PARP1 Activation Induces HMGB1 Secretion Promoting Intestinal Inflammation in Mice and Human Intestinal Organoids
Abstract
:1. Introduction
2. Results
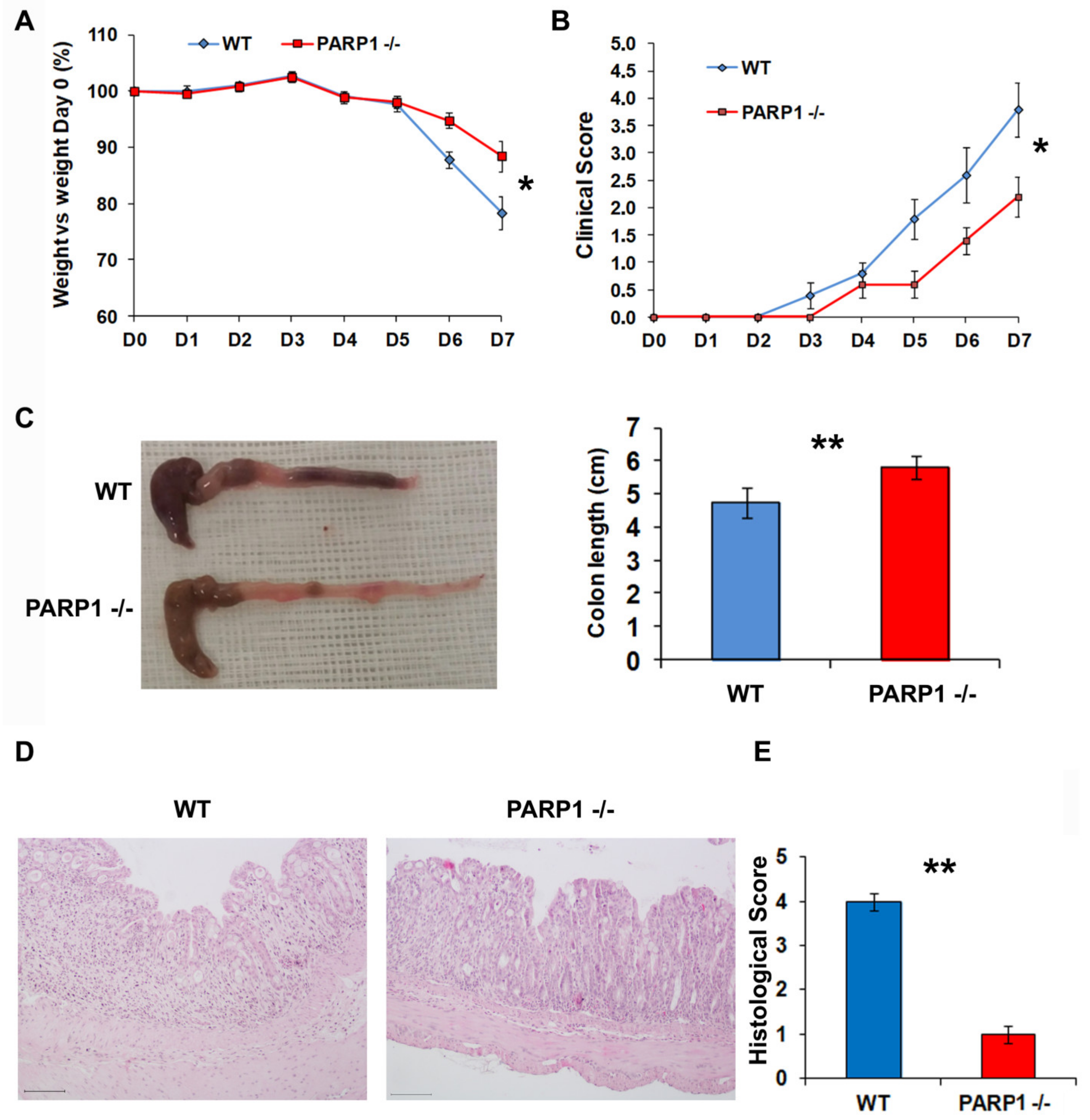
2.1. PARP1−/− Mice Develop Less Severe Colitis than Wild type (WT) Mice
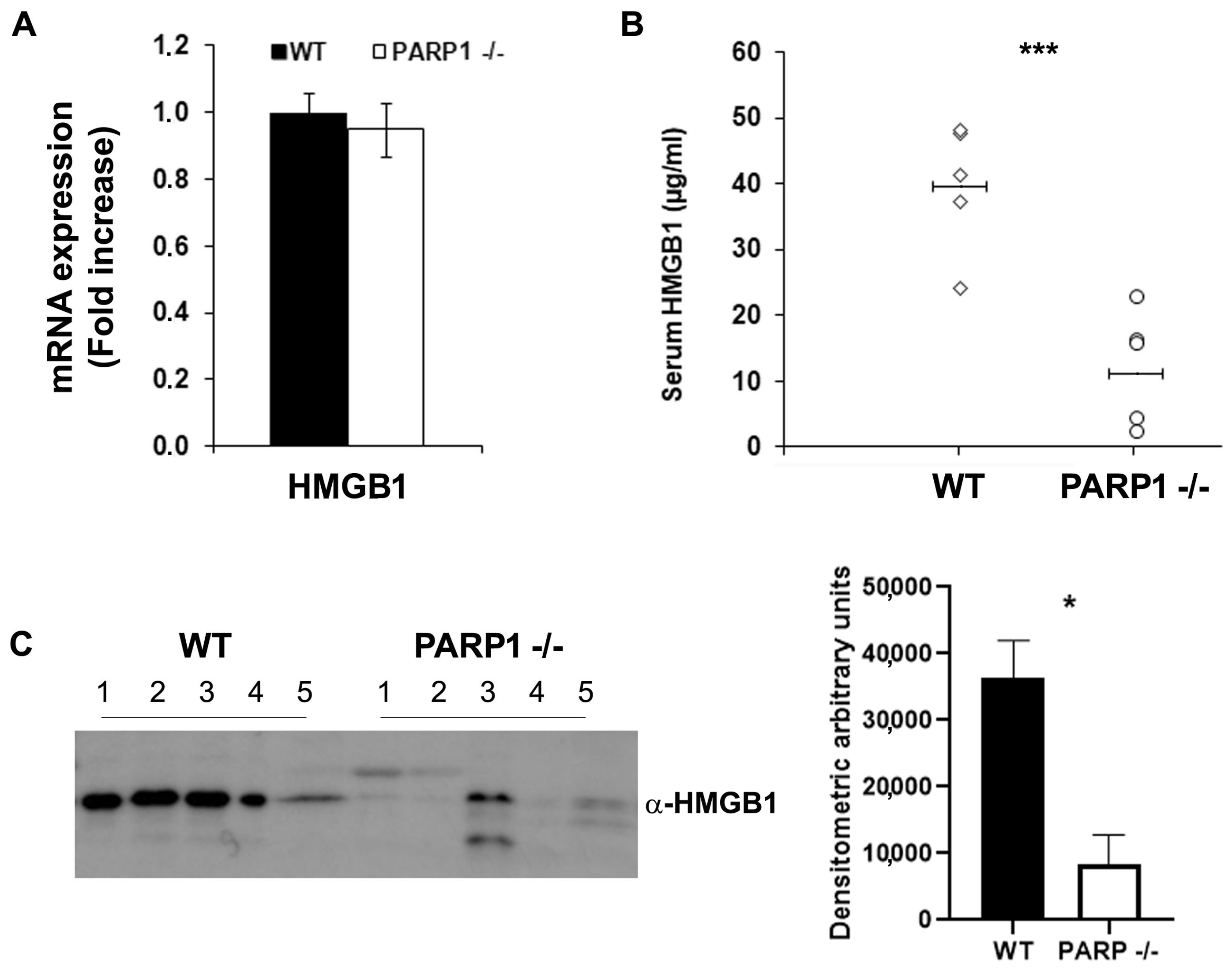
2.2. Extracellular HMGB1 Is Significantly Reduced in the Stools and Serum of PARP1−/− Compared to WT Mice
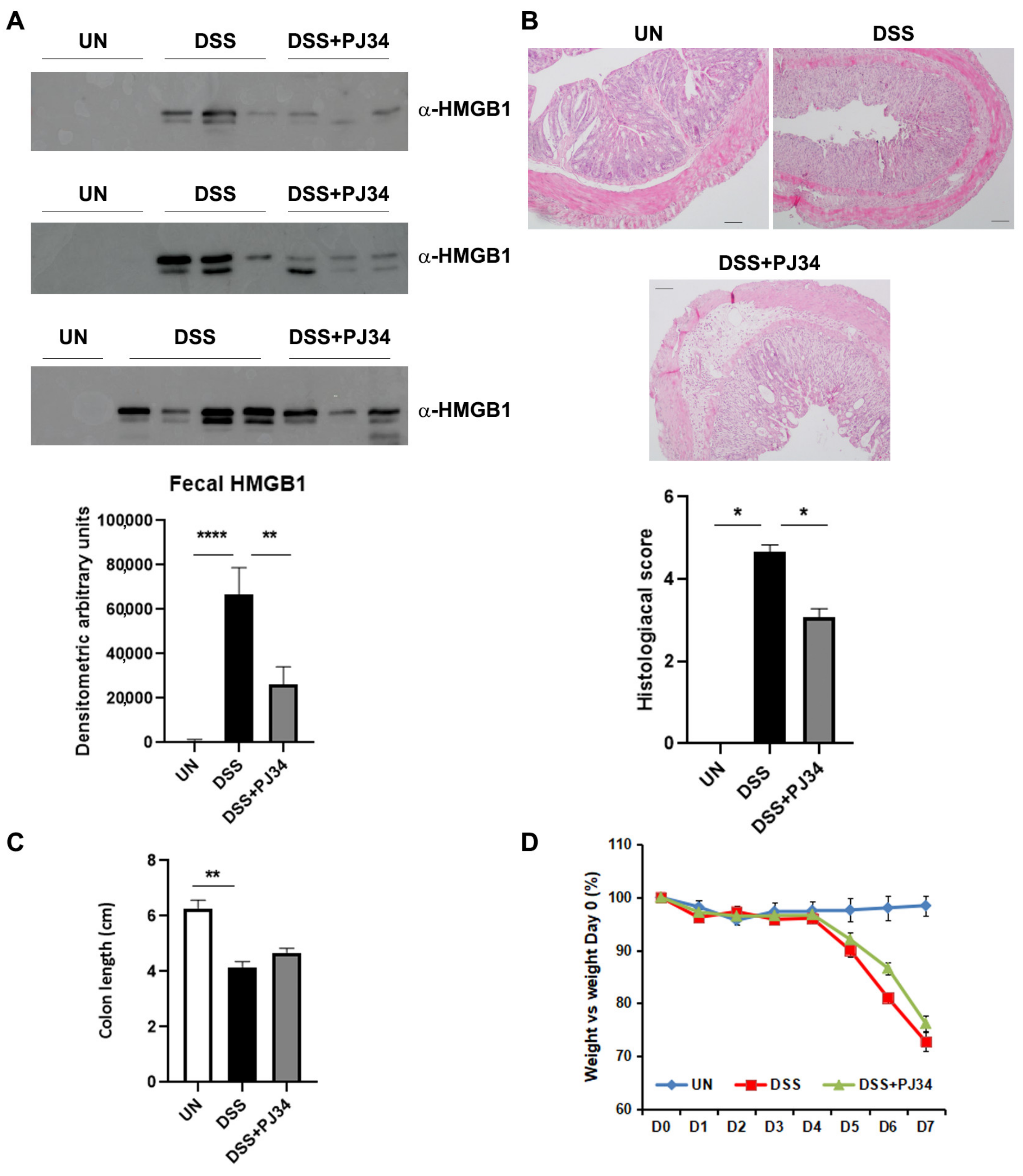
2.3. PARP1-Inhibitor, PJ34, Significantly Decreases the Amount of HMGB1 in the Stools of DSS-Treated WT Mice and Restores the Architecture of Intestinal Mucosa
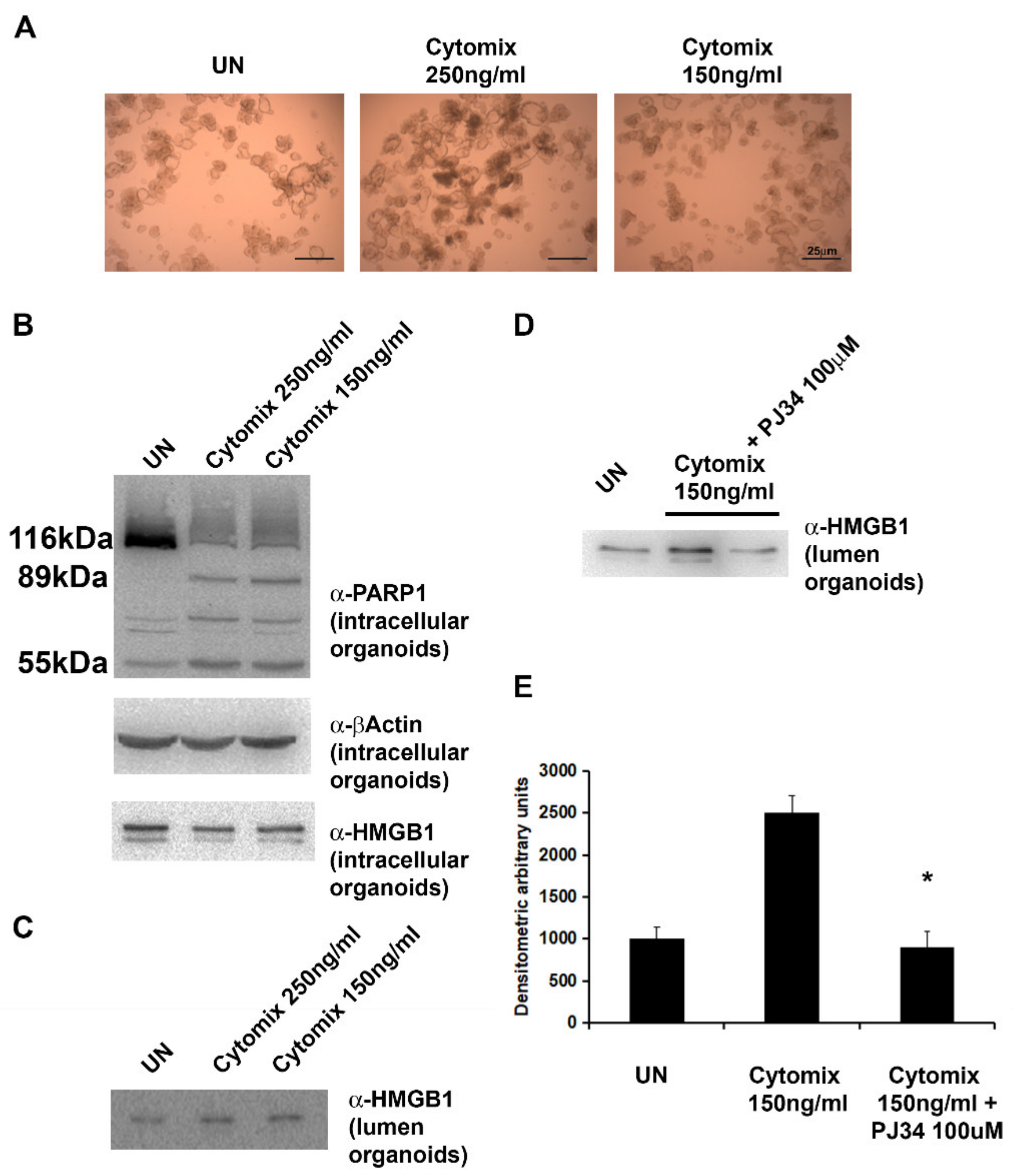
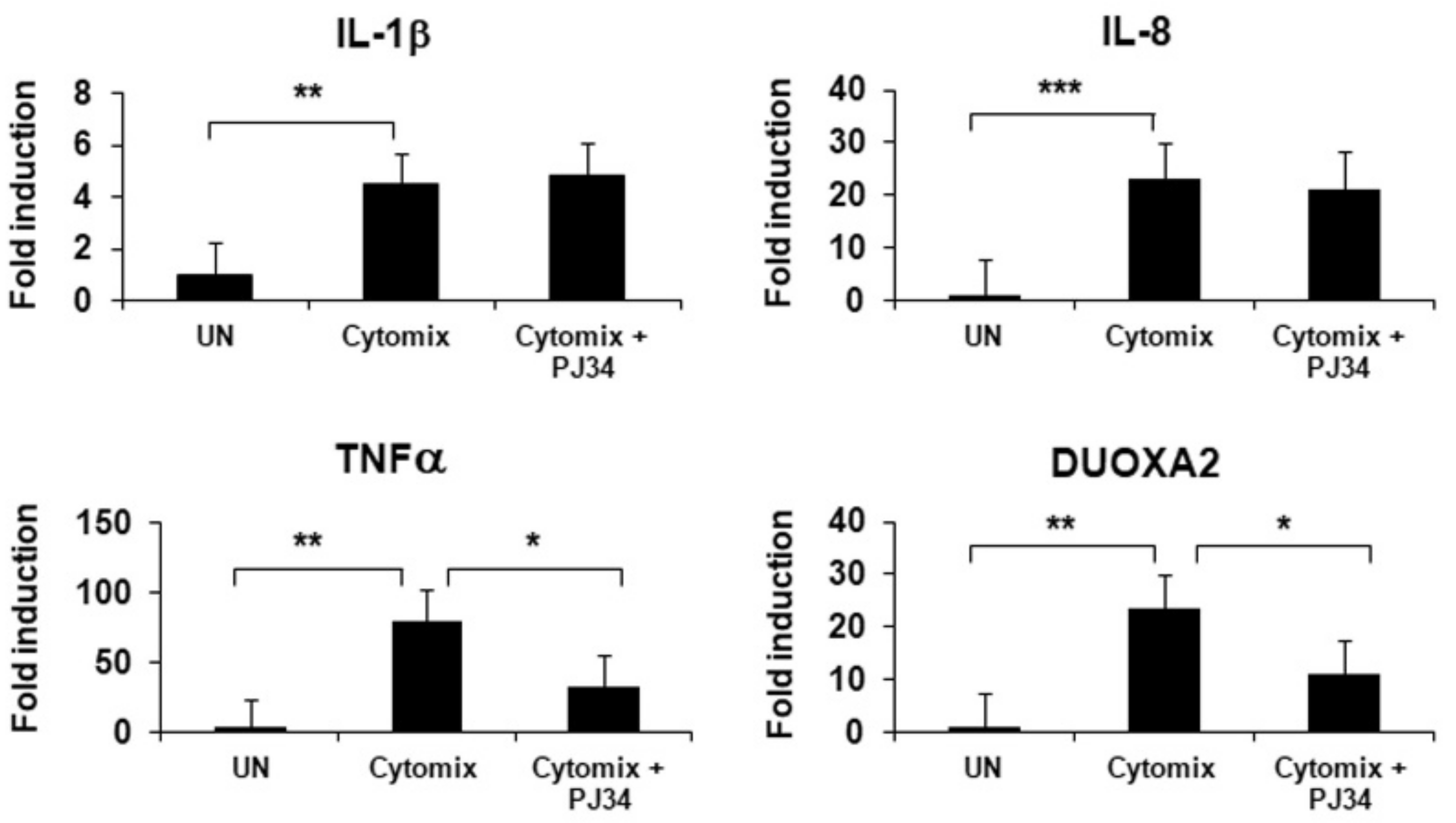
2.4. Exposure of HIOs to Pro-Inflammatory Cytokines (INFγ + TNFα) Results in PARP1 Activation and HMGB1 Secretion
2.5. PARP1-Inhibitor, PJ34, Significantly Reduces the Release of HMGB1 in the Lumen of HIOs
2.6. Inhibition of PARP1 Results in a Significant Decrease in Inflammation and Oxidative Stress in HIOs
2.7. HMGB1 Release during Inflammation Is Associated with PARP1-Induced PARylation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Treatments
4.3. Histology
4.4. RT-PCR
4.5. Quantification of Serum HMGB1 by Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Fecal Extraction
4.7. Patients
4.8. HIOs
4.9. HIOs Treatments
4.10. HIOs Protein Analysis
4.11. Cell Culture
4.12. Immunoprecipitation
4.13. Western Blot
4.14. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int. J. Mol. Sci. 2022, 23, 6966. [Google Scholar] [CrossRef] [PubMed]
- Sekhri, S.; Yarur, A.J. Integrating New and Emerging Therapies into Inflammatory Bowel Disease Clinical Practice. Curr. Opin. Gastroenterol. 2022, 38, 328–336. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R.; van Deventer, S. 25 Years of Anti-TNF Treatment for Inflammatory Bowel Disease: Lessons from the Past and a Look to the Future. Gut 2021, 70, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.F.; Nathans, D. Multivalent DNA-Binding Properties of the HMG-1 Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 6716–6720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A Multifunctional Alarmin Driving Autoimmune and Inflammatory Disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The Mechanism of HMGB1 Secretion and Release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Crippa, M.P.; Manfredi, A.A.; Mezzapelle, R.; Rovere Querini, P.; Venereau, E. High-Mobility Group Box 1 Protein Orchestrates Responses to Tissue Damage via Inflammation, Innate and Adaptive Immunity, and Tissue Repair. Immunol. Rev. 2017, 280, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Shao, Y.; Tian, Y.; Ouyang, C.; Wang, X. Nuclear Alarmin Cytokines in Inflammation. J. Immunol. Res. 2020, 2020, 7206451. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. High-Mobility Group Box 1 Protein (HMGB1) Operates as an Alarmin Outside as Well as inside Cells. Semin. Immunol. 2018, 38, 40–48. [Google Scholar] [CrossRef]
- Kang, R.; Chen, R.; Zhang, Q.; Hou, W.; Wu, S.; Cao, L.; Huang, J.; Yu, Y.; Fan, X.-G.; Yan, Z.; et al. HMGB1 in Health and Disease. Mol. Asp. Med. 2014, 40, 1–116. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Huang, M.; Yao, Y.-M. The Effect and Regulatory Mechanism of High Mobility Group Box-1 Protein on Immune Cells in Inflammatory Diseases. Cells 2021, 10, 1044. [Google Scholar] [CrossRef]
- Ni, Y.-A.; Chen, H.; Nie, H.; Zheng, B.; Gong, Q. HMGB1: An Overview of Its Roles in the Pathogenesis of Liver Disease. J. Leukoc. Biol. 2021, 110, 987–998. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tajima, Y. HMGB1 Is a Promising Therapeutic Target for Acute Liver Failure. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 673–682. [Google Scholar] [CrossRef]
- Wang, M.; Gauthier, A.; Daley, L.; Dial, K.; Wu, J.; Woo, J.; Lin, M.; Ashby, C.; Mantell, L.L. The Role of HMGB1, a Nuclear Damage-Associated Molecular Pattern Molecule, in the Pathogenesis of Lung Diseases. Antioxid. Redox Signal. 2019, 31, 954–993. [Google Scholar] [CrossRef]
- Manivannan, S.; Marei, O.; Elalfy, O.; Zaben, M. Neurogenesis after Traumatic Brain Injury—The Complex Role of HMGB1 and Neuroinflammation. Neuropharmacology 2021, 183, 108400. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Shaikh, M.F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, Á.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A Common Biomarker and Potential Target for TBI, Neuroinflammation, Epilepsy, and Cognitive Dysfunction. Front. Neurosci. 2018, 12, 628. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-Mobility Group Box 1 Serves as an Inflammation Driver of Cardiovascular Disease. Biomed. Pharmacother. 2021, 139, 111555. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Z.; Barnie, P.A.; Su, Z. Dual Faced HMGB1 Plays Multiple Roles in Cardiomyocyte Senescence and Cardiac Inflammatory Injury. Cytokine Growth Factor Rev. 2019, 47, 74–82. [Google Scholar] [CrossRef]
- Vitali, R.; Stronati, L.; Negroni, A.; Di Nardo, G.; Pierdomenico, M.; del Giudice, E.; Rossi, P.; Cucchiara, S. Fecal HMGB1 Is a Novel Marker of Intestinal Mucosal Inflammation in Pediatric Inflammatory Bowel Disease. Am. J. Gastroenterol. 2011, 106, 2029–2040. [Google Scholar] [CrossRef]
- Palone, F.; Vitali, R.; Cucchiara, S.; Pierdomenico, M.; Negroni, A.; Aloi, M.; Nuti, F.; Felice, C.; Armuzzi, A.; Stronati, L. Role of HMGB1 as a Suitable Biomarker of Subclinical Intestinal Inflammation and Mucosal Healing in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2014, 20, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Palone, F.; Vitali, R.; Cucchiara, S.; Mennini, M.; Armuzzi, A.; Pugliese, D.; D’Incà, R.; Barberio, B.; Stronati, L. Fecal HMGB1 Reveals Microscopic Inflammation in Adult and Pediatric Patients with Inflammatory Bowel Disease in Clinical and Endoscopic Remission. Inflamm. Bowel. Dis. 2016, 22, 2886–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitali, R.; Palone, F.; Cucchiara, S.; Negroni, A.; Cavone, L.; Costanzo, M.; Aloi, M.; Dilillo, A.; Stronati, L. Dipotassium Glycyrrhizate Inhibits HMGB1-Dependent Inflammation and Ameliorates Colitis in Mice. PLoS ONE 2013, 8, e66527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, L.; Gong, Z. Regulation of Acetylation in High Mobility Group Protein B1 Cytosol Translocation. DNA Cell Biol. 2019, 38, 491–499. [Google Scholar] [CrossRef]
- Ito, I.; Fukazawa, J.; Yoshida, M. Post-Translational Methylation of High Mobility Group Box 1 (HMGB1) Causes Its Cytoplasmic Localization in Neutrophils. J. Biol. Chem. 2007, 282, 16336–16344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonaldi, T.; Talamo, F.; Scaffidi, P.; Ferrera, D.; Porto, A.; Bachi, A.; Rubartelli, A.; Agresti, A.; Bianchi, M.E. Monocytic Cells Hyperacetylate Chromatin Protein HMGB1 to Redirect It towards Secretion. EMBO J. 2003, 22, 5551–5560. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhao, X.; Antoine, D.; Xiao, X.; Wang, H.; Andersson, U.; Billiar, T.R.; Tracey, K.J.; Lu, B. Regulation of Posttranslational Modifications of HMGB1 During Immune Responses. Antioxid. Redox Signal 2016, 24, 620–634. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Nussenzweig, A. The Multifaceted Roles of PARP1 in DNA Repair and Chromatin Remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Gupte, R.; Liu, Z.; Kraus, W.L. PARPs and ADP-Ribosylation: Recent Advances Linking Molecular Functions to Biological Outcomes. Genes Dev. 2017, 31, 101–126. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.; Banerjee, S.; Friggeri, A.; Bell, C.; Abraham, E.; Zerfaoui, M. Poly(ADP-Ribosyl)Ation of High Mobility Group Box 1 (HMGB1) Protein Enhances Inhibition of Efferocytosis. Mol. Med. 2012, 18, 359–369. [Google Scholar] [CrossRef]
- Noguchi, T.; Sekiguchi, Y.; Kudoh, Y.; Naganuma, R.; Kagi, T.; Nishidate, A.; Maeda, K.; Ishii, C.; Toyama, T.; Hirata, Y.; et al. Gefitinib Initiates Sterile Inflammation by Promoting IL-1β and HMGB1 Release via Two Distinct Mechanisms. Cell Death Dis. 2021, 12, 49. [Google Scholar] [CrossRef]
- Pal Singh, M.; Pal Khaket, T.; Bajpai, V.K.; Alfarraj, S.; Kim, S.-G.; Chen, L.; Huh, Y.S.; Han, Y.-K.; Kang, S.C. Morin Hydrate Sensitizes Hepatoma Cells and Xenograft Tumor towards Cisplatin by Downregulating PARP-1-HMGB1 Mediated Autophagy. Int. J. Mol. Sci. 2020, 21, 8253. [Google Scholar] [CrossRef]
- Walko, T.D.; Di Caro, V.; Piganelli, J.; Billiar, T.R.; Clark, R.S.B.; Aneja, R.K. Poly(ADP-Ribose) Polymerase 1-Sirtuin 1 Functional Interplay Regulates LPS-Mediated High Mobility Group Box 1 Secretion. Mol. Med. 2015, 20, 612–624. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, Z.-M.; Sun, S.-Y.; Wang, L.-P.; Wang, P.-X.; Guo, Z.; Yang, H.-W.; Ye, J.-T.; Lu, J.; Liu, P.-Q. PARP1 Interacts with HMGB1 and Promotes Its Nuclear Export in Pathological Myocardial Hypertrophy. Acta Pharmacol. Sin. 2019, 40, 589–598. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Chen, L.; Yuan, W.; Dong, L.; Zhang, Y.; Wu, H.; Wang, C. PARP-1 Mediates LPS-Induced HMGB1 Release by Macrophages through Regulation of HMGB1 Acetylation. J. Immunol. 2014, 193, 6114–6123. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, J.; Wang, B.; Li, Y. Protective Effect of LncRNA CRNDE on Myocardial Cell Apoptosis in Heart Failure by Regulating HMGB1 Cytoplasm Translocation through PARP-1. Arch. Pharm. Res. 2020, 43, 1325–1334. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, Y.; Qin, X.-M.; Jiang, T.; Tan, Y.-F.; Ouyang, W.-X.; Xiao, Z.-H.; Li, S.-J. CircTLK1 Modulates Sepsis-Induced Cardiomyocyte Apoptosis via Enhancing PARP1/HMGB1 Axis-Mediated Mitochondrial DNA Damage by Sponging MiR-17-5p. J. Cell Mol. Med. 2021, 25, 8244–8260. [Google Scholar] [CrossRef]
- Kong, Q.; Li, Y.; Liang, Q.; Xie, J.; Li, X.; Fang, J. SIRT6-PARP1 Is Involved in HMGB1 PolyADP-Ribosylation and Acetylation and Promotes Chemotherapy-Induced Autophagy in Leukemia. Cancer Biol. Ther. 2020, 21, 320–331. [Google Scholar] [CrossRef]
- VanPatten, S.; Al-Abed, Y. High Mobility Group Box-1 (HMGb1): Current Wisdom and Advancement as a Potential Drug Target. J. Med. Chem. 2018, 61, 5093–5107. [Google Scholar] [CrossRef] [Green Version]
- Faro, R.; Toyoda, Y.; McCully, J.D.; Jagtap, P.; Szabo, E.; Virag, L.; Bianchi, C.; Levitsky, S.; Szabo, C.; Sellke, F.W. Myocardial Protection by PJ34, a Novel Potent Poly (ADP-Ribose) Synthetase Inhibitor. Ann. Thorac. Surg. 2002, 73, 575–581. [Google Scholar] [CrossRef]
- Huang, F.-C. Upregulation of Salmonella-Induced IL-6 Production in Caco-2 Cells by PJ-34, PARP-1 Inhibitor: Involvement of PI3K, P38 MAPK, ERK, JNK, and NF-KappaB. Mediat. Inflamm. 2009, 2009, 103890. [Google Scholar] [CrossRef] [Green Version]
- Black, J.H.; Casey, P.J.; Albadawi, H.; Cambria, R.P.; Watkins, M.T. Poly Adenosine Diphosphate-Ribose Polymerase Inhibitor PJ34 Abolishes Systemic Proinflammatory Responses to Thoracic Aortic Ischemia and Reperfusion. J. Am. Coll. Surg. 2006, 203, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Xiong, M.; Chen, X.-P.; Xiao, Z.-Y.; Zhao, Y.-F.; Huang, Z.-Y. PJ34, an Inhibitor of PARP-1, Suppresses Cell Growth and Enhances the Suppressive Effects of Cisplatin in Liver Cancer Cells. Oncol. Rep. 2008, 20, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, D.H.; Albadawi, H.; Conrad, M.F.; Entabi, F.; Stoner, M.C.; Casey, P.J.; Cambria, R.P.; Watkins, M.T. PJ34, a Poly-ADP-Ribose Polymerase Inhibitor, Modulates Visceral Mitochondrial Activity and CD14 Expression Following Thoracic Aortic Ischemia-Reperfusion. Am. J. Surg. 2009, 198, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, X.; Gong, L.; Wu, G.; Peng, X.; Tang, X. Role of High-Mobility Group Box 1 Protein in Inflammatory Bowel Disease. Inflamm. Res. 2015, 64, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Chen, Q.; Jiao, F.; Shi, C.; Pei, M.; Lv, J.; Zhang, H.; Wang, L.; Gong, Z. TNF-α/HMGB1 Inflammation Signalling Pathway Regulates Pyroptosis during Liver Failure and Acute Kidney Injury. Cell Prolif. 2020, 53, e12829. [Google Scholar] [CrossRef]
- Venereau, E.; De Leo, F.; Mezzapelle, R.; Careccia, G.; Musco, G.; Bianchi, M.E. HMGB1 as Biomarker and Drug Target. Pharmacol. Res. 2016, 111, 534–544. [Google Scholar] [CrossRef]
- Maxwell, J.R.; Brown, W.A.; Smith, C.L.; Byrne, F.R.; Viney, J.L. Methods of Inducing Inflammatory Bowel Disease in Mice. Curr. Protoc. Pharmacol. 2009, 47, 5.58.1–5.58.37. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4+ T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]

| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| mHMGB1 | GCCTCGCGGAGGAAAATC | AAGTTTGCACAAAGAATGCATATGA |
| mGAPDH | AACTTTGGCATTGTGGAAGG | CACATTGGGGGTAGGAACAC |
| hIL-1β | TTCGACACATGGGATAACGAGG | TTTTTGCTGTGAGTCCCGGAG |
| hIL-8 | TTGGCAGCCTGATTTC | TTGGAGTATGTCTTTATGCACTGAC |
| hTNF-α | ATCTTCTCGAACCCCGAGTGA | GGAGCTGCCCCTCAGCTT |
| hDual Oxidase Maturation Factor 2 (Duoxa2) | TCCGCAACGATGGACAGA | AGGGTCGGTTGGAAACGAA |
| hRibosomal Protein S14 (RSP14) | TCACCGCCCTACACATCAAAC | GCCCGATCTTCATACCCGA |
| hHypoxanthine Phosphoribosyltransferase 1 (HPRT1) | GAAAAGGACCCCACGAAGTGT | AGTCAAGGGCATATCCTACAACA |
| hBeta-2-Microglobulin (B2M) | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitali, R.; Mancuso, A.B.; Palone, F.; Pioli, C.; Cesi, V.; Negroni, A.; Cucchiara, S.; Oliva, S.; Carissimi, C.; Laudadio, I.; et al. PARP1 Activation Induces HMGB1 Secretion Promoting Intestinal Inflammation in Mice and Human Intestinal Organoids. Int. J. Mol. Sci. 2023, 24, 7096. https://doi.org/10.3390/ijms24087096
Vitali R, Mancuso AB, Palone F, Pioli C, Cesi V, Negroni A, Cucchiara S, Oliva S, Carissimi C, Laudadio I, et al. PARP1 Activation Induces HMGB1 Secretion Promoting Intestinal Inflammation in Mice and Human Intestinal Organoids. International Journal of Molecular Sciences. 2023; 24(8):7096. https://doi.org/10.3390/ijms24087096
Chicago/Turabian StyleVitali, Roberta, Anna Barbara Mancuso, Francesca Palone, Claudio Pioli, Vincenzo Cesi, Anna Negroni, Salvatore Cucchiara, Salvatore Oliva, Claudia Carissimi, Ilaria Laudadio, and et al. 2023. "PARP1 Activation Induces HMGB1 Secretion Promoting Intestinal Inflammation in Mice and Human Intestinal Organoids" International Journal of Molecular Sciences 24, no. 8: 7096. https://doi.org/10.3390/ijms24087096





