Advances in the Electrophysiological Recordings of Long-Term Potentiation
Abstract
1. Introduction
- Why did most researchers use field potential to detect LTP at excitatory synapses while using the single-cell potential to detect LTP at inhibitory synapses, respectively?
- What is the mechanism of LTP at the inhibitory synapses? Is this similar to excitatory synapses?
- Do LTP and LTP of inhibition (iLTP) occur independently? What does an inhibitory neuron do while excitatory neurons are stimulated to induce LTP?
| Year | Mechanism or Event | Induction | Recording Method | Brain Area | Ref. |
|---|---|---|---|---|---|
| 1970–1980 | Discovery of LTP | 10–20 Hz 100 Hz | Extracellular micro-electrodes Population EPSP | HP, CA1, CA3 | [10] |
| Brain slice recording on LTP | 3–50 Hz | Population EPSP | HP, CA1 | [16] | |
| LTP needs synaptic transmission | 100 Hz | Population EPSP | HP | [17] | |
| Ca2+- dependent | 100 Hz | Extracellular population spike EPSP | HP, CA1 | [18] | |
| 1980–1990 | NMDAR Postsynaptic Ca2+ | >35 Hz | Extracellular recording | HP, CA1 | [19,20,21,22] |
| Activation of NMDA receptors blocks GABAergic inhibition | Tetanic electrical stimuli | Extra and intracellular recording IPSP | HP, CA1 | [23] | |
| LTP needs NMDAR | HFS | Intracellular recording | Visual cortex | [24] | |
| 1990–2000 | Single-cell recording EPSP-spike | HFS | Intracellular recording | CA1 | [25] |
| GABABR regulates NMDA to induce LTP | 0.5–100 Hz | Monosynaptic inhibitory pathway IPSC | HP, granule cells | [26,27] | |
| Induction of LTP needs mGluRs | HFS 100 Hz | Extracellular field potentials Whole-cell patch clamp | HP, CAl CA3 | [28,29] | |
| NMDAR dependent Ca2+ | 100 Hz | Field potentials EPSP | HP | [30] | |
| NO mediate LTP | 100 Hz | Field potentials EPSP | HP | [31] | |
| GABA AR Independent | HFS 50 Hz | Intracellular recording IPSP | visual cortex (LV) | [32] | |
| GABABR dependent Ca2+ Release | HFS 50 Hz | Intracellular and whole-cell recording IPSP/IPSC | visual cortex (LV) | [33] | |
| NMDA-dependent inhibition | 100 Hz | Whole-cell and extracellular recording | HP, CA1 | [34] | |
| Bi-directional plasticity | 100 Hz | Intracellular recording | HP | [35] | |
| NO mediate LTP | 50 Hz | Whole-cell ruptured patch recording EPSCs | HP | [36] | |
| GABAergic synaptic LTP | 0.1 Hz | Intracellular recording | Neonatal rats, HP | [37] | |
| 2000–2010 | mGluR GABA B R Postsynaptic Ca2+ | TBS | Whole-cell recording IPSP | HP CA1 | [38] |
| NMDAR-nondependent | HFS (30 Hz) | Whole-cell recording IPSP and EPSP | Lateral Amygdala | [39] | |
| The pairing of presynaptic activity with sub-threshold postsynaptic depolarization Postsynaptic | 50 Hz postsynaptic depolarization-60 mV | Patch clamp IPSC | Visual Cortex(LIV) | [40] | |
| GABA AR NO initiates iLTP NMDA-independent | HFS | Whole-cell patch clamp IPSC | VTA | [41] | |
| BDNF-TrkB | HFS (50 Hz) | Whole-cell patch clamp Voltage clamp IPSC | Visual cortex (LV) | [42,43] | |
| mGluR5 postsynaptic calcium, NMDAR- nondependent | TBS | Whole-cell patch clamp EPSP | The visual cortex, L II/III | [44] | |
| BDNF and cAMP-dependent PKA | LFS (0.05 Hz) | Patch clamp–Voltage clamp IPSP and IPSC | HP, CA3 | [45] | |
| Astrocyte-induced independent-LTP | 0.5 Hz | Whole-cell patch clamp | HP, CA1, CA3 | [46] | |
| D-serine from astrocytes activates NMDAR | 100 Hz | Whole-cell recordings | HP | [47,48] | |
| 2010–2020 | BDNF-TrkB | 50 Hz | Whole-cell patch clamp IPSC | Auditory Cortex (AC) | [49] |
| Cholecystokinin (CCK) modulates the plasticity of GABA Synapses | HFS | Whole-cell recording IPSC | Dorsomedial Hypothalamus | [50] | |
| NMDAR triggers CCK release | HFS (100 Hz) | In vivo, fEPSP | AC | [51] | |
| Dual-channel optogenetic LTP-induction | Optogenetic HFS (oHFS) 50 Hz | Field potential recording fEPSP Whole-cell recording NMDAR/AMPA-EPSP | Dorsal striatum | [52] | |
| Astrocytic ATP is necessary for LTPCCK | HFS (100 Hz) | Whole-cell recording | Hypothalamus | [50] | |
| Small increase in Astrocytic ATP release | HFS (100 Hz) | Extracellular field recordings, fEPSP | HP, CA1 | [53] | |
| 2020–2023 | CCK Potentiates GABAergic Synapses | 20 Hz | Whole-cell patch clamp | VTA | [54] |
| Spatial regulation of excitatory and inhibitory synaptic plasticity | LFS 2 Hz, 4 Hz | Whole-cell patch clamp | HP | [55] | |
| Astrocyte dystrophy parallels impaired LTP | HFS 100 Hz | Patch clamp | HP, CA1 | [56] | |
| Optogenetic induction of orbitostriatal LTP | oHFS 50 Hz | Whole-cell recording oEPSCs | Dorsomedial Striatum | [57] | |
| Novel CCKR: GPR173 Mediates iLTP | HFLS | In vivo extracellular and in vitro patch clamp | Neocortex | [58] | |
| Capacitive energy storage in the phospholipid bilayer | LFS 0.01 Hz | Patch clamp | DPhPC multilamellar vesicles (MLVs) | [59] |
2. Field Potential and Single-Cell Potential Recording in LTP
2.1. Field Potential Recording at Excitatory Synapses
2.2. Extracellular Ionic Currents That Are of Dual Nature
2.3. Single-Cell Potential Recording at Inhibitory Synapses
2.3.1. Intracellular Recordings
2.3.2. Patch Clamp: Whole-Cell Recording
3. LTP Mechanisms of Excitatory and Inhibitory Synapses
3.1. Nitric Oxide (NO)
3.2. BDNF-TrkB
3.3. NMDAR-Dependent
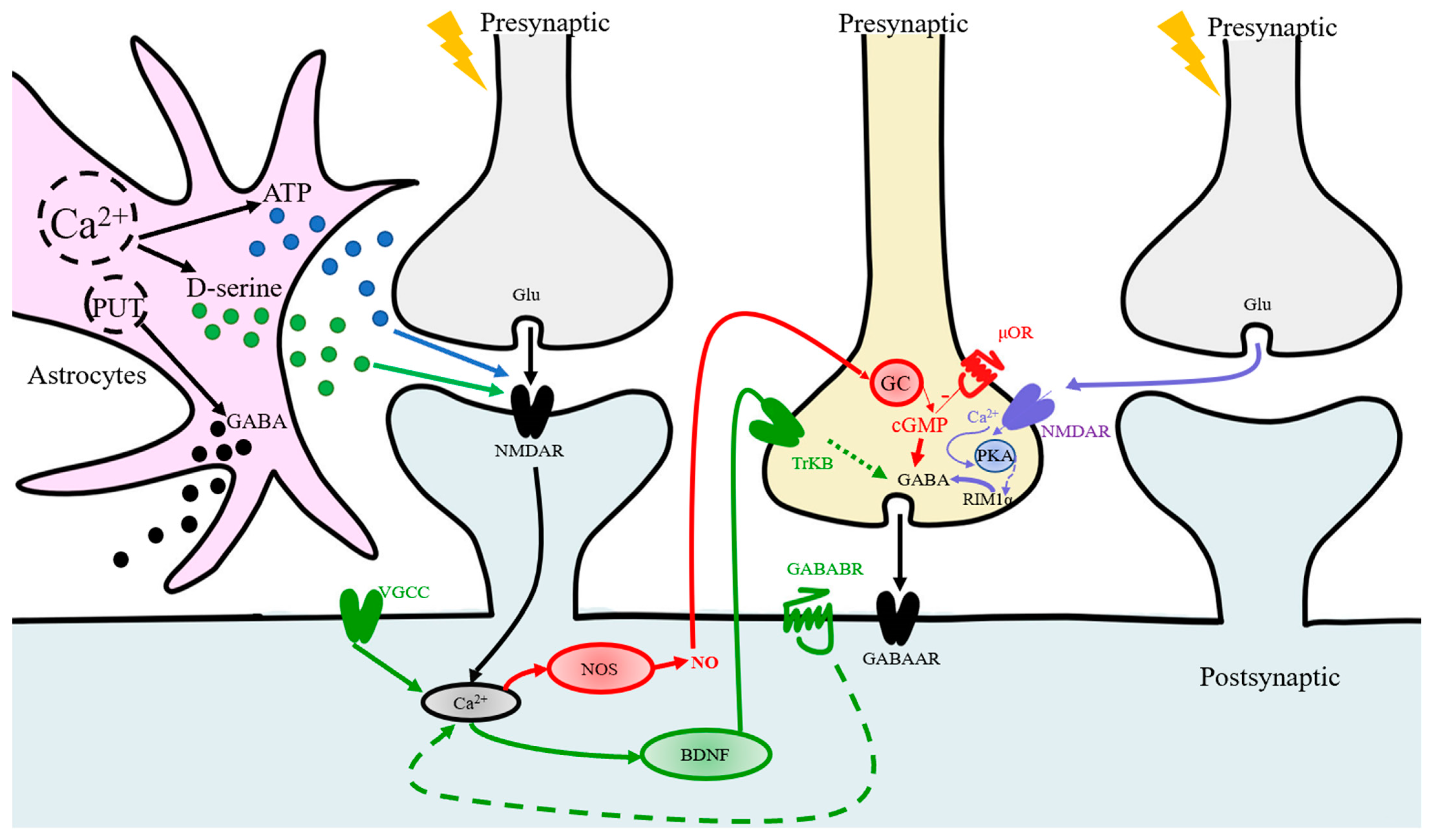
3.4. Glial Cells
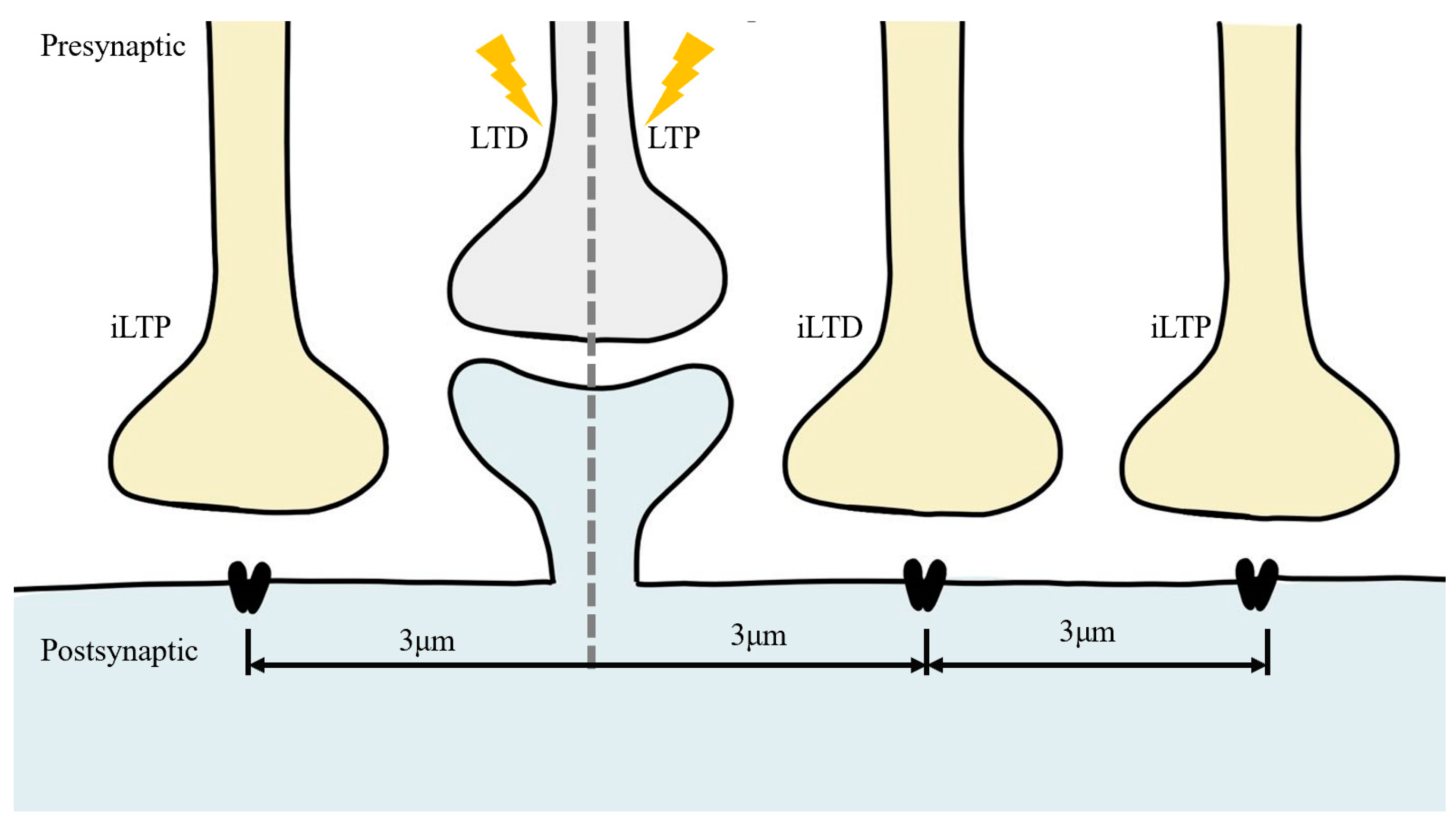
4. Coordinated Plasticity of Excitatory and Inhibitory Synapses
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTP | Long-term potentiation |
| iLTP | Long-term potentiation of the inhibitory synapses |
| Chem-iLTP | Chemically induced iLTP |
| EPSP | Excitatory postsynaptic potential |
| IPSC | Inhibitory postsynaptic current |
| HP | Hippocampus |
| VTA | Ventral tegmental area |
| HFS | High-frequency stimulation |
| SC | Stellate cells |
| PKC | Protein kinase C |
| CaMKII | Calcium–calmodulin (CaM) dependent protein kinase II |
| PKA | Protein kinase A system |
| BDNF-TrkB | Brain-derived neurotrophic factor_ Tyrosine kinase B |
| NMDAR | N-methyl-D-aspartate receptor |
| GABA | γ-aminobutyric acid |
| GABAAR | GABA A receptor |
| GABABR | GABA B receptor |
| cGMP | Cyclic guanosine monophosphate |
| PUT | Polyamine putrescine |
| SPM | Polyamine spermine |
| APs | Action potentials |
| LFPs | Local field potentials |
| edNEG | Electrodiffusive neuron-extracellular-glia |
| MEA | microelectrode array |
| CNS | central nervous system |
| Trk | tyrosine-related receptor kinase |
| STDP | Spike timing-dependent plasticity |
| MeCP2 | methyl CpG binding protein 2 |
| mHTT | mutant huntingtin |
| HD | Huntington’s disease |
| AD | Alzheimer’s disease |
| mHTT | mutant huntingtin |
| p75NTR | p75 neurotrophin receptor |
| ACC | Anterior cingulate cortex |
| oEPSCs | Optically evoked excitatory postsynaptic currents |
| oHFS | Optogenetic HFS |
| HFLS | High-frequency laser stimulation |
| CCKR | Cholecystokinin receptor |
| PUT | putrescine |
| LTPCCK | A form of activity-dependent synaptic plasticity mediated by CCK |
| AC | Auditory cortex |
References
- Herculano-Houzel, S.; Lent, R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 2005, 25, 2518–2521. [Google Scholar] [CrossRef] [PubMed]
- Charvet, C.J.; Cahalane, D.J.; Finlay, B.L. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb. Cortex 2015, 25, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S.; Mota, B.; Lent, R. Cellular scaling rules for rodent brains. Proc. Natl. Acad. Sci. USA 2006, 103, 12138–12143. [Google Scholar] [CrossRef] [PubMed]
- Lent, R.; Azevedo, F.A.; Andrade-Moraes, C.H.; Pinto, A.V. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur. J. Neurosci. 2012, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Nayak, M.; Das, D.; Pradhan, J.; Ahmed, R.; Laureano-Melo, R.; Dandapat, J. Epigenetic signature in neural plasticity: The journey so far and journey ahead. Heliyon 2022, 8, e12292. [Google Scholar] [CrossRef]
- Trojan, S.; Pokorny, J. Threoretical aspects of neuroplasticity. Physiol. Res. 1999, 48, 87–98. [Google Scholar]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef]
- Matynia, A.; Kushner, S.A.; Silva, A.J. Genetic approaches to molecular and cellular cognition: A focus on LTP and learning and memory. Annu. Rev. Genet. 2002, 36, 687–720. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Abraham, W.C. How long will long-term potentiation last? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 735–744. [Google Scholar] [CrossRef]
- Henze, D.A.; Borhegyi, Z.; Csicsvari, J.; Mamiya, A.; Harris, K.D.; Buzsáki, G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 2000, 84, 390–400. [Google Scholar] [CrossRef]
- Oka, H.; Shimono, K.; Ogawa, R.; Sugihara, H.; Taketani, M. A new planar multielectrode array for extracellular recording: Application to hippocampal acute slice. J. Neurosci. Methods 1999, 93, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakmann, B. The patch clamp technique. Sci. Am. 1992, 266, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lomo, T. Frequency Potentiation of Excitatory Synaptic Activity in Dentate Area of Hippocampal Formation; Acta Physiologica Scandinavica, 1966; Blackwell Science Ltd.: Oxford, UK, 1966; p. 128. [Google Scholar]
- Schwartzkroin, P.A.; Wester, K. Long-lasting facilitation of a synaptic potential following tetanization in the in vitro hippocampal slice. Brain Res. 1975, 89, 107–119. [Google Scholar] [CrossRef]
- Dunwiddie, T.; Madison, D.; Lynch, G. Synaptic transmission is required for initiation of long-term potentiation. Brain Res. 1978, 150, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Dunwiddie, T.V.; Lynch, G. The relationship between extracellular calcium concentrations and the induction of hippocampal long-term potentiation. Brain Res. 1979, 169, 103–110. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Kehl, S.; McLennan, H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J. Physiol. 1983, 334, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Joly, M.; Lynch, G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science 1988, 242, 1694–1697. [Google Scholar] [CrossRef]
- Davies, J.; Francis, A.A.; Jones, A.W.; Watkins, J.C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci. Lett. 1981, 21, 77–81. [Google Scholar] [CrossRef]
- Lynch, G.; Baudry, M. The biochemistry of memory: A new and specific hypothesis. Science 1984, 224, 1057–1063. [Google Scholar] [CrossRef]
- Stelzer, A.; Slater, N.T.; ten Bruggencate, G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature 1987, 326, 698–701. [Google Scholar] [CrossRef]
- Artola, A.; Singer, W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature 1987, 330, 649–652. [Google Scholar] [CrossRef]
- Chavez-Noriega, L.E.; Halliwell, J.V.; Bliss, T.V. A decrease in firing threshold observed after induction of the EPSP-spike (E-S) component of long-term potentiation in rat hippocampal slices. Exp. Brain Res. 1990, 79, 633–641. [Google Scholar] [CrossRef]
- Davies, C.H.; Starkey, S.J.; Pozza, M.F.; Collingridge, G.L. GABA autoreceptors regulate the induction of LTP. Nature 1991, 349, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Mott, D.D.; Lewis, D.V. Facilitation of the induction of long-term potentiation by GABAB receptors. Science 1991, 252, 1718–1720. [Google Scholar] [CrossRef]
- Bashir, Z.I.; Bortolotto, Z.A.; Davies, C.H.; Berretta, N.; Irving, A.J.; Seal, A.J.; Henley, J.M.; Jane, D.E.; Watkins, J.C.; Collingridge, G.L. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 1993, 363, 347–350. [Google Scholar] [CrossRef]
- Bortolotto, Z.A.; Bashir, Z.I.; Davies, C.H.; Collingridge, G.L. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature 1994, 368, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Williams, J.H.; Li, Y.G.; Nayak, A.; Errington, M.L.; Murphy, K.P.; Bliss, T.V. The suppression of long-term potentiation in rat hippocampus by inhibitors of nitric oxide synthase is temperature and age dependent. Neuron 1993, 11, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J. Neurosci. 1994, 14 Pt 1, 6488–6499. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J. Neurosci. 1996, 16, 6342–6352. [Google Scholar] [CrossRef] [PubMed]
- Grunze, H.C.; Rainnie, D.G.; Hasselmo, M.E.; Barkai, E.; Hearn, E.F.; McCarley, R.W.; Greene, R.W. NMDA-dependent modulation of CA1 local circuit inhibition. J. Neurosci. 1996, 16, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- McLean, H.A.; Caillard, O.; Ben-Ari, Y.; Gaiarsa, J.L. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. J. Physiol. 1996, 496 Pt 2, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O.; Kiebler, M.; Lee, C.J.; Lev-Ram, V.; Tsien, R.Y.; Kandel, E.R.; Hawkins, R.D. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 1996, 87, 1025–1035. [Google Scholar] [CrossRef]
- Caillard, O.; Ben-Ari, Y.; Gaiarsa, J.L. Long-term potentiation of GABAergic synaptic transmission in neonatal rat hippocampus. J. Physiol. 1999, 518 Pt 1, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, C.; Chapman, C.A.; Bertrand, S.; Congar, P.; Lacaille, J.C. GABAB receptor- and metabotropic glutamate receptor-dependent cooperative long-term potentiation of rat hippocampal GABAA synaptic transmission. J. Physiol. 2003, 553 Pt 1, 155–167. [Google Scholar] [CrossRef]
- Bauer, E.P.; LeDoux, J.E. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J. Neurosci. 2004, 24, 9507–9512. [Google Scholar] [CrossRef]
- Maffei, A.; Nataraj, K.; Nelson, S.B.; Turrigiano, G.G. Potentiation of cortical inhibition by visual deprivation. Nature 2006, 443, 81–84. [Google Scholar] [CrossRef]
- Nugent, F.S.; Penick, E.C.; Kauer, J.A. Opioids block long-term potentiation of inhibitory synapses. Nature 2007, 446, 1086–1090. [Google Scholar] [CrossRef]
- Inagaki, T.; Begum, T.; Reza, F.; Horibe, S.; Inaba, M.; Yoshimura, Y.; Komatsu, Y. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci. Res. 2008, 61, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nugent, F.S.; Niehaus, J.L.; Kauer, J.A. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology 2009, 34, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Sarihi, A.; Jiang, B.; Komaki, A.; Sohya, K.; Yanagawa, Y.; Tsumoto, T. Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J. Neurosci. 2008, 28, 1224–1235. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Mohajerani, M.H.; Cherubini, E. At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J. Neurosci. 2009, 29, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.; Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, W.; Chen, Y.; Zhang, Z.; Shen, W.; Wu, C.; Poo, M.; Duan, S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. USA 2003, 100, 15194–15199. [Google Scholar] [CrossRef]
- Xu, H.; Kotak, V.C.; Sanes, D.H. Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex. J. Neurosci. 2010, 30, 331–341. [Google Scholar] [CrossRef]
- Crosby, K.M.; Murphy-Royal, C.; Wilson, S.A.; Gordon, G.R.; Bains, J.S.; Pittman, Q.J. Cholecystokinin Switches the Plasticity of GABA Synapses in the Dorsomedial Hypothalamus via Astrocytic ATP Release. J. Neurosci. 2018, 38, 8515–8525. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Wong, Y.T.; Zheng, X.; Wang, H.; Peng, Y.; Feng, H.; Feng, J.; Baibado, J.T.; Jesky, R.; et al. Cholecystokinin release triggered by NMDA receptors produces LTP and sound-sound associative memory. Proc. Natl. Acad. Sci. USA 2019, 116, 6397–6406. [Google Scholar] [CrossRef]
- Ma, T.; Cheng, Y.; Roltsch Hellard, E.; Wang, X.; Lu, J.; Gao, X.; Huang, C.C.Y.; Wei, X.Y.; Ji, J.Y.; Wang, J. Bidirectional and long-lasting control of alcohol-seeking behavior by corticostriatal LTP and LTD. Nat. Neurosci. 2018, 21, 373–383. [Google Scholar] [CrossRef]
- Lee, H.U.; Yamazaki, Y.; Tanaka, K.F.; Furuya, K.; Sokabe, M.; Hida, H.; Takao, K.; Miyakawa, T.; Fujii, S.; Ikenaka, K. Increased astrocytic ATP release results in enhanced excitability of the hippocampus. Glia 2013, 61, 210–224. [Google Scholar] [CrossRef]
- Martinez Damonte, V.; Pomrenze, M.B.; Manning, C.E.; Casper, C.; Wolfden, A.L.; Malenka, R.C.; Kauer, J.A. Somatodendritic Release of Cholecystokinin Potentiates GABAergic Synapses Onto Ventral Tegmental Area Dopamine Cells. Biol. Psychiatry 2023, 93, 197–208. [Google Scholar] [CrossRef]
- Ravasenga, T.; Ruben, M.; Regio, V.; Polenghi, A.; Petrini, E.M.; Barberis, A. Spatial regulation of coordinated excitatory and inhibitory synaptic plasticity at dendritic synapses. Cell Rep. 2022, 38, 110347. [Google Scholar] [CrossRef]
- Popov, A.; Brazhe, A.; Denisov, P.; Sutyagina, O.; Li, L.; Lazareva, N.; Verkhratsky, A.; Semyanov, A. Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 2021, 20, e13334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xie, X.; Lu, J.; Gangal, H.; Wang, W.; Melo, S.; Wang, X.; Jerger, J.; Woodson, K.; Garr, E.; et al. Optogenetic induction of orbitostriatal long-term potentiation in the dorsomedial striatum elicits a persistent reduction of alcohol-seeking behavior in rats. Neuropharmacology 2021, 191, 108560. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shi, H.; Zhang, G.; Peng, Y.; Ghosh, A.; Zhang, M.; Hu, X.; Liu, C.; Shao, Y.; Wang, S.; et al. A Novel CCK Receptor GPR173 Mediates Potentiation of GABAergic Inhibition. J. Neurosci. 2023, 43, 2305–2325. [Google Scholar] [CrossRef]
- Scott, H.L.; Bolmatov, D.; Podar, P.T.; Liu, Z.; Kinnun, J.J.; Doughty, B.; Lydic, R.; Sacci, R.L.; Collier, C.P.; Katsaras, J. Evidence for long-term potentiation in phospholipid membranes. Proc. Natl. Acad. Sci. USA 2022, 119, e2212195119. [Google Scholar] [CrossRef] [PubMed]
- Lømo, T. The discovery of long-term potentiation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 617–620. [Google Scholar] [CrossRef]
- Kimura, F.; Nishigori, A.; Shirokawa, T.; Tsumoto, T. Long-term potentiation and N-methyl-D-aspartate receptors in the visual cortex of young rats. J. Physiol. 1989, 414, 125–144. [Google Scholar] [CrossRef]
- Grover, L.M.; Teyler, T.J. Two components of long-term potentiation induced by different patterns of afferent activation. Nature 1990, 347, 477–479. [Google Scholar] [CrossRef]
- Zakharenko, S.S.; Zablow, L.; Siegelbaum, S.A. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat. Neurosci. 2001, 4, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.W.; Williams, A.N.; Lucas, J.H. Recording of spontaneous activity with photoetched microelectrode surfaces from mouse spinal neurons in culture. J. Neurosci. Methods 1982, 5, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Garad, M.; Edelmann, E.; Leßmann, V. Impairment of Spike-Timing-Dependent Plasticity at Schaffer Collateral-CA1 Synapses in Adult APP/PS1 Mice Depends on Proximity of Aβ Plaques. Int. J. Mol. Sci. 2021, 22, 1378. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, S.; Luan, Y.; Zhu, Z.; Cai, Y.; Liu, Y. Ginkgo biloba extracts inhibit post-ischemic LTP through attenuating EPSCs in rat hippocampus. Metab. Brain Dis. 2021, 36, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Griego, E.; Galván, E.J. Biophysical and synaptic properties of regular spiking interneurons in hippocampal area CA3 of aged rats. Neurobiol. Aging 2022, 112, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Garcia-Oscos, F.; Kourrich, S. Whole-cell Patch-clamp Recordings in Brain Slices. J. Vis. Exp. 2016, 112, e54024. [Google Scholar] [CrossRef]
- Erofeev, A.; Antifeev, I.; Bolshakova, A.; Bezprozvanny, I.; Vlasova, O. In Vivo Penetrating Microelectrodes for Brain Electrophysiology. Sensors 2022, 22, 9085. [Google Scholar] [CrossRef]
- Kirkwood, A.; Dudek, S.M.; Gold, J.T.; Aizenman, C.D.; Bear, M.F. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science 1993, 260, 1518–1521. [Google Scholar] [CrossRef]
- Wang, X.F.; Daw, N.W. Long term potentiation varies with layer in rat visual cortex. Brain Res. 2003, 989, 26–34. [Google Scholar] [CrossRef]
- Liu, H.N.; Kurotani, T.; Ren, M.; Yamada, K.; Yoshimura, Y.; Komatsu, Y. Presynaptic activity and Ca2+ entry are required for the maintenance of NMDA receptor-independent LTP at visual cortical excitatory synapses. J. Neurophysiol. 2004, 92, 1077–1087. [Google Scholar] [CrossRef]
- Huemmeke, M.; Eysel, U.T.; Mittmann, T. Metabotropic glutamate receptors mediate expression of LTP in slices of rat visual cortex. Eur. J. Neurosci. 2002, 15, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A.; Frambach, D.A.; Odette, L.L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 1984, 225, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Bykov, K.A.; Dmitriev, A.V.; Skachkov, S.N. Relationship between photoinduced changes in the intercellular concentration of potassium ions and transretinal potential generation by the Muller cells of the retina. Biofizika 1981, 26, 104–107. [Google Scholar] [PubMed]
- Newman, E.A.; Odette, L.L. Model of electroretinogram b-wave generation: A test of the K+ hypothesis. J. Neurophysiol. 1984, 51, 164–182. [Google Scholar] [CrossRef]
- Karwoski, C.J.; Lu, H.K.; Newman, E.A. Spatial buffering of light-evoked potassium increases by retinal Muller (glial) cells. Science 1989, 244, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Matsuda, N.; Ishibashi, Y.; Suzuki, I. Detection of astrocytic slow oscillatory activity and response to seizurogenic compounds using planar microelectrode array. Front. Neurosci. 2022, 16, 1050150. [Google Scholar] [CrossRef]
- Sætra, M.J.; Einevoll, G.T.; Halnes, G. An electrodiffusive neuron-extracellular-glia model for exploring the genesis of slow potentials in the brain. PLoS Comput. Biol. 2021, 17, e1008143. [Google Scholar] [CrossRef]
- Abraham, W.C.; Gustafsson, B.; Wigström, H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J. Physiol. 1987, 394, 367–380. [Google Scholar] [CrossRef]
- Gray, R.; Johnston, D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature 1987, 327, 620–622. [Google Scholar] [CrossRef]
- Edwards, F.A.; Konnerth, A.; Sakmann, B.; Takahashi, T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflug. Arch. 1989, 414, 600–612. [Google Scholar] [CrossRef]
- Tepper, J.M.; Bolam, J.P. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004, 14, 685–692. [Google Scholar] [CrossRef]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2007, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, G.; Alonso-Nanclares, L.; Anderson, S.; Barrionuevo, G.; Benavides-Piccione, R.; Burkhalter, A.; Buzsáki, G.; Cauli, B.; DeFelipe, J.; Fairén, A. Yuste The Petilla Interneuron Nomenclature Group (PING) R. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008, 9, 557–568. [Google Scholar] [PubMed]
- Kullmann, D.M.; Lamsa, K.P. LTP and LTD in cortical GABAergic interneurons: Emerging rules and roles. Neuropharmacology 2011, 60, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kittler, J.T.; Moss, S.J. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: Implications for the efficacy of synaptic inhibition. Curr. Opin. Neurobiol. 2003, 13, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E. Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb. Perspect. Biol. 2012, 4, a005728. [Google Scholar] [CrossRef]
- Monday, H.R.; Younts, T.J.; Castillo, P.E. Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu. Rev. Neurosci. 2018, 41, 299–322. [Google Scholar] [CrossRef]
- Bauer, V.; Sotníková, R. Nitric oxide—The endothelium-derived relaxing factor and its role in endothelial functions. Gen. Physiol. Biophys. 2010, 29, 319. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Wu, G. l-Arginine increases AMPK phosphorylation and the oxidation of energy substrates in hepatocytes, skeletal muscle cells, and adipocytes. Amino Acids 2022, 54, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.P. Synaptic plasticity: A role for nitric oxide in LTP. Curr. Biol. 1997, 7, R141–R143. [Google Scholar] [CrossRef] [PubMed]
- Tropea, M.R.; Gulisano, W.; Vacanti, V.; Arancio, O.; Puzzo, D.; Palmeri, A. Nitric oxide/cGMP/CREB pathway and amyloid-beta crosstalk: From physiology to Alzheimer’s disease. Free Radic. Biol. Med. 2022, 193 Pt 2, 657–668. [Google Scholar] [CrossRef]
- Jehle, A.; Garaschuk, O. The Interplay between cGMP and Calcium Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 7048. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, J.L.; Murali, M.; Kauer, J.A. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur. J. Neurosci. 2010, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, T.J.; Hawkins, R.D.; Kandel, E.R.; Arancio, O. Tests of the roles of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc. Natl. Acad. Sci. USA 1991, 88, 11285–11289. [Google Scholar] [CrossRef]
- Ge, Y.-X.; Xin, W.-J.; Hu, N.-W.; Zhang, T.; Xu, J.-T.; Liu, X.-G. Clonidine depresses LTP of C-fiber evoked field potentials in spinal dorsal horn via NO-cGMP pathway. Brain Res. 2006, 1118, 58–65. [Google Scholar] [CrossRef]
- Alkadhi, K.; Alzoubi, K.; Aleisa, A. Plasticity of synaptic transmission in autonomic ganglia. Prog. Neurobiol. 2005, 75, 83–108. [Google Scholar] [CrossRef]
- Acquarone, E.; Argyrousi, E.K.; Van Den Berg, M.; Gulisano, W.; Fà, M.; Staniszewski, A.; Calcagno, E.; Zuccarello, E.; D’Adamio, L.; Deng, S.-X. Synaptic and memory dysfunction induced by tau oligomers is rescued by up-regulation of the nitric oxide cascade. Mol. Neurodegener. 2019, 14, 26. [Google Scholar] [CrossRef]
- Argyrousi, E.K.; Heckman, P.R.; Prickaerts, J. Role of cyclic nucleotides and their downstream signaling cascades in memory function: Being at the right time at the right spot. Neurosci. Biobehav. Rev. 2020, 113, 12–38. [Google Scholar] [CrossRef]
- Chachlaki, K.; Prevot, V. Nitric oxide signalling in the brain and its control of bodily functions. Br. J. Pharmacol. 2020, 177, 5437–5458. [Google Scholar] [CrossRef]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Qiu, L.L.; Pan, W.; Luo, D.; Zhang, G.F.; Zhou, Z.Q.; Sun, X.Y.; Yang, J.J.; Ji, M.H. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca(2+)/calpain might contribute to postoperative cognitive dysfunction in aging mice. J. Neuroinflammation 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Gubellini, P.; Ben-Ari, Y.; Gaïarsa, J.L. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J. Neurosci. 2005, 25, 5796–5802. [Google Scholar] [CrossRef]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, S.S.; Patterson, S.L.; Dragatsis, I.; Zeitlin, S.O.; Siegelbaum, S.A.; Kandel, E.R.; Morozov, A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 2003, 39, 975–990. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF effects on dendritic spine morphology and hippocampal function. Cell. Tissue Res. 2018, 373, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Magariños, A.M.; Li, C.J.; Gal Toth, J.; Bath, K.G.; Jing, D.; Lee, F.S.; McEwen, B.S. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 2011, 21, 253–264. [Google Scholar] [CrossRef]
- Alkadhi, K.A. NMDA receptor-independent LTP in mammalian nervous system. Prog. Neurobiol. 2021, 200, 101986. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Li, X.H.; Zhuo, M. NMDA receptors and synaptic plasticity in the anterior cingulate cortex. Neuropharmacology 2021, 197, 108749. [Google Scholar] [CrossRef]
- Lachamp, P.M.; Liu, Y.; Liu, S.J. Glutamatergic modulation of cerebellar interneuron activity is mediated by an enhancement of GABA release and requires protein kinase A/RIM1alpha signaling. J. Neurosci. 2009, 29, 381–392. [Google Scholar] [CrossRef]
- Liu, S.J.; Lachamp, P. The activation of excitatory glutamate receptors evokes a long-lasting increase in the release of GABA from cerebellar stellate cells. J. Neurosci. 2006, 26, 9332–9339. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Chiu, C.Q.; Carroll, R.C. Long-term plasticity at inhibitory synapses. Curr. Opin. Neurobiol. 2011, 21, 328–338. [Google Scholar] [CrossRef]
- Aizenman, C.D.; Manis, P.B.; Linden, D.J. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 1998, 21, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Chistiakova, M.; Bannon, N.M.; Bazhenov, M.; Volgushev, M. Heterosynaptic plasticity: Multiple mechanisms and multiple roles. Neuroscientist 2014, 20, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Boué-Grabot, E.; Pankratov, Y. Modulation of Central Synapses by Astrocyte-Released ATP and Postsynaptic P2X Receptors. Neural Plast. 2017, 2017, 9454275. [Google Scholar] [CrossRef]
- Kovács, Z.; Skatchkov, S.N.; Veh, R.W.; Szabó, Z.; Németh, K.; Szabó, P.T.; Kardos, J.; Héja, L. Critical Role of Astrocytic Polyamine and GABA Metabolism in Epileptogenesis. Front. Cell. Neurosci. 2021, 15, 787319. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y. ATP-mediated signalling in the central synapses. Neuropharmacology 2023, 229, 109477. [Google Scholar] [CrossRef]
- Rebola, N.; Lujan, R.; Cunha, R.A.; Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 2008, 57, 121–134. [Google Scholar] [CrossRef]
- Laming, P.R.; Kimelberg, H.; Robinson, S.; Salm, A.; Hawrylak, N.; Muller, C.; Roots, B.; Ng, K. Neuronal-glial interactions and behaviour. Neurosci. Biobehav. Rev. 2000, 24, 295–340. [Google Scholar] [CrossRef]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71 e14. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.R.; Esguerra, M.; Kim, P.M.; Newman, E.A.; Snyder, S.H.; Zahs, K.R.; Miller, R.F. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 6789–6794. [Google Scholar] [CrossRef]
- Lalo, U.; Rasooli-Nejad, S.; Bogdanov, A.; More, L.; Koh, W.; Muller, J.; Wall, M.; Lee, C.J.; Pankratov, Y. Synergy between vesicular and non-vesicular gliotransmission regulates synaptic plasticity and working memory. BioRxiv 2021. [Google Scholar] [CrossRef]
- Benedikt, J.; Malpica-Nieves, C.J.; Rivera, Y.; Méndez-González, M.; Nichols, C.G.; Veh, R.W.; Eaton, M.J.; Skatchkov, S.N. The Polyamine Spermine Potentiates the Propagation of Negatively Charged Molecules through the Astrocytic Syncytium. Biomolecules 2022, 12, 1812. [Google Scholar] [CrossRef]
- Kiyoshi, C.M.; Du, Y.; Zhong, S.; Wang, W.; Taylor, A.T.; Xiong, B.; Ma, B.; Terman, D.; Zhou, M. Syncytial isopotentiality: A system-wide electrical feature of astrocytic networks in the brain. Glia 2018, 66, 2756–2769. [Google Scholar] [CrossRef]
- Kovács, Z.; Skatchkov, S.N.; Szabó, Z.; Qahtan, S.; Méndez-González, M.P.; Malpica-Nieves, C.J.; Eaton, M.J.; Kardos, J.; Héja, L. Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures. Int. J. Mol. Sci. 2022, 23, 8191. [Google Scholar] [CrossRef]
- Chiu, C.Q.; Barberis, A.; Higley, M.J. Preserving the balance: Diverse forms of long-term GABAergic synaptic plasticity. Nat. Rev. Neurosci. 2019, 20, 272–281. [Google Scholar] [CrossRef]
- Barberis, A. Postsynaptic plasticity of GABAergic synapses. Neuropharmacology 2020, 169, 107643. [Google Scholar] [CrossRef]
- Liu, G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 2004, 7, 373–379. [Google Scholar] [CrossRef]
- Lagrange, A.H.; Hu, N.; Macdonald, R.L. GABA beyond the synapse: Defining the subtype-specific pharmacodynamics of non-synaptic GABA(A) receptors. J. Physiol. 2018, 596, 4475–4495. [Google Scholar] [CrossRef]
- Tyagarajan, S.K.; Fritschy, J.M. Gephyrin: A master regulator of neuronal function? Nat. Rev. Neurosci. 2014, 15, 141–156. [Google Scholar] [CrossRef]
- Choii, G.; Ko, J. Gephyrin: A central GABAergic synapse organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef]
- Petrini, E.M.; Ravasenga, T.; Hausrat, T.J.; Iurilli, G.; Olcese, U.; Racine, V.; Sibarita, J.B.; Jacob, T.C.; Moss, S.J.; Benfenati, F.; et al. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 2014, 5, 3921. [Google Scholar] [CrossRef]
- Ostrovskaya, O.; Xie, K.; Masuho, I.; Fajardo-Serrano, A.; Lujan, R.; Wickman, K.; Martemyanov, K.A. RGS7/Gβ5/R7BP complex regulates synaptic plasticity and memory by modulating hippocampal GABABR-GIRK signaling. Elife 2014, 3, e02053. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.A.; Loreth, D.; Gee, A.L.; Watanabe, M.; Kind, P.C.; Wyllie, D.J.A.; Kulik, Á.; Vida, I. Postsynaptic GABA(B)Rs Inhibit L-Type Calcium Channels and Abolish Long-Term Potentiation in Hippocampal Somatostatin Interneurons. Cell. Rep. 2018, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, M.X.; Poo, M.M.; Zhang, X.H. GABA(B) receptor activation mediates frequency-dependent plasticity of developing GABAergic synapses. Nat. Neurosci. 2008, 11, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, P.; Ferrand, N.; Colin-Le Brun, I.; Gaïarsa, J.L. Epileptiform activity triggers long-term plasticity of GABA(B) receptor signalling in the developing rat hippocampus. J. Physiol. 2005, 568, 951–966. [Google Scholar] [CrossRef]
- Butt, S.J.; Stacey, J.A.; Teramoto, Y.; Vagnoni, C. A role for GABAergic interneuron diversity in circuit development and plasticity of the neonatal cerebral cortex. Curr. Opin. Neurobiol. 2017, 43, 149–155. [Google Scholar] [CrossRef]
- Wang, L.; Maffei, A. Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J. Neurosci. 2014, 34, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Neveu, D.; Zucker, R.S. Postsynaptic levels of [Ca2+] i needed to trigger LTD and LTP. Neuron 1996, 16, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Davies, G.C. Two-photon microscopy for chemical neuroscience. ACS Chem. Neurosci. 2011, 2, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Pettit, D.L.; Augustine, G.J. Chemical two-photon uncaging. CSH Protoc. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, J.; Nagaoka, A.; Watanabe, S.; Ellis-Davies, G.C.; Kitamura, K.; Kano, M.; Matsuzaki, M.; Kasai, H. In vivo two-photon uncaging of glutamate revealing the structure–function relationships of dendritic spines in the neocortex of adult mice. J. Physiol. 2011, 589, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Kruijssen, D.L.H.; Wierenga, C.J. Single Synapse LTP: A Matter of Context? Front. Cell. Neurosci. 2019, 13, 496. [Google Scholar] [CrossRef]
- Noguchi, J.; Nagaoka, A.; Hayama, T.; Ucar, H.; Yagishita, S.; Takahashi, N.; Kasai, H. Bidirectional in vivo structural dendritic spine plasticity revealed by two-photon glutamate uncaging in the mouse neocortex. Sci. Rep. 2019, 9, 13922. [Google Scholar] [CrossRef]
- Denk, W.; Svoboda, K. Photon upmanship: Why multiphoton imaging is more than a gimmick. Neuron 1997, 18, 351–357. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Wilt, B.A.; Burns, L.D.; Wei Ho, E.T.; Ghosh, K.K.; Mukamel, E.A.; Schnitzer, M.J. Advances in light microscopy for neuroscience. Annu. Rev. Neurosci. 2009, 32, 435–506. [Google Scholar] [CrossRef]
- Schreurs, A.; Leenders, N.; Sabanov, V.; Van Den Haute, C.; Welkenhuysen, M.; Hoffman, L.; Braeken, D.; Baekelandt, V.; Balschun, D. Optogenetic Inhibition of Long-Term Potentiation in the Mouse Dentate Gyrus. In Proceedings of the Abstract: 12th National Congress of the Belgian Society for Neuroscience, Gent, Belgium, 22 May 2017. [Google Scholar]
- Quinlan, M.A.; Strong, V.M.; Skinner, D.M.; Martin, G.M.; Harley, C.W.; Walling, S.G. Locus coeruleus optogenetic light activation induces long-term potentiation of perforant path population spike amplitude in rat dentate gyrus. Front. Syst. Neurosci. 2019, 12, 67. [Google Scholar] [CrossRef]
- Rost, B.R.; Wietek, J.; Yizhar, O.; Schmitz, D. Optogenetics at the presynapse. Nat. Neurosci. 2022, 25, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Asgarihafshejani, A.; Honoré, È.; Michon, F.X.; Laplante, I.; Lacaille, J.C. Long-term potentiation at pyramidal cell to somatostatin interneuron synapses controls hippocampal network plasticity and memory. iScience 2022, 25, 104259. [Google Scholar] [CrossRef] [PubMed]
- Udakis, M.; Pedrosa, V.; Chamberlain, S.E.L.; Clopath, C.; Mellor, J.R. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat. Commun. 2020, 11, 4395. [Google Scholar] [CrossRef]
- Froemke, R.C. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci. 2015, 38, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, G.; Agnes, E.J.; Vogels, T.P. Inhibitory Plasticity: Balance, Control, and Codependence. Annu. Rev. Neurosci. 2017, 40, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, O.; Sejnowski, T.J. Natural patterns of activity and long-term synaptic plasticity. Curr. Opin. Neurobiol. 2000, 10, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Monier, C.; Fournier, J.; Frégnac, Y. In vitro and in vivo measures of evoked excitatory and inhibitory conductance dynamics in sensory cortices. J. Neurosci. Methods 2008, 169, 323–365. [Google Scholar] [CrossRef]
- Willems, J.G.P.; Wadman, W.J.; Cappaert, N.L.M. Interaction of Cortical and Amygdalar Synaptic Input Modulates the Window of Opportunity for Information Processing in the Rhinal Cortices. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Angelo, K.; Margrie, T.W. Population diversity and function of hyperpolarization-activated current in olfactory bulb mitral cells. Sci. Rep. 2011, 1, 50. [Google Scholar] [CrossRef]
- Bear, M.F.; Malenka, R.C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Heynen, A.J.; Abraham, W.C.; Bear, M.F. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature 1996, 381, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Suppa, A.; Marsili, L.; Belvisi, D.; Conte, A.; Iezzi, E.; Modugno, N.; Fabbrini, G.; Berardelli, A. Lack of LTP-like plasticity in primary motor cortex in Parkinson’s disease. Exp. Neurol. 2011, 227, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Matsuura, T.; Xue, M.; Chen, Q.Y.; Liu, R.H.; Lu, J.S.; Shi, W.; Fan, K.; Zhou, Z.; Miao, Z.; et al. Oxytocin in the anterior cingulate cortex attenuates neuropathic pain and emotional anxiety by inhibiting presynaptic long-term potentiation. Cell Rep. 2021, 36, 109411. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Motta, C.; Bonnì, S.; Mercuri, N.B.; Caltagirone, C.; Martorana, A.; Koch, G. LTP-like cortical plasticity is associated with verbal memory impairment in Alzheimer’s disease patients. Brain Stimul. 2019, 12, 148–151. [Google Scholar] [CrossRef]
- Bialas, M.; Mandziuk, J. Spike-Timing-Dependent Plasticity With Activation-Dependent Scaling for Receptive Fields Development. IEEE Trans. Neural Netw. Learn. Syst. 2022, 33, 5215–5228. [Google Scholar] [CrossRef]
- Cutsuridis, V. GABA inhibition modulates NMDA-R mediated spike timing dependent plasticity (STDP) in a biophysical model. Neural Netw. 2011, 24, 29–42. [Google Scholar] [CrossRef]
- Inglebert, Y.; Debanne, D. Calcium and Spike Timing-Dependent Plasticity. Front. Cell Neurosci. 2021, 15, 727336. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.Y.; Cheung, K.; Hung, K.W.; Chen, C.; Geng, H.; Yung, W.H.; Qu, J.Y.; Fu, A.K.Y.; Ip, N.Y. Astrocyte-secreted IL-33 mediates homeostatic synaptic plasticity in the adult hippocampus. Proc. Natl. Acad. Sci. USA 2021, 118, e2020810118. [Google Scholar] [CrossRef]
- Shin, R.M.; Tully, K.; Li, Y.; Cho, J.H.; Higuchi, M.; Suhara, T.; Bolshakov, V.Y. Hierarchical order of coexisting pre- and postsynaptic forms of long-term potentiation at synapses in amygdala. Proc. Natl. Acad. Sci. USA 2010, 107, 19073–19078. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, K.; Schayek, R.; Kritman, M.; Maroun, M. Differential Age-dependent Mechanisms of High-frequency Stimulation-induced Potentiation in the Prefrontal Cortex-Basolateral Amygdala Pathway Following Fear Extinction. Neuroscience 2022, 491, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.L.; Quigley, L.D.; Dunham, T.; Volk, L.J. KIBRA regulates activity-induced AMPA receptor expression and synaptic plasticity in an age-dependent manner. iScience 2022, 25, 105623. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.W.; Chong, Y.S.; Lin, W.; Kisiswa, L.; Sim, E.; Ibáñez, C.F.; Sajikumar, S. Age-related changes in hippocampal-dependent synaptic plasticity and memory mediated by p75 neurotrophin receptor. Aging Cell. 2021, 20, e13305. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S. Interspecies differences. Nat. Rev. Neurosci. 2022, 23, 69. [Google Scholar] [CrossRef]
- Olsen, M.L.; Khakh, B.S.; Skatchkov, S.N.; Zhou, M.; Lee, C.J.; Rouach, N. New insights on astrocyte ion channels: Critical for homeostasis and neuron-glia signaling. J. Neurosci. 2015, 35, 13827–13835. [Google Scholar] [CrossRef]
- Lioy, D.T.; Garg, S.K.; Monaghan, C.E.; Raber, J.; Foust, K.D.; Kaspar, B.K.; Hirrlinger, P.G.; Kirchhoff, F.; Bissonnette, J.M.; Ballas, N.; et al. A role for glia in the progression of Rett’s syndrome. Nature 2011, 475, 497–500. [Google Scholar] [CrossRef]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Bello, S.T.; Gao, Q.; Lai, Y.; Li, X.; He, L. Advances in the Electrophysiological Recordings of Long-Term Potentiation. Int. J. Mol. Sci. 2023, 24, 7134. https://doi.org/10.3390/ijms24087134
Jiang F, Bello ST, Gao Q, Lai Y, Li X, He L. Advances in the Electrophysiological Recordings of Long-Term Potentiation. International Journal of Molecular Sciences. 2023; 24(8):7134. https://doi.org/10.3390/ijms24087134
Chicago/Turabian StyleJiang, Feixu, Stephen Temitayo Bello, Qianqian Gao, Yuanying Lai, Xiao Li, and Ling He. 2023. "Advances in the Electrophysiological Recordings of Long-Term Potentiation" International Journal of Molecular Sciences 24, no. 8: 7134. https://doi.org/10.3390/ijms24087134
APA StyleJiang, F., Bello, S. T., Gao, Q., Lai, Y., Li, X., & He, L. (2023). Advances in the Electrophysiological Recordings of Long-Term Potentiation. International Journal of Molecular Sciences, 24(8), 7134. https://doi.org/10.3390/ijms24087134






