Influence of Fasting until Noon (Extended Postabsorptive State) on Clock Gene mRNA Expression and Regulation of Body Weight and Glucose Metabolism
Abstract
1. Introduction
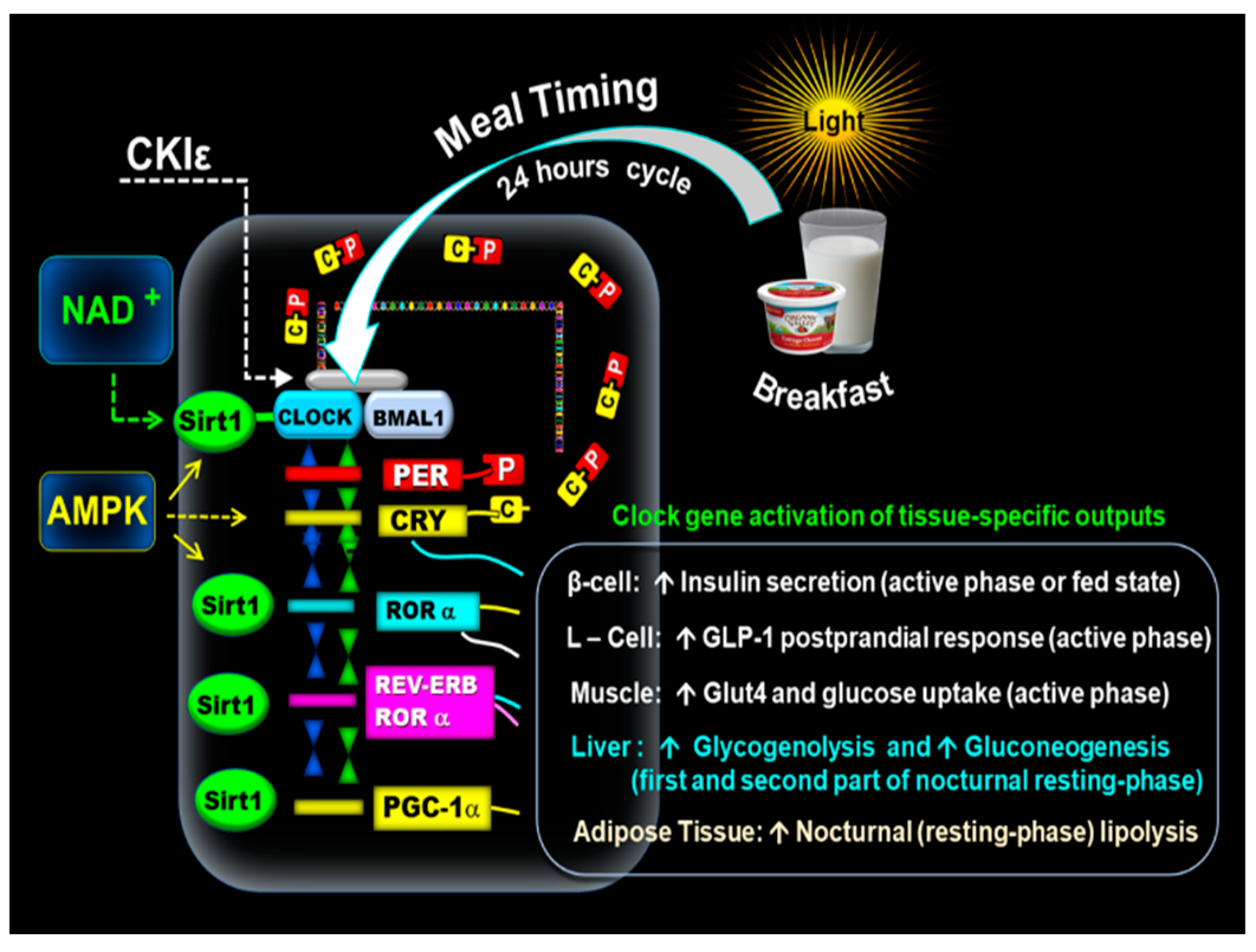
2. Circadian Clock Molecular Mechanism
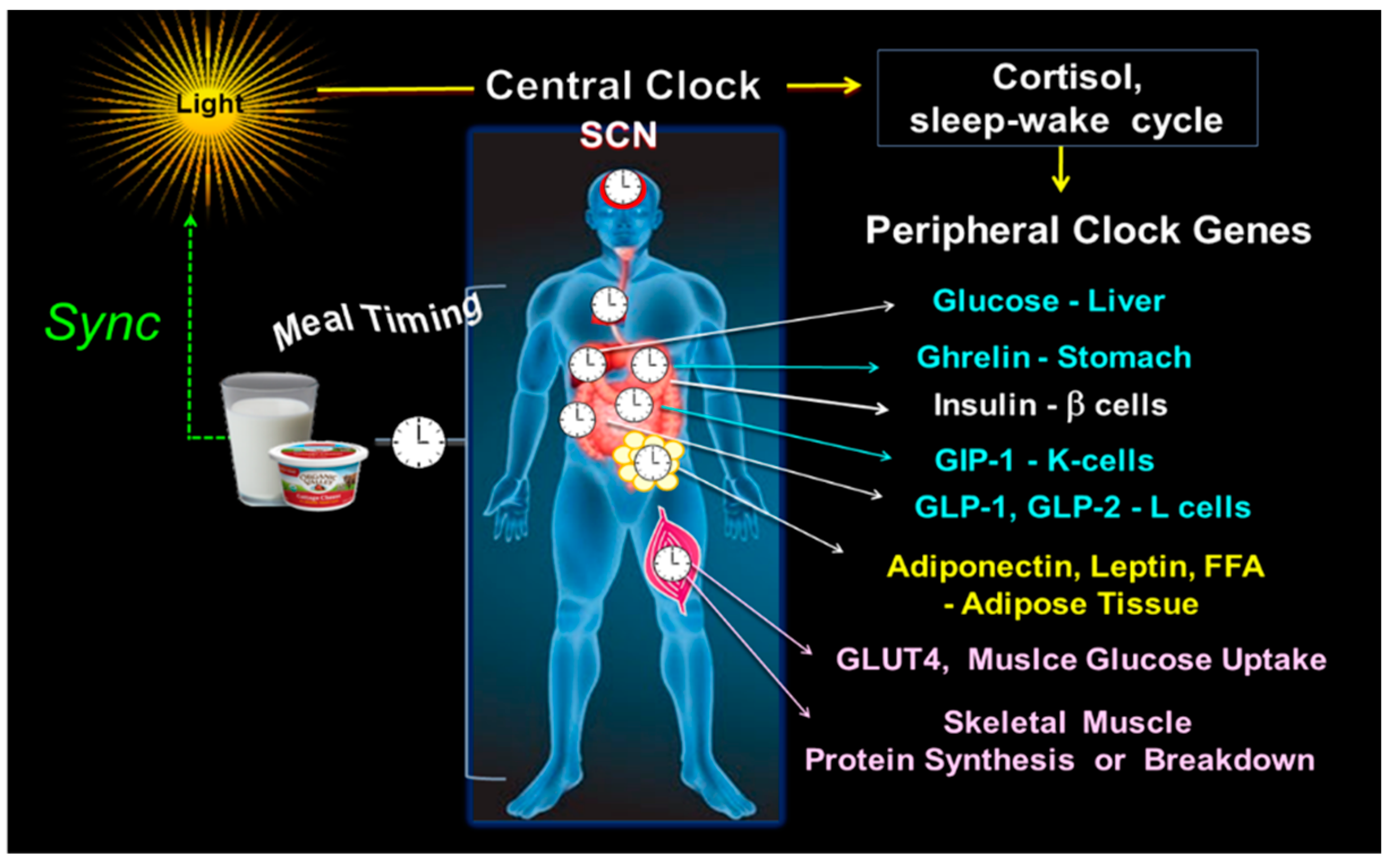
3. Synchrony between Central and Peripheral Clock Genes
4. Circadian Variation of Metabolism during Active and Resting Phase
4.1. Clock Controlled Active and Resting Phase
4.2. Clock Controlled Metabolism Is Enhanced in the Early Hours of the Active Phase
4.3. Potential Benefits of Early-Timed Breakfast
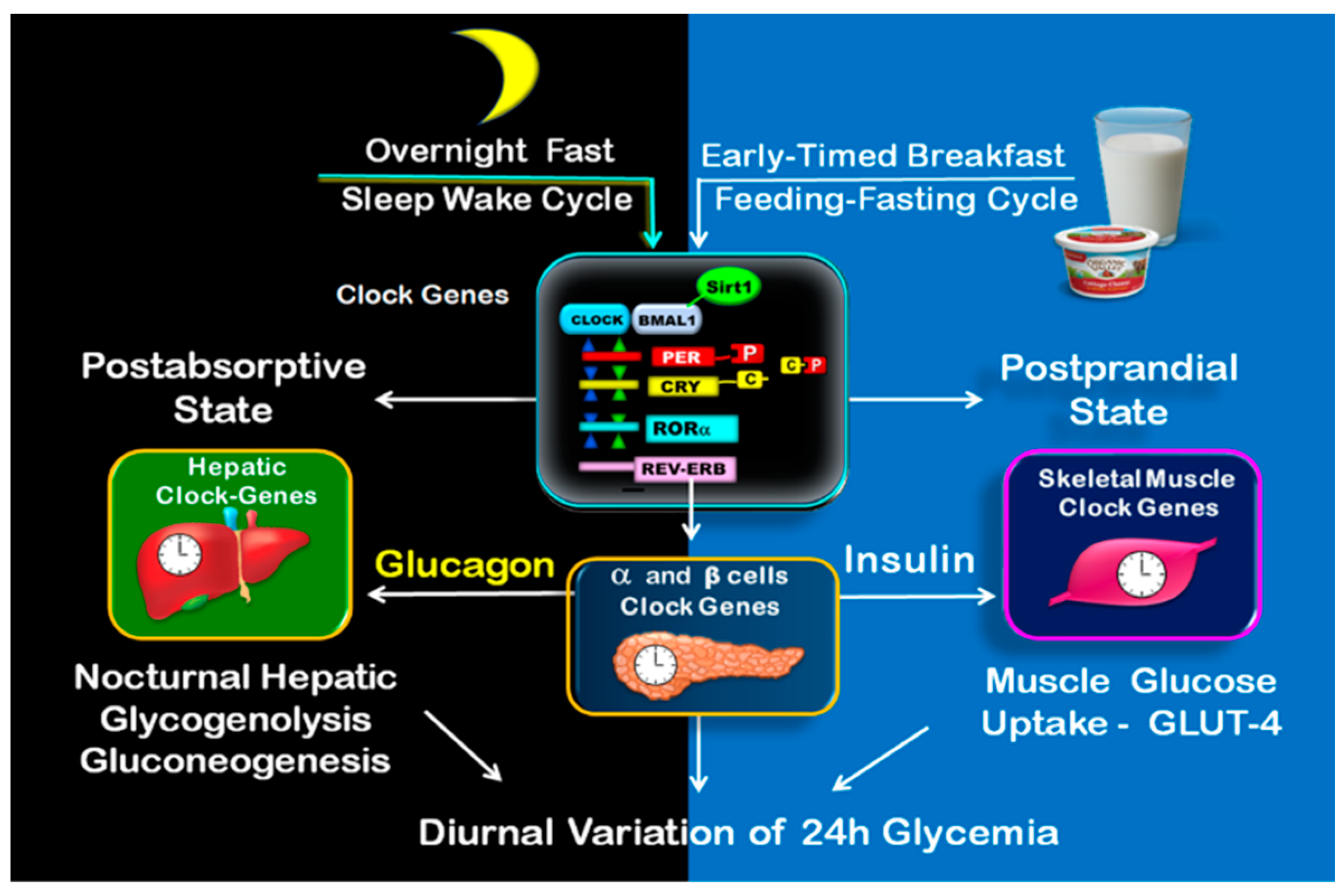
5. Circadian Clock Regulation of Postprandial and Postabsorptive Glucose Metabolism and the Transition (Switch) from Overnight Fast to Postprandial Fed State
5.1. Clock Controlled Metabolism during Postprandial State
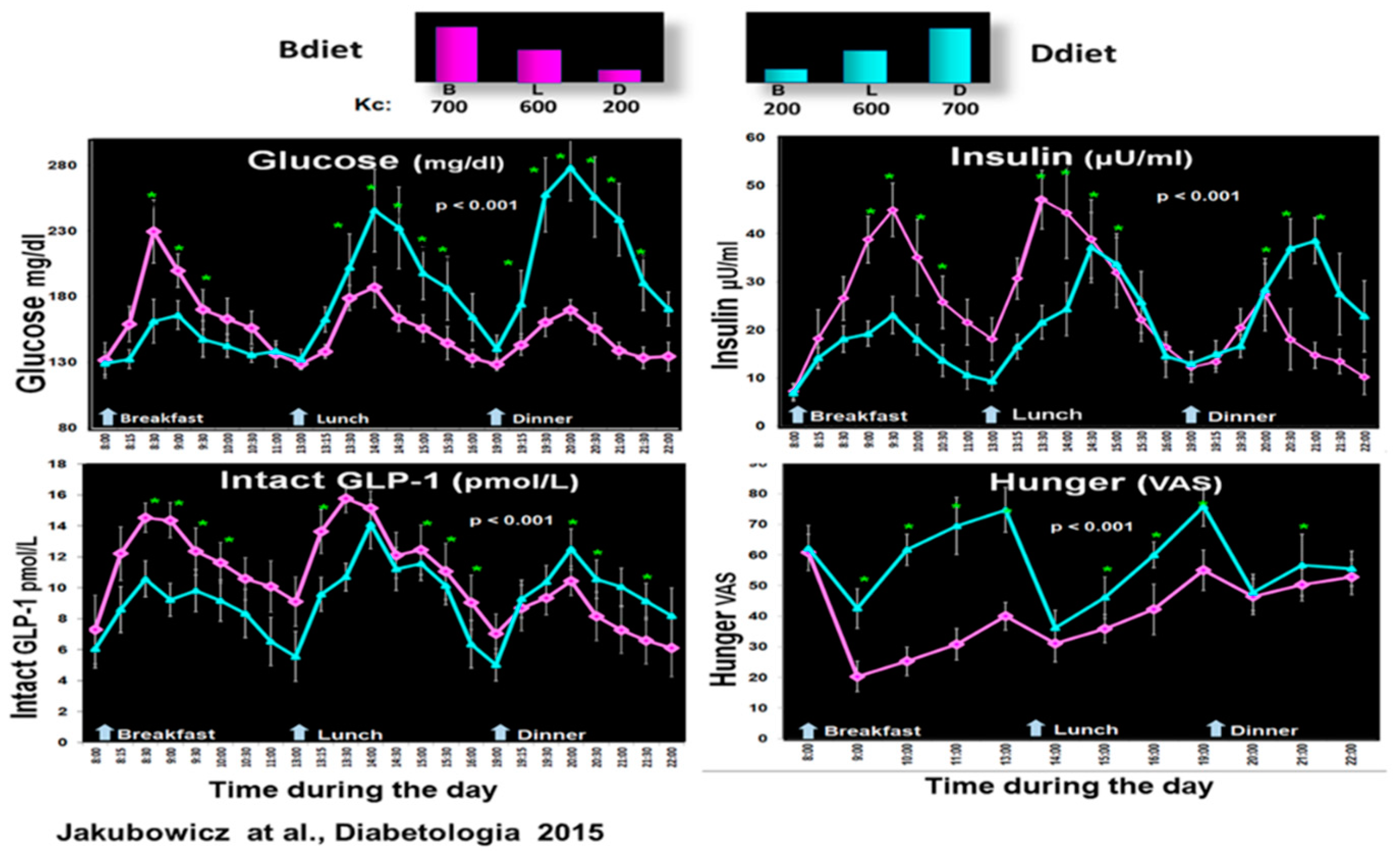
5.2. Influence of High Energy Breakfast versus High Energy Dinner on Clock Controlled Postprandial Glucose Metabolism
5.3. Clock Controlled Metabolism during Postabsorptive State
5.4. Clock-Controlled Glucose Metabolism during the Transition (Switch) from Overnight Fast to Postprandial State
6. Effect of Fasting until Noon (Extended Postabsorptive State) on Clock Genes mRNA Expression and Regulation of Body Weight, Glucose Metabolism, Appetite, and Energy Expenditure
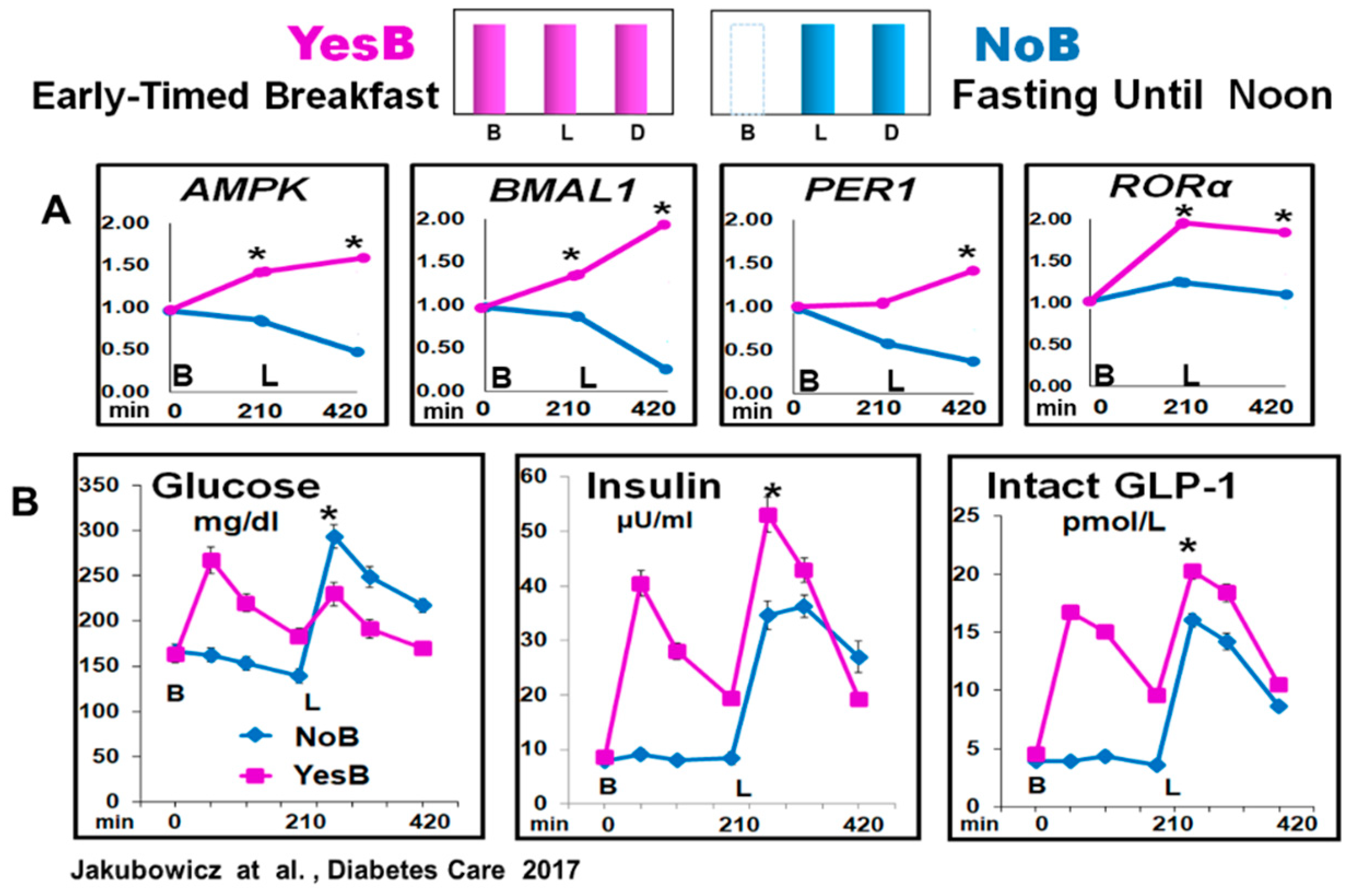
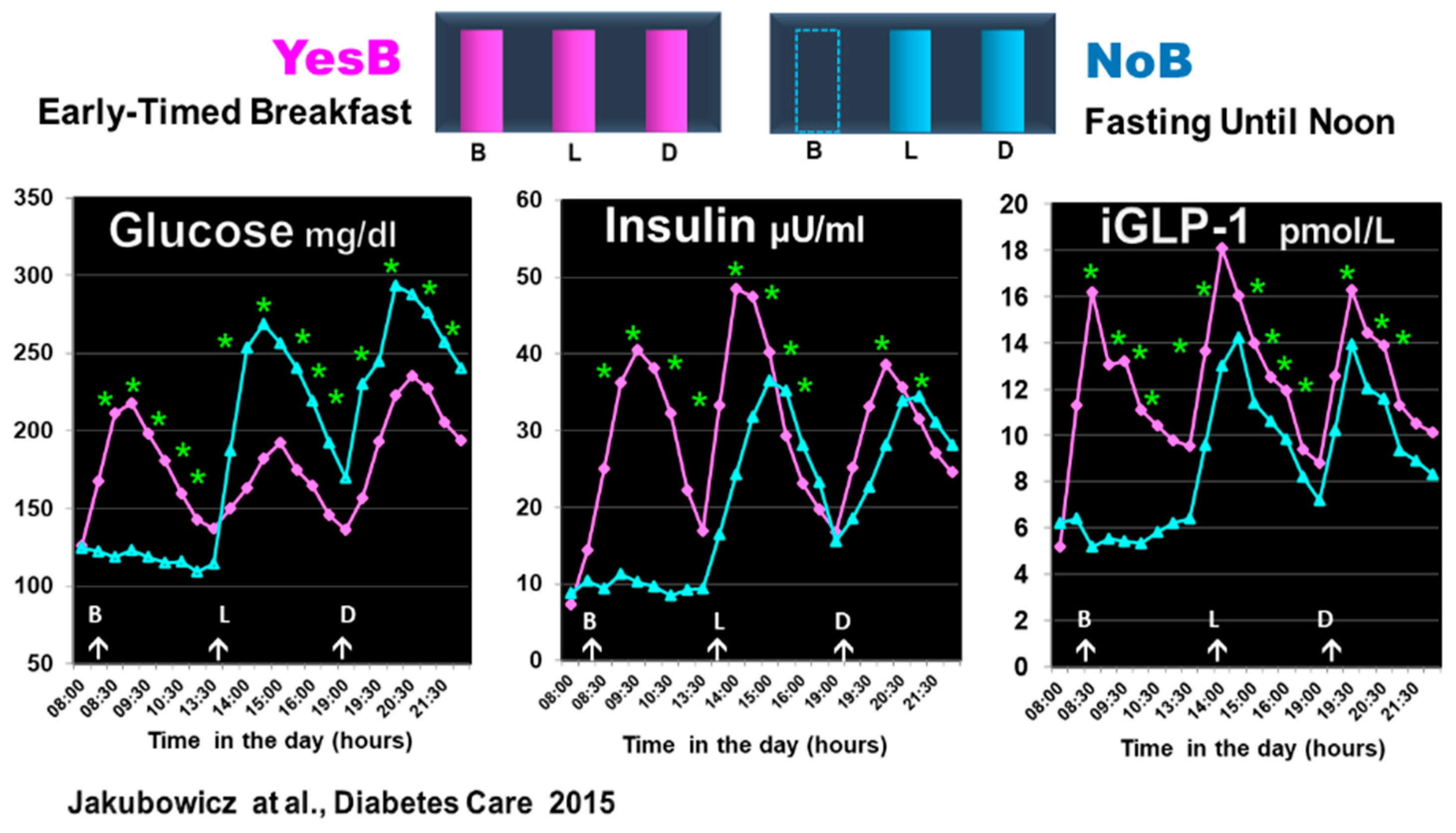
6.1. Effect of Fasting until Noon on Clock Genes mRNA Expression and Glucose, Insulin, and Incretin Responses after Subsequent Meals
6.2. Effect of Fasting until Noon at the Switch from Overnight Fast to Fed State on Clock-Controlled Glucose Metabolism and Skeletal Muscle Protein Synthesis and Breakdown
6.3. Influence of Fasting until Noon on Circadian Clock-Controlled Regulation of Body Weight, Appetite, and Energy Expenditure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Ruddick-Collins, L.C.; Johnston, J.D.; Morgan, P.J.; Johnstone, A.M. The Big Breakfast Study: Chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 2018, 43, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Charlot, A.; Hutt, F.; Sabatier, E.; Zoll, J. Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients 2021, 13, 1405. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Zhao, L.; Hutchison, A.T.; Heilbronn, L.K. Carbohydrate intake and circadian synchronicity in the regulation of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 2021, 22, bnab027. [Google Scholar] [CrossRef]
- Clayton, D.J.; Mode, W.J.A.; Slater, T. Optimising intermittent fasting: Evaluating the behavioural and metabolic effects of extended morning and evening fasting. Nutr. Bull. 2020, 45, 444–455. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Morgan, P.J.; Fyfe, C.L.; Filipe, J.A.N.; Horgan, G.W.; Westerterp, K.R.; Johnston, J.D.; Johnstone, A.M. Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity. Cell Metab. 2020, 34, 1472–1485.e6. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.P.; Ellacott, K.L.J.; Chen, H.; McGuinness, O.P.; Johnson, C.H. Time-optimized feeding is beneficial without enforced fasting. Open Biol. 2021, 11, 210183. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Ahrén, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef]
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfast skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669. [Google Scholar] [CrossRef]
- Kinouchi, K.; Magnan, C.; Ceglia, N.; Liu, Y.; Cervantes, M.; Pastore, N.; Huynh, T.; Ballabio, A.; Baldi, P.; Masri, S.; et al. Fasting Imparts a Switch to Alternative Daily Pathways in Liver and Muscle. Cell Rep. 2018, 25, 3299–3314.e6. [Google Scholar] [CrossRef]
- Wu, T.; Sun, L.; Zhuge, F.; Guo, X.; Zhao, Z.; Tang, R.; Chen, Q.; Chen, L.; Kato, H.; Fu, Z. Differential roles of breakfast and supper in rats of a daily three-meal schedule upon circadian regulation and physiology. Chronobiol. Int. 2011, 28, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y.; et al. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals With Diabetes: A Randomized Clinical Trial. Diabetes Care 2017, 40, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Tomi, R.; Shinzawa, M.; Yoshimura, R.; Ozaki, S.; Nakanishi, K.; Ide, S.; Nagatomo, I.; Nishida, M.; Yamauchi-Takihara, K.; et al. Associations of Skipping Breakfast, Lunch, and Dinner with Weight Gain and Overweight/Obesity in University Students: A Retrospective Cohort Study. Nutrients 2021, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Vujović, N.; Piron, M.J.; Qian, J.; Chellappa, S.L.; Nedeltcheva, A.; Barr, D.; Heng, S.W.; Kerlin, K.; Srivastav, S.; Wang, W.; et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 2022, 34, 1486–1498.e7. [Google Scholar] [CrossRef]
- Xiao, K.; Furutani, A.; Sasaki, H.; Takahashi, M.; Shibata, S. Effect of a High Protein Diet at Breakfast on Postprandial Glucose Level at Dinner Time in Healthy Adults. Nutrients 2022, 15, 85. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ogata, H.; Omi, N.; Nagasaka, S.; Yamaguchi, S.; Hibi, M.; Tokuyama, K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pract. 2014, 8, e201–e298. [Google Scholar] [CrossRef]
- Ogata, H.; Hatamoto, Y.; Goto, Y.; Tajiri, E.; Yoshimura, E.; Kiyono, K.; Uehara, Y.; Kawanaka, K.; Omi, N.; Tanaka, H. Association between breakfast skipping and postprandial hyperglycaemia after lunch in healthy young individuals. Br. J. Nutr. 2019, 122, 431–440. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Landau, Z.; Tsameret, S.; Wainstein, J.; Raz, I.; Ahren, B.; Chapnik, N.; Barnea, M.; Ganz, T.; Menaged, M.; et al. Reduction in Glycated Hemoglobin and Daily Insulin Dose Alongside Circadian Clock Upregulation in Patients With Type 2 Diabetes Consuming a Three-Meal Diet: A Randomized Clinical Trial. Diabetes Care 2019, 42, 2171–2180. [Google Scholar] [CrossRef]
- Williamson, E.; Moore, D.R. A Muscle-Centric Perspective on Intermittent Fasting: A Suboptimal Dietary Strategy for Supporting Muscle Protein Remodeling and Muscle Mass. Front. Nutr. 2021, 8, 640621. [Google Scholar] [CrossRef]
- Aoyama, S.; Kim, H.K.; Hirooka, R.; Tanaka, M.; Shimoda, T.; Chijiki, H.; Kojima, S.; Sasaki, K.; Takahashi, K.; Makino, S.; et al. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021, 36, 109336. [Google Scholar] [CrossRef]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014, 144, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O.; Wainstein, J.; Boaz, M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012, 77, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695. [Google Scholar] [CrossRef]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef]
- Richter, J.; Herzog, N.; Janka, S.; Baumann, T.; Kistenmacher, A.; Oltmanns, K.M. Twice as High Diet-Induced Thermogenesis After Breakfast vs Dinner On High-Calorie as Well as Low-Calorie Meals. J. Clin. Endocrinol. Metab. 2020, 105, dgz311. [Google Scholar] [CrossRef] [PubMed]
- Romon, M.; Edme, J.L.; Boulenguez, C.; Lescroart, J.L.; Frimat, P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993, 57, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Bideyan, L.; Nagari, R.; Tontonoz, P. Hepatic transcriptional responses to fasting and feeding. Genes Dev. 2021, 35, 635–657. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Q.; Pu, Y.; Guo, M.; Jiang, Z.; Huang, W.; Long, Y.; Xu, Y. Skipping breakfast is associated with overweight and obesity: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Otaki, N.; Obayashi, K.; Saeki, K.; Kitagawa, M.; Tone, N.; Kurumatani, N. Relationship between Breakfast Skipping and Obesity among Elderly: Cross-Sectional Analysis of the HEIJO-KYO Study. J. Nutr. Health Aging 2017, 21, 501–504. [Google Scholar] [CrossRef]
- Kiriyama, K.; Yamamoto, M.; Kim, D.; Sun, S.; Yamamoto, H.; Oda, H. Skipping breakfast regimen induces an increase in body weight and a decrease in muscle weight with a shifted circadian rhythm in peripheral tissues of mice. Br. J. Nutr. 2022, 128, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Siwasaranond, N.; Saetung, S.; Thakkinstian, A.; Ongphiphadhanakul, B.; Reutrakul, S. The relationship among breakfast time, morningness–eveningness preference and body mass index in Type 2 diabetes. Diabet. Med. 2018, 35, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Landau, Z.; Bar-Dayan, Y.; Froy, O. Fasting Until Noon Triggers Increased Postprandial Hyperglycemia and Impaired Insulin Response After Lunch and Dinner in Individuals With Type 2 Diabetes: A Randomized Clinical Trial. Diabetes Care 2015, 38, 1820–1826. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef]
- Hodge, B.A.; Wen, Y.; Riley, L.A.; Zhang, X.; England, J.H.; Harfmann, B.D.; Schroder, E.A.; Esser, K.A. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5, 17. [Google Scholar] [CrossRef]
- Erion, D.M.; Kotas, M.E.; McGlashon, J.; Yonemitsu, S.; Hsiao, J.J.; Nagai, Y.; Iwasaki, T.; Murray, S.F.; Bhanot, S.; Cline, G.W.; et al. cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 2 (CRTC2) promotes glucagon clearance and hepatic amino acid catabolism to regulate glucose homeostasis. J. Biol. Chem. 2013, 288, 16167–16176. [Google Scholar] [CrossRef]
- Eide, E.J.; Woolf, M.F.; Kang, H.; Woolf, P.; Hurst, W.; Camacho, F.; Vielhaber, E.L.; Giovanni, A.; Virshup, D.M. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005, 25, 2795–2807. [Google Scholar] [CrossRef]
- Takahashi, M.; Tahara, Y.; Tsubosaka, M.; Fukazawa, M.; Ozaki, M.; Iwakami, T.; Nakaoka, T.; Shibata, S. Chronotype and social jetlag influence human circadian clock gene expression. Sci. Rep. 2018, 8, 10152. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Kinouchi, K.; Welz, P.S.; Smith, J.G.; Zinna, V.M.; Shi, J.; Samad, M.; Chen, S.; Magnan, C.N.; Kinchen, J.M.; et al. Defining the Independence of the Liver Circadian Clock. Cell 2019, 177, 1448–1462.e14. [Google Scholar] [CrossRef]
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythm. 2005, 20, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 2014, 2014, 653017. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Garaulet, M. The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef]
- Basu, A.; Joshi, N.; Miles, J.; Carter, R.E.; Rizza, R.A.; Basu, R. Paradigm shifts in nocturnal glucose control in type 2 diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3801–3809. [Google Scholar] [CrossRef]
- Saad, A.; Man, C.D.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 2011, 104, 535–545. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Pinho, A.V.; Bensellam, M.; Wauters, E.; Rees, M.; Giry-Laterriere, M.; Mawson, A.; Ly, L.Q.; Biankin, A.V.; Wu, J.; Laybutt, D.R.; et al. Pancreas-specific Sirt1-deficiency in mice compromises β-cell function without development of hyperglycemia. PLoS ONE 2015, 10, e0128012. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Imai, S.I. A clock ticks in pancreatic β cells. Cell Metab. 2010, 12, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Sadacca, L.A.; Lamia, K.A.; DeLemos, A.S.; Blum, B.; Weitz, C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 2011, 54, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Hou, X.; Zhang, J.; Chen, Y.; Su, Z. Identification of insulin as a novel retinoic acid receptor-related orphan receptor α target gene. FEBS Lett. 2014, 588, 1071–1079. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Mingomataj, E.L.; Wu, W.K.; Ridout, S.A.; Brubaker, P.L. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 2014, 63, 3674–3685. [Google Scholar] [CrossRef]
- Biancolin, A.D.; Martchenko, A.; Mitova, E.; Gurges, P.; Michalchyshyn, E.; Chalmers, J.A.; Doria, A.; Mychaleckyj, J.C.; Adriaenssens, A.E.; Reimann, F.; et al. The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol. Metab. 2020, 31, 124–137. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.P.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Sato, S.; Parr, E.B.; Devlin, B.L.; Hawley, J.A.; Sassone-Corsi, P. Human metabolomics reveal daily variations under nutritional challenges specific to serum and skeletal muscle. Mol. Metab. 2018, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Gnocchi, D.; Bruscalupi, G. Circadian Rhythms and Hormonal Homeostasis: Pathophysiological Implications. Biology 2017, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nature reviews. Endocrinology 2014, 10, 466–475. [Google Scholar] [PubMed]
- Skene, D.J.; Arendt, J. Human circadian rhythms: Physiological and therapeutic relevance of light and melatonin. Ann. Clin. Biochem. 2006, 43 Pt 5, 344–353. [Google Scholar] [CrossRef]
- Gavrila, A.; Peng, C.K.; Chan, J.L.; Mietus, J.E.; Goldberger, A.L.; Mantzoros, C.S. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J. Clin. Endocrinol. Metab. 2003, 88, 2838–2843. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kadowaki, T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int. J. Obes. 2008, 32 (Suppl. S7), S13–S18. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef]
- Morgan, L.M.; Shi, J.W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Tada, Y.; Hida, A.; Sunami, A.; Yokoyama, Y.; Yasuda, J.; Nakai, A.; Togo, F.; Kawano, Y. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur. J. Appl. Physiol. 2013, 113, 2603–2611. [Google Scholar] [CrossRef]
- Zitting, K.M.; Vujovic, N.; Yuan, R.K.; Isherwood, C.M.; Medina, J.E.; Wang, W.; Buxton, O.M.; Williams, J.S.; Czeisler, C.A.; Duffy, J.F. Human Resting Energy Expenditure Varies with Circadian Phase. Curr. Biol. CB 2018, 28, 3685–3690.e3. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. S1), S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Fielding, B.; Ilic, V.; Macdonald, I.A.; Summers, L.; Frayn, K.N. Adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 2002, 51, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.; Oishi, K.; Ishida, N. CLOCK regulates circadian rhythms of hepatic glycogen synthesis through transcriptional activation of Gys2. J. Biol. Chem. 2010, 285, 22114–22121. [Google Scholar] [CrossRef] [PubMed]
- Zani, F.; Breasson, L.; Becattini, B.; Vukolic, A.; Montani, J.-P.; Albrecht, U.; Provenzani, A.; Ripperger, J.A.; Solinas, G. PER2 promotes glucose storage to liver glycogen during feeding and acute fasting by inducing Gys2 PTG and G L expression. Mol. Metab. 2013, 2, 292–305. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Jordan, S.D.; Lamia, K.A. AMPK at the crossroads of circadian clocks and metabolism. Mol. Cell. Endocrinol. 2013, 366, 163–169. [Google Scholar] [CrossRef]
- Wefers, J.; Van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; Van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef]
- Vetter, C.; Vetter, C.; Devore, E.E.; Ramin, C.A.; Speizer, F.E.; Willett, W.C.; Schernhammer, E.S. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 2015, 38, 1707–1713. [Google Scholar] [CrossRef]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef]
- Vagn Korsgaard, T.; Colding-Jørgensen, M. Time-dependent mechanisms in β-cells glucose sensing. J. Biol. Phys. 2006, 32, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Goginashvili, A.; Zhang, Z.; Erbs, E.; Spiegelhalter, C.; Kessler, P.; Mihlan, M.; Pasquier, A.; Krupina, K.; Schieber, N.; Cinque, L.; et al. Insulin granules. Insulin secretory granules control autophagy in pancreatic β cells. Science 2015, 347, 878–882. [Google Scholar] [CrossRef]
- Cherrington, A. Control of glucose uptake and release by the liver in vivo. Diabetes 1999, 48, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Taira, A.; Arita, E.; Matsumoto, E.; Oohira, A.; Iwase, K.; Hiwasa, T.; Yokote, K.; Shibata, S.; Takiguchi, M. Systemic oscillator-driven and nutrient-responsive hormonal regulation of daily expression rhythms for gluconeogenic enzyme genes in the mouse liver. Chronobiol. Int. 2019, 36, 591–615. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mendoza, M.; Rivera-Zavala, J.B.; Díaz-Muñoz, M. Daytime restricted feeding modifies the daily variations of liver gluconeogenesis: Adaptations in biochemical and endocrine regulators. Chronobiol. Int. 2014, 31, 815–828. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef]
- Menting, J.G.; Whittaker, J.; Margetts, M.B.; Whittaker, L.J.; Kong, G.K.; Smith, B.J.; Watson, C.J.; Zakova, L.; Kletvikova, E.; Jiracek, J.; et al. How insulin engages its primary binding site on the insulin receptor. Nature 2013, 493, 241–245. [Google Scholar] [CrossRef]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Haring, H.U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K.; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Lee, S.H.; Tura, A.; Mari, A.; Ko, S.H.; Kwon, H.S.; Song, K.H.; Yoon, K.H.; Lee, K.W.; Ahn, Y.B. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E984–E990. [Google Scholar] [CrossRef]
- Jovanovic, A.; Gerrard, J.; Taylor, R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009, 32, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Hatamoto, Y.; Tajiri, E.; Matsumoto, N.; Tanaka, S.; Yoshimura, E.; Hatamoto, Y.; Tajiri, E.; Matsumoto, N.; Tanaka, S.; et al. An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program. Nutrients 2022, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, controlled daytime versus delayed eating impacts weight and metabolism. Curr. Biol. 2021, 31, 650–657.e3. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.P.; McGuinness, O.P.; Buchowski, M.; Hughey, J.J.; Chen, H.; Powers, J.; Page, T.; Johnson, C.H. Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biol. 2020, 18, e3000622. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Pozefsky, T.; Marliss, E.; Cahill, G.F., Jr. Alanine: Key role in gluconeogenesis. Science 1970, 167, 1003–1004. [Google Scholar] [CrossRef]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R. The Effect of Fasting on Human Metabolism and Psychological Health. Dis. Markers 2022, 2022, 5653739. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Breakfast keeps hunger in check. Cell Metab. 2022, 34, 1420–1421. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowicz, D.; Rosenblum, R.C.; Wainstein, J.; Twito, O. Influence of Fasting until Noon (Extended Postabsorptive State) on Clock Gene mRNA Expression and Regulation of Body Weight and Glucose Metabolism. Int. J. Mol. Sci. 2023, 24, 7154. https://doi.org/10.3390/ijms24087154
Jakubowicz D, Rosenblum RC, Wainstein J, Twito O. Influence of Fasting until Noon (Extended Postabsorptive State) on Clock Gene mRNA Expression and Regulation of Body Weight and Glucose Metabolism. International Journal of Molecular Sciences. 2023; 24(8):7154. https://doi.org/10.3390/ijms24087154
Chicago/Turabian StyleJakubowicz, Daniela, Rachel Chava Rosenblum, Julio Wainstein, and Orit Twito. 2023. "Influence of Fasting until Noon (Extended Postabsorptive State) on Clock Gene mRNA Expression and Regulation of Body Weight and Glucose Metabolism" International Journal of Molecular Sciences 24, no. 8: 7154. https://doi.org/10.3390/ijms24087154
APA StyleJakubowicz, D., Rosenblum, R. C., Wainstein, J., & Twito, O. (2023). Influence of Fasting until Noon (Extended Postabsorptive State) on Clock Gene mRNA Expression and Regulation of Body Weight and Glucose Metabolism. International Journal of Molecular Sciences, 24(8), 7154. https://doi.org/10.3390/ijms24087154





