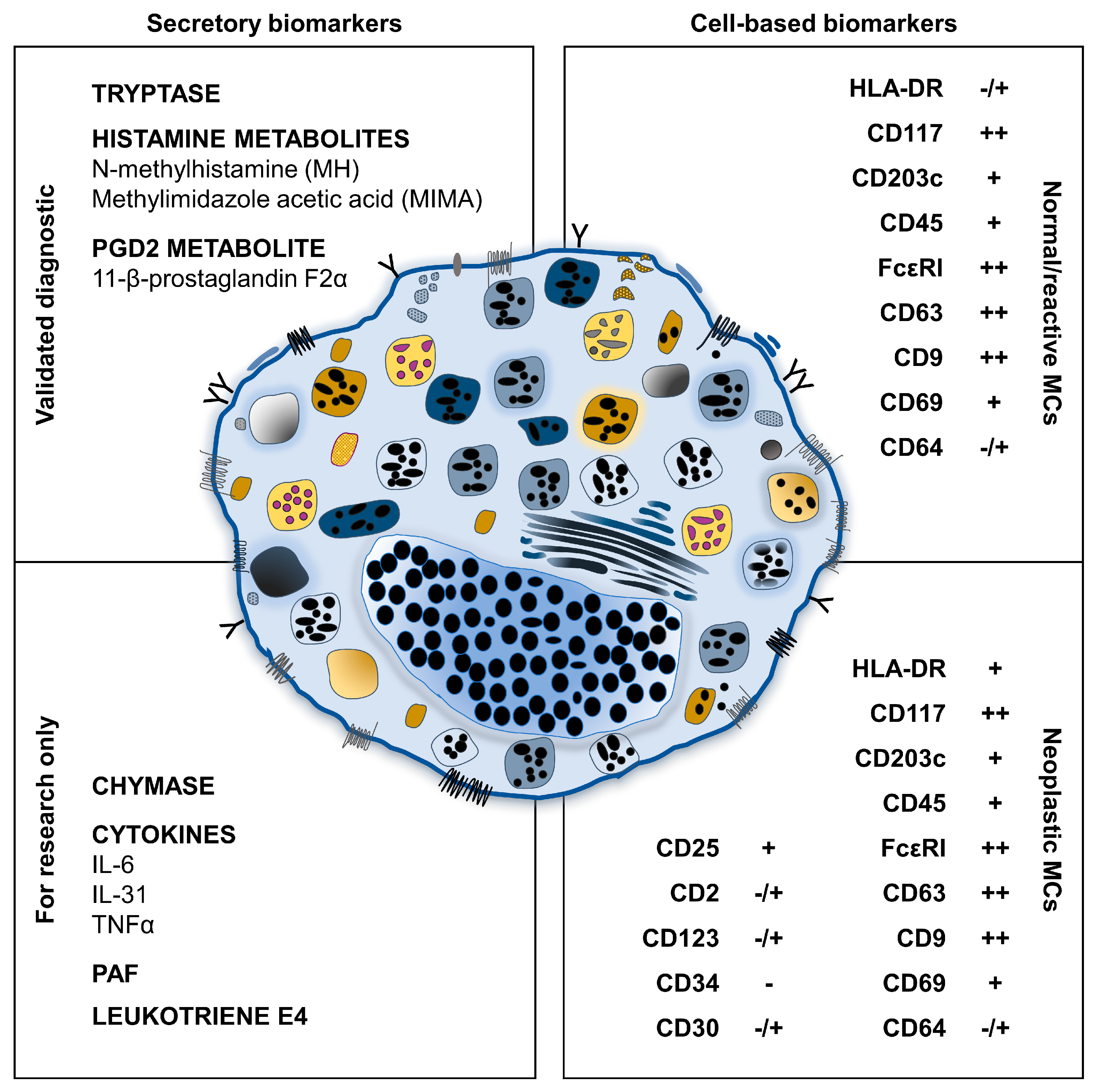
3. Soluble Biomarkers of MC Activation
3.1. Preformed Mediators
3.1.1. Histamine
3.1.2. Tryptase
3.1.3. Chymase
3.2. De Novo Synthesized Mediators
3.2.1. Prostaglandin D2
3.2.2. Platelet Activating Factor (PAF)
3.2.3. Cysteinyl Leukotrienes
3.2.4. Cytokines
IL-6
IL-31
4. Flow Cytometry Biomarkers of MC Activation
4.1. Identification of Clonal MC by Flow Cytometry
4.2. Receptors Involved in MC Activation
4.3. Potential Markers of MC Activation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilfillan, A.M.; Austin, S.J.; Metacalfe, D.D. Mast cells biology: Introduction and overview. Adv. Exp. Med. Biol. 2011, 716, 2–12. [Google Scholar] [PubMed] [Green Version]
- Valent, P.; Akin, C.; Hartamann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef] [PubMed]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [Green Version]
- De Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Theoharides, C.; Valent, P.; Akin, C. Mast cells, mastocytosis, and related disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.R. Mast cell activation syndrome: Tools for diagnosis and differential diagnosis. J. Allergy Clin. Immunol. 2020, 8, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Escribano, L.; Garcia Montero, A.C.; Nunez, R.; Orfao, A. Flow cytometric analysis of normal and neoplastic mast cells: Role in diagnosis and follow-up of mast cell disease. Immunol. Allergy Clin. N. Am. 2006, 26, 535–547. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, C. Mast cell activation syndromes. J. Allergy Clin. Immunol. 2017, 140, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Giavina-Bianchi, P.; Mezzano, V.; Castells, M. Expanding spectrum of mast cell activation disorders: Monoclonal and idiopathic mast cell activation syndromes. Clin. Ther. 2013, 35, 548–562. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017, 77, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Updated diagnostic criteria and classification of mast cell disorders. A consensus proposal. Hemasphere 2021, 5, e646. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 465–485. [Google Scholar] [CrossRef]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT mutation analysis in mast cell neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, A.; George, T.I.; Gotlib, J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood 2020, 135, 1365–1376. [Google Scholar] [CrossRef]
- Trizuljak, J.; Sperr, W.R.; Nekvindova, L.; Elberink, H.O.; Gleixner, K.V.; Gorska, A.; Lange, M.; Hartmann, K.; Illerhaus, A.; Bonifacio, M.; et al. Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification. Allergy 2020, 75, 1927–1938. [Google Scholar] [CrossRef]
- Castells, M.; Austen, K.F. Mastocytosis: Mediator related signs and symptoms. Int. Arch. Allergy Immunol. 2002, 127, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Power, M.R.; Lin, T.J. De novo synthesis of early growth response factor-1 is required for the full responsiveness of mast cells to produce TNF and IL-13 by IgE and antigen stimulation. Blood 2006, 107, 2814–2820. [Google Scholar] [CrossRef] [Green Version]
- Kruger-Krasagakes, S.; Moller, A.; Kolde, G.; Lippert, U.; Weber, M.; Henz, B.M. Production of interleukin-6 by human mast cells and basophilic cells. J. Investig. Dermatol. 1996, 106, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Conti, P.; Kempuraj, D.; Di Gioacchino, M.; Boucher, W.; Letourneau, R.; Kandare, K.; Barbacane, R.C.; Reale, M.; Felaco, M.; Frydas, S.; et al. Interleukin-6 and mast cells. Allergy Asthma. Proc. 2002, 23, 331–335. [Google Scholar]
- Theoharides, T.C.; Tisilioni, I.; Ren, H. Recent advances in our understanding of mast cell activation—Or should it be mast cell mediator disorders? Expert Rev. Clin. Immunol. 2019, 15, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Nedoszytko, B.; Bonadonna, P.; Hartmann, K.; Niedoszytko, M.; Brockow, K.; Siebenhaar, F.; Triggiani, M.; Arock, M.; et al. Diagnosis, Classification and Management of Mast Cell Activation Syndromes (MCAS) in the Era of Personalized Medicine. Int. J. Mol. Sci. 2020, 21, 9030. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.R.; Austen, K.F.; Akin, C.; Barkoff, M.S.; Bernstein, J.A.; Bonadonna, P.; Butterfield, J.H.; Carter, M.; Fox, C.C.; Maitland, A.; et al. AAAAI Mast Cell Disorders Committee Work Group Report: Mast cell activation syndrome (MCAS) diagnosis and management. J. Allergy Clin. Immunol. 2019, 144, 883–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valent, P. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 1125–1133. [Google Scholar] [CrossRef]
- Valent, P.; Bonadonna, P.; Hartmann, K.; Broesby-Olsen, S.; Brockow, K.; Butterfield, J.H.; Triggiani, M.; Lyons, J.J.; Oude Elberink, J.N.G.; Arok, M.; et al. Why the 20% + 2 Tryptase Formula Is a Diagnostic Gold Standard for Severe Systemic Mast Cell Activation and Mast Cell Activation Syndrome. Int. Arch. Allergy Immunol. 2019, 180, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI guidelines: Anaphylaxis (2021 updates). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J. Hereditary alpha tryptasemia: Genotyping and associated clinical features. Immunol. Allergy Clin. N. Am. 2019, 38, 483–495. [Google Scholar] [CrossRef]
- Greiner, G.; Sprinzl, B.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; Esterbauer, H.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef]
- Sordi, B.; Vanderwert, F.; Crupi, F.; Gesullo, F.; Zanotti, R.; Bonadonna, P.; Crosera, L.; Elena, C.; Fiorelli, N.; Ferrari, J.; et al. Disease correlates and clinical relevance of hereditary alpha-tryptasemia in patients with systemic mastocytosis. J. Allergy Clin. Immunol. 2023, 151, 485–493. [Google Scholar] [CrossRef]
- Wu, R.; Lyons, J.J. Hereditary alpha-tryptasemia: A commonly inherited modifier of anaphylaxis. Curr. Allergy Asthma. Rep. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.H.; Ravi, A.; Pongdee, T. Mast cell mediators of significance in clinical practice in mastocytosis. Immunol. Allergy Clin. N. Am. 2018, 28, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.H. Survey of Mast Cell Mediator Levels from Patients Presenting with Symptoms of Mast Cell Activation. Int. Arch. Allergy Clin. Immunol. 2020, 181, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Leru, P.M.; Anton, V.F.; Ureche, C.; Zurac, S.; Bratu, O.; Neagoe, C.D. Mast cell activation syndromes—Evaluation of current diagnostic criteria and laboratory tools in clinical practice (Review). Exp. Ther. Med. 2020, 20, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P. The basics of histamine biology. Ann. Allergy Asthma. Immunol. 2011, 106, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Nicklas, R.A.; Randolph, C.; Oppenheimer, J.; Bernstein, D.; Bernstein, J.; Ellis, A.; Golden, D.B.; Greenberger, P.; Kemp, S.; et al. Anaphylaxis—A practice parameter update 2015. Ann. Allergy Asthma. Immunol. 2015, 115, 341–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Y.; Schwartz, L.B.; Curry, A.; Pesola, G.R.; Knight, R.J.; Lee, H.S.; Tenenbaum, C.; Westfal, R.E. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J. Allergy Clin. Immunol. 2000, 106, 65–71. [Google Scholar] [CrossRef]
- Friedman, B.S.; Steinberg, S.C.; Meggs, W.J.; Kaliner, M.A.; Frieri, M.; Metacalfe, D.D. Analysis of plasma histamine levels in patients with mast cell disorders. Am. J. Med. 1989, 87, 649–654. [Google Scholar] [CrossRef]
- Simons, F.E.R. Anaphylaxis: Recent advances in assessment and treatment. J. Allergy Clin. Immunol. 2009, 124, 625–636. [Google Scholar] [CrossRef]
- Van Doormaal, J.J.; van der Veer, E.; van Voorst Vader, P.C.; Kluin, P.M.; Mulder, A.B.; van der Heide, S.; Arends, S.; Kluin-Nelemans, J.C.; Oude Elberink, J.N.; de Monchy, J.G. Tryptase and histamine metabolites as diagnostic indicators of indolent systemic mastocytosis without skin lesions. Allergy 2012, 67, 683–690. [Google Scholar] [CrossRef]
- Oranje, A.P.; Mulder, P.G.; Heide, R.; Tank, B.; van Toorenenbergen, A.W. Urinary N-methylhistamine as an indicator of bone marrow involvement in mastocytosis. Clin. Exp. Dermatol. 2002, 27, 502–506. [Google Scholar] [CrossRef]
- Divekar, R.; Butterfield, J. Urinary 11β-PGF2α and N-methyl histamine correlate with bone marrow biopsy findings in mast cell disorders. Allergy 2015, 70, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Nakashima, C.; Nonomura, Y.; Otsuka, A.; Cardamone, C.; Parente, R.; De Feo, G.; Triggiani, M. Biomarkers for evaluation of mast cell and basophil activation. Immunol. Rev. 2018, 282, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol. Immunol. 2015, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B. Clinical utility of tryptase levels in systemic mastocytosis and associated hematologic disorders. Leuk. Res. 2001, 25, 553–562. [Google Scholar] [CrossRef]
- Schwartz, L.B. Diagnostic Value of Tryptase in Anaphylaxis and Mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 451–463. [Google Scholar] [CrossRef]
- Itoh, Y.; Sendo, T.; Oishi, R. Physiology and pathophysiology of proteinase-activated receptors (PARs): Role of tryptase/PAR-2 in vascular endothelial barrier function. J. Pharmacol. Sci. 2005, 97, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Quang, T.L.; Lyons, J.J.; Naranjo, A.N.; Olivera, A.; Lazarus, R.A.; Metcalfe, D.D.; Milner, J.D.; Schwartz, L.B. Impact of naturally forming human α/β tryptase heterotetramers in the pathogenesis of hereditary α tryptasemia. J. Exp. Med. 2019, 216, 2348–2361. [Google Scholar]
- Sperr, W.R.; Stehberger, B.; Wimazal, F.; Baghestanian, M.; Schwartz, L.B.; Kundi, M.; Semper, H.; Jordan, J.H.; Chott, A.; Drach, J.; et al. Serum tryptase measurement in patients with myelodysplastic syndromes. Leuk. Lymphoma. 2002, 43, 1097–1105. [Google Scholar] [CrossRef]
- Vadas, P.; Perelman, B.; Liss, G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 144–149. [Google Scholar] [CrossRef]
- Sala-Cunill, A.; Cardona, V.; Labrador-Horrillo, M.; Luengo, O.; Esteso, O.; Garriga, T.; Vicario, M.; Guilarte, M. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int. Arch. Allergy Immunol. 2013, 160, 192–199. [Google Scholar] [CrossRef]
- Sperr, W.R.; Jordan, J.H.; Fiegl, M.; Escribano, L.; Bellas, C.; Dirnhofer, S.; Semper, H.; Simonitsch-Klupp, I.; Horny, H.P.; Valent, P. Serum tryptase levels in patients with mastocytosis: Correlation with mast cell burden and implication for defining the category of disease. Int. Arch. Allergy Immunol. 2002, 128, 136–141. [Google Scholar] [CrossRef]
- Kristensen, T.; Broesby-Olsen, S.; Vestergaard, H.; Bindslev-Jensen, C.; Boe Moller, M. Serum tryptase correlates with the KIT D816V mutation burden in adults with indolent systemic mastocytosis. Eur. J. Haematol 2013, 91, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sperr, W.R.; Kundi, M.; Alvarez-Twose, I.; van Anrooij, B.; Oude Elberink, J.N.G.; Gorska, A.; Niedoszytko, M.; Gleixner, K.V.; Hadzijusufovic, E.; Zanotti, R.; et al. International prognostic scoring system for mastocytosis (IPSM): A retrospective cohort study. Lancet Haematol. 2019, 6, e638–e649. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am. J. Hematol. 2021, 96, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Cardama, A.; Server, M.; Cortes, J.; Kantarjian, H.; Verstovsek, S. Bone marrow mast cell burden and serum tryptase level as markers of response in patients with systemic mastocytosis. Leuk. Lymphoma. 2013, 54, 1959–1964. [Google Scholar] [CrossRef]
- Van Anrooij van der Verr, E.; de Monchy, J.G.; van der Heide, S.; Kluin-Nelemans, J.C.; van Voorst Vader, P.C.; van Doormaal, J.J.; Oude Elberink, J.N. Higher mast cell load decreases the risk of hymenoptera venom-induced anaphylaxis in patients with mastocytosis. J. Allergy Clin. Immunol. 2013, 132, 125–130. [Google Scholar] [CrossRef]
- Klion, A.D.; Noel, P.; Akin, C.; Law, M.A.; Gilliland, D.G.; Cools, J.; Metcalfe, D.D.; Nutman, T.B. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 2003, 101, 4660–4666. [Google Scholar] [CrossRef] [Green Version]
- Mateya, A.; Wang, Q.; Chovanec, J.; Kim, J.; Wilson, K.J.; Schwartz, L.B.; Glover, S.C.; Carter, M.C.; Metcalfe, D.D.; Brittain, E.; et al. Defining baseline variability of serum tryptase levels improves accuracy in identifying anaphylaxis. J. Allergy Clin. Immunol. 2022, 149, 1010–1017. [Google Scholar] [CrossRef]
- Sala- Cunill, A.; Cardona, V. Markers of anaphylaxis, beyond tryptase. Curr. Opin. Allergy Clin. Immunol 2015, 15, 329–336. [Google Scholar] [CrossRef]
- Nishio, H.; Takai, S.; Miyazaki, M.; Horiuchi, H.; Osawa, M.; Uemura, K.; Yoshida, K.; Mukaida, M.; Ueno, Y.; Suzuki, K. Usefulness of serum mast cell-specific chymase levels for postmortem diagnosis of anaphylaxis. Int. J. Legal. Med. 2015, 119, 331–334. [Google Scholar] [CrossRef]
- Raymond, W.W.; Su, S.; Makarova, A.; Wilson, T.M.; Carter, M.C.; Metcalfe, D.D.; Caughey, G.H. Alpha 2-macroglobuline capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity. J. Immunol. 2009, 182, 5770–5777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, J.H.; Weiler, C.R. The Utility of Measuring Urinary Metabolites of Mast Cell Mediators in Systemic Mastocytosis and Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2020, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, M.; Eckermann, O.; Babina, M.; Edenharter, G.; Worm, M. Serum levels of 9alpha, 11beta PGF2 and cysteinyl leukotrienes are useful biomarkers of anaphylaxis. J. Allergy Clin. Immunol. 2016, 137, 312–314.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, J.H.; Weiler, C.R. Prevention of mast cell activation disorder-associated clinical sequelae of excessive prostaglandin D(2) production. Int. Arch. Allergy Immunol. 2008, 147, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Butterfield, J.; Weiler, C.R. Mast cell activation syndrome: Improved identification by combined determinations of serum tryptase and 24-hour urine 11beta-prostaglandin2alpha. J. Allergy Clin. Immunol. Pract. 2014, 2, 775–778. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Grunebaum, E.; Sussman, G.; Vadas, P. Platelet activating factor (PAF): A mediator of inflammation. Biofactors 2022, 48, 1189–1202. [Google Scholar] [CrossRef]
- Vadas, P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann. Allergy Asthma. Immunol. 2016, 117, 455–457. [Google Scholar] [CrossRef]
- Vadas, P.; Gold, M.; Perelman, B.; Liss, G.M.; Lack, G.; Blyth, T.; Simons, F.E.R.; Simons, K.J.; Cass, D.; Yeung, J. Platelet-activating factor, PF acetylhydrolase, and severe anaphylaxis. N. Eng. J. Med. 2008, 358, 28–35. [Google Scholar] [CrossRef]
- Macpherson, J.L.; Kemp, A.; Rogers, M.; Mallet, A.I.; Toia, R.F.; Spur, B.; Earl, J.W.; Chesterman, C.N.; Krills, S. A Occurrence of platelet-activating factor (PAF) and an endogenous inhibitor of platelet aggregation in diffuse cutaneous mastocytosis. Clin. Exp. Immunol. 1989, 77, 391–396. [Google Scholar]
- Austen, K.F. The cysteinyl leukotrienes: Where do they come from? What are they? Where are they going? Nat. Immunol. 2008, 9, 113–115. [Google Scholar] [CrossRef]
- Butterfield, J.H. Increased leukotriene E4 excretion in systemic mastocytosis. Prostaglandins Other Lipid Mediat. 2010, 92, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Valent, P. KIT D816V and the cytokine storm in mastocytosis: Production and role of interleukin-6. Haematologica 2020, 105, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Boucher, W.; Spear, K. Serum interleukin-6 reflects disease severity and osteoporosis in mastocytosis patients. Int. Arch. Allergy Immunol. 2002, 128, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Akin, C.; Huber, M.; Metcalfe, D.D. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin. Immunol. 2005, 115, 216–223. [Google Scholar] [CrossRef]
- Mayado, A.; Teodosio, C.; Garcia-Montero, A.C.; Matito, A.; Rodriguez-Caballero, A.; Morgado, J.M.; Muniz, C.; Jara-Acevedo, M.; Alvarez-Twose, I.; Sanchez-Munoz, L.; et al. Increased IL6 plasma levels in indolent systemic mastocytosis patients are associated with high risk of disease progression. Leukemia 2015, 30, 124–130. [Google Scholar] [CrossRef]
- Hartmann, K.; Wagner, N.; Rabenhorst, A.; Pflanz, L.; Leja, S.; Forster, A.; Gehring, M.; Kapp, A.; Raap, U. Serum Il-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adults patients. J. Allergy Clin. Immunol. 2013, 132, 232–235. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, L.; Teodosio, C.; Morgado, J.M.; Perbellini, O.; Mayado, A.; Alvarez-Twose, I.; Matito, A.; Jara-Acevedo, M.; Garcia-Montero, A.C.; Orfao, A.; et al. Flow cytometry in mastocytosis: Utility as a diagnostic and prognostic tool. Immunol. Allergy Clin. N. Am. 2014, 34, 297–313. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, L.; Teodósio, C.; Morgado, J.M.; Escribano, L. Immunophenotypic characterization of bone marrow mast cells in mastocytosis and other mast cell disorders. Methods Cell Biol. 2011, 103, 333–359. [Google Scholar]
- Escribano, L.; Díaz-Agustín, B.; Bellas, C.; Navalon, R.; Nunez, R.; Sperr, W.R.; Schernhaner, G.H.; Valent, P.; Orfao, A. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk. Res. 2001, 25, 563–570. [Google Scholar] [CrossRef]
- Escribano, L.; Orfao, A.; Villarrubia, J.; Diaz-Agustin, B.; Cerverò, C.; Rios, A.; Velasco, J.L.; Ciudad, J.; Navarro, J.L.; San Miguel, J.F. Immunophenotypic characterization of human bone marrow mast cells. A flow cytometric study of normal and pathological bone marrow samples. Annal. Cell. Pathol. 1998, 16, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Teodosio, C.; Mayado, A.; SanchezMunoz, L.; Morgado, J.M.; Jara-Acevedo, M.; Alvarez-Twose, I.; Garcia-Montero, A.C.; Matito, A.; Caldas, C.; Escribano, L.; et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J. Leuk. Biol 2015, 97, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, L.; Henriques, A.F.; Alvarez-Twose, I.; Matito, A. Bone marrow expression of mast cell disorders. Immunol. Allergy Clin. N. Am. 2018, 38, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Escribano, L.; Orfao, A.; Diaz-Agustin, B.; Villarrubia, J.; Cervero, C.; Lopez, A.; Marcos, M.A.; Bellas, C.; Fernandez-Canadas, S.; Cuevas, M.; et al. Indolent systemic mast cell disease in adults: Immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood 1998, 91, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Teodosio, C.; Garcia-Montero, A.C.; Jara-Acevedo, M.; Sanchez-munoz, L.; Alvarez-Twose, I.; Nunez, R.; Schwartz, L.B.; Walls, A.F.; Escribano, L.; Orfao, A. Mast cells from different molecular and prognostic subtypes of systemic mastocytosis display distinct immunophenotypes. J. Allergy Clin. Immunol. 2010, 125, 719–726. [Google Scholar] [CrossRef]
- Álvarez-Twose, I.; Jara-Acevedo, M.; Morgado, J.M.; Garcia-Montero, A.; Sanchez-Munoz, L.; Teodosio, C.; Matito, A.; Mayado, A.; Caldas, C.; Mollejo, M.; et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 2016, 137, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Morgado, J.M.; Sánchez-Muñoz, L.; Teodosio, C.G.; Jara-Acevedo, M.; Alvarez-Twose, I.; Matito, A.; Fernandez-Nunez, E.; Garcia-Montero, A.; Orfao, A.; Escribano, L. Immunophenotyping in systemic mastocytosis diagnosis: ‘CD25 positive’ alone is more informative than the ‘CD25 and/or CD2′ WHO criterion. Mod. Pathol. 2012, 25, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Sotlar, K.; Cerny-Reiterer, S.; Petat-Dutter, K.; Hessel, H.; Berezowska, S.; Mullauer, L.; Valent, P.; Horny, H. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod. Pathol. 2011, 24, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Horie, R.; Watanabe, T. CD30, expression and function in health and disease. Semin. Immunol. 1998, 10, 457–470. [Google Scholar] [CrossRef]
- Bellos, F.; Sotlar, K.; Schnittger, S.; Haferlach, C.; Haferlach, T.; Kern, W. Correlation of CD30 Expression on Neoplastic Mast Cells in Systemic Mastocytosis Assessed By Immunohistochemistry Versus Multiparameter Flow Cytometry and Correlation to Clinical Parameters. Blood 2015, 126, 1616. [Google Scholar] [CrossRef]
- Morgado, J.M.; Perbellini, O.; Johnson, R.C.; Teodosio, C.; Matito, A.; Bonadonna, P.; Zamò, A.; Jara-Acevedo, M.; Mayado, A.; Garcia-Montero, A.; et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology 2013, 63, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Akin, C.; Schwartz, L.B.; Kitoh, T.; Obayashi, H.; Worobec, A.S.; Scott, L.M.; Metcalfe, D.D. Soluble stem cell factor receptor (CD117) and IL-2 receptor alpha chain (CD25) levels in the plasma of patients with mastocytosis: Relationships to disease severity and bone marrow pathology. Blood 2000, 96, 1267–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardanani, A.; Finke, C.; Abdelrahman, R.A.; Lasho, T.L.; Hanson, C.A.; Tefferi, A. Increased circulating IL-2Rα (CD25) predicts poor outcome in both indolent and aggressive forms of mastocytosis: A comprehensive cytokine-phenotype study. Leukemia 2013, 27, 1430–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, S.; Jouvin, M.H.; Kulkarni, N.; Kissing, S.; Morgan, E.S.; Dvorak, A.M.; Schroder, B.; Saftig, P.; Kinet, J.P. The tetraspanin CD63 is required for efficient IgE-mediated mast cell degranulation and anaphylaxis. J. Immunol. 2013, 191, 2871–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruzkau, A.; Smorodchenko, A.; Lippert, U.; Kirchhof, L.; Artuc, M.; Henz, B.M. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 61, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Bahri, R.; Custovic, A.; Korosec, P.; Tsoumani, M.; Barron, M.; Wu, J.; Sayers, R.; Weimann, A.; Ruiz-Garcia, M.; Patel, N.; et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J. Allergy Clin. Immunol. 2018, 142, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D.; Pawankar, R.; Ackerman, S.J.; Akin, C.; Clayton, F.; Falcone, F.H.; Gleich, G.J.; Irani, A.M.; Johansson, M.W.; Klion, A.D.; et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ. J. 2016, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Escribano, L.; Dıaz-Agustın, B.; Nunez, R.; Prados, A.; Rodriguez, R.; Orfao, A. Abnormal expression of CD antigens in mastocytosis. Int. Arch. Allergy Immunol. 2002, 127, 127–132. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Lazki-Hagenbach, P.; Ali, H.; Sagi-Eisemberg, R. Authentic and Ectopically Expressed MRGPRX2 Elicit Similar Mechanisms to Stimulate Degranulation of Mast Cells. Cells 2021, 10, 376. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Stakl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Harnandez, J.D.; Sagi-Eisemberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef] [Green Version]
- Tatemoto, K.; Nozaki, Y.; Tusada, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuruda, K.; Nunomura, S.; Hayama, K.; Tenui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chompunud, N.A.; Thapaliya, M.; Deepak, V.; Ali, H. Multifaceted MRGPRX2, New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 2021, 148, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Datsi, A.; Steinhoff, M.; Ahamd, M.; Alam, M.; Buddenkotte, J. Interleukin-31, The “itchy” cytokine in inflammation and therapy. Allergy 2021, 76, 2982–2997. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Kolkhir, P.; Babina, M.; Dull, M.; Frischbutter, S.; Fok, J.S.; Jiao, Q.; Metz, M.; Scheffel, J.; Wolf, K.; et al. Mas-related G protein–coupled receptor X2 and its activators in dermatologic allergies. J. Allergy Clin. Immunol. 2021, 147, 456–469. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Giavina Bianchi, P.; Goncalves, D.G.; Zanandrea, A.; Borges de Castro, R.; Garro, L.S.; Kalil, J.; Castells, M. Anaphylaxis to quinolones in mastocytosis: Hypothesis on the mechanism. J. Allergy Clin. Immunol. Pract. 2019, 7, 2089–2090. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Nilsson, G.; Draber, P.; Blank, U.; Levi-Shaffer, F. Positive and negative signals in mast cell activation. Trends Immunol. 2017, 38, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Borrego, F. The CD300 molecules: An emerging family of regulators of the immune system. Blood 2013, 121, 1951–1960. [Google Scholar] [CrossRef] [Green Version]
- Bachelet, I.; Munitz, A.; Moretta, A.; Moretta, L.; Levi-Shaffer, F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J. Immunol. 2005, 175, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Munitz, A.; Bachelet, I.; Eliashar, R.; Moretta, A.; Moretta, L.; Levi-Shaffer, F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 2006, 107, 1996–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabato, V.; Verweij, M.M.; Bridts, C.H.; Levi-Shaffer, F.; Gibbs, B.F.; De Clerck, S.D.; Shiavino, D.; Ebo, D.G. CD300a is expressed on human basophils and seems to inhibit IgE/FcεRI-dependent anaphylactic degranulation. Cytom. Part B Clin. Cytom. 2012, 82, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, I.; Munitz, A.; Mankutad, D.; Levi-Shaffer, F. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): A novel interface in the allergic process. J. Biol. Chem. 2006, 281, 27190–27196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachelet, I.; Munitz, A.; Levi-Shaffer, F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J. Allergy Clin. Immunol. 2006, 117, 1314–1320. [Google Scholar] [CrossRef]
- Bachelet, I.; Munitz, A.; Berent-Maoz, B.; Mankuta, D.; Levi-Shaffer, F. Suppression of normal and malignant kit signaling by a bispecific antibody linking kit with CD300a. J. Immunol. 2008, 180, 6064–6069. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Izawa, K.; Kashiwakura, J.I.; Yamanishi, Y.; Enomoto, Y.; Kaitani, A.; Maehara, A.; Isobe, M.; Ito, S.; Matsukawa, T.; et al. Human CD300C delivers an Fc receptor-γ-dependent activating signal in mast cells and monocytes and differs from CD300A in ligand recognition. J. Biol. Chem. 2013, 288, 7662–7675. [Google Scholar] [CrossRef] [Green Version]
- Izawa, K.; Isobe, M.; Matsukawa, T.; Ito, S.; Maehara, A.; Takahashi, M.; Yamanishi, Y.; Kaitani, A.; Oki, T.; Okumura, K.; et al. Sphingomyelin and ceramide are physiological ligands for human LMIR3/CD300f, inhibiting FcεRI-mediated mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 270–273. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Arkesteijn, G.J.; van de Lest, C.H.A.; Geerts, W.J.C.; Goerdayal, S.S.; Altelaar, M.A.F.; Redegeld, F.A.; Nolte-‘t Hoen, E.N.M.; Wauben, M.H.M. Mast Cell Degranulation Is Accompanied by the Release of a Selective Subset of Extracellular Vesicles That Contain Mast Cell-Specific Proteases. J. Immunol. 2016, 197, 3382–3392. [Google Scholar] [CrossRef] [Green Version]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Lowev, D.; Tkach, M.; Thèry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, e968–e977. [Google Scholar] [CrossRef] [Green Version]
- Dahlin, J.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]

| Disorder/Condition | Main Features | Clonality |
|---|---|---|
| Primary/monoclonal | KIT D816V mutation and/or CD25+ in most cases | Yes |
| Secondary | Caused by allergic or immunologic diseases | No |
| Idiopathic | Diagnostic MCAS criteria fulfilled with unknown causes | No |
| Mastocytosis | MC Hyperplasia | Primary MCAS (Clonal) | Secondary MCAS | Hereditary α Tryptasemia | |
|---|---|---|---|---|---|
| Increased number of bone marrow MCs | ++ | +/- | +/- | - | - |
| Enhanced release of MC mediators | + | + | ++ | ++ | +/- |
| KIT mutations or other genetic abnormalities | KIT mutations (e.g., D816V) | - | KIT D816V | - | TPSAB1 duplication |
| CD2/CD25 expression on MC | + | - | +/- | - | - |
| MC morphology alterations | + (spindle-shaped MC) | - | +/- | - | - |
| Increased basal serum tryptase | ++ | - | - | - | + |
| Increased acute serum tryptase | +/- | - | + | + | + |
| Biomarker | Diagnostic Value | Potential Limitations |
|---|---|---|
| Tryptase (serum) | Specific for MC activation and proliferation Diagnostic and prognostic value in SM Acute (2–12 h after onset of symptoms) and basal measurement for MCAS diagnosis | Increased levels in: HAT End-stage renal failure Non-mast cell hematologic disease |
| Histamine metabolites (24 h urine collection) | Correlate with MC proliferation in SM Potentially useful for SM diagnosis in patients with slight elevation of serum tryptase and without skin lesions | Influenced by diet, bacterial contamination, and storage conditions Specific cut-off not established |
| PGD2 metabolites (24 h urine collection) | Correlate with symptoms in SM and MCAS Can guide decision to initiate aspirin therapy | Not recommended as a single test of MC activation Heterogeneity in proposed cut-off |
| Leukotriene E4 (24 h urine collection) | Correlate with symptoms in SM and MCAS Can guide decision to initiate antileukotriene therapy | Weak clinical evidence |
| CD | BM Precursor | Normal MC | ISM | WDSM | ASM | Diagnostic Value |
|---|---|---|---|---|---|---|
| CD34 | + | - | - | - | + | Correlates with disease aggressiveness |
| CD45 | + | + | MC identification The expression could be reduced in aggressive forms | |||
| CD133 | + | |||||
| HLA-DR | - | -/+ | ++ | + | -/+ | |
| CD117 | + | ++ | + | + | -/+ | |
| CD203c | - | + | ++ | + | -/+ | Highly present in ISM/WDSM forms |
| FcεRI | - | ++ | ++ | ++ | -/+ | |
| CD25 | - | - | + | -/+ | ++ | Aberrant on neoplastic MCs Highly expressed in aggressive forms |
| CD30 | - | -/+ | -/+ | + | ||
| CD123 | - | |||||
| CD2 | - | + | -/+ | - | Aberrant on neoplastic MCs Mainly found in ISM | |
| CD63 | ++ | ++ | + | -/+ | Activation markers Present on both normal MCs and ISM/WDSM | |
| CD9 | ++ | |||||
| CD18 | + | Activation markers Candidate biomarkers of MCAS Not useful for differential diagnosis of SM | ||||
| CD29 | ++ | |||||
| CD33 | + | ++ | ||||
| CD44 | ++ | |||||
| CD49d | + | |||||
| CD49e | + | |||||
| CD51 | + | |||||
| CD55 | + | |||||
| CD59 | + | |||||
| CD32 | + | |||||
| CD69 | + | ++ | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parente, R.; Giudice, V.; Cardamone, C.; Serio, B.; Selleri, C.; Triggiani, M. Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation. Int. J. Mol. Sci. 2023, 24, 7071. https://doi.org/10.3390/ijms24087071
Parente R, Giudice V, Cardamone C, Serio B, Selleri C, Triggiani M. Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation. International Journal of Molecular Sciences. 2023; 24(8):7071. https://doi.org/10.3390/ijms24087071
Chicago/Turabian StyleParente, Roberta, Valentina Giudice, Chiara Cardamone, Bianca Serio, Carmine Selleri, and Massimo Triggiani. 2023. "Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation" International Journal of Molecular Sciences 24, no. 8: 7071. https://doi.org/10.3390/ijms24087071
APA StyleParente, R., Giudice, V., Cardamone, C., Serio, B., Selleri, C., & Triggiani, M. (2023). Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation. International Journal of Molecular Sciences, 24(8), 7071. https://doi.org/10.3390/ijms24087071






