Edema after CNS Trauma: A Focus on Spinal Cord Injury
Abstract
:1. Introduction
2. Edema in the CNS
2.1. Edema Classification in the CNS
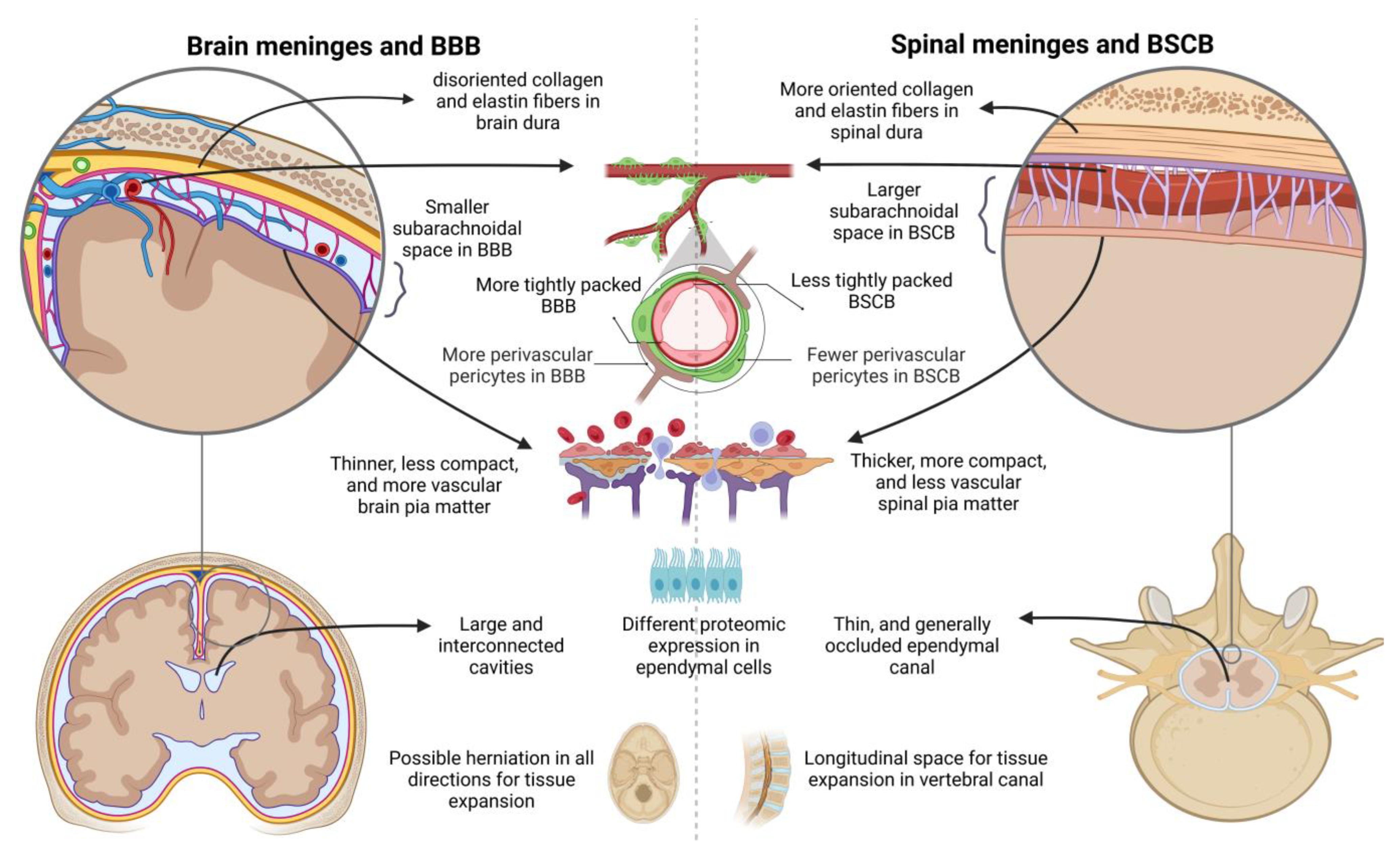
2.2. Local Particularities of Edema in the Spinal Card
3. Edema Formation after SCI: A Loop of Interconnected Mechanisms
4. Time Course of Spinal Cord Edema Formation after Injury
5. Toward a Further Treatment of Spinal Cord Edema
6. Conclusions
Funding
Conflicts of Interest
References
- Touwaide, A.; De Santo, N.G. Edema in the Corpus Hippocraticum. Am. J. Nephrol. 1999, 19, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Celsus, A.C. De Medicina. In Latin, Circa 25 A.D.; Spencer, W.G., Translator; Harvard University Press: Cambridge, MA, USA, 1938. [Google Scholar]
- Opitz, R.B. A history of the theory of edema. JAMA 1899, XXXII, 51. [Google Scholar] [CrossRef] [Green Version]
- Rose, B.D. (Ed.) Pathophysiology and Etiology of Edema; UpToDate: Wellesley, MA, USA, 2004. [Google Scholar]
- Whytt, R. Observations on the Dropsy in the Brain; John Balfour, by Balfour, Auld, & Smellie: Edinburgh, UK, 1768. [Google Scholar]
- Reichardt, M. Zur Entstehung des Hirndrucks bei Hirngeschwülsten und anderen Hirnkrankheiten und über eine hei diesen zu beobachtende besondere Art der Hirnschwellung. J. Neurol. 1905, 28, 306–355. [Google Scholar] [CrossRef]
- Badaut, J.; Plesnila, N. Introduction: Brain Edema Formation—Significance for Patient Outcome, History of Brain Edema. Brain Edema: From Molecular Mechanisms to Clinical Practice. In Brain Edema: From Molecular Mechanisms to Clinical Practice; Badaut, J., Plesnila, N., Eds.; Academic Press: Cambridge, MA, USA, 2017; p. xvii. [Google Scholar]
- Klatzo, I. Presidential Address. J. Neuropathol. Exp. Neurol. 1967, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.G.; Hansebout, R.R.; Pappius, H.M. Chemical characteristics of traumatic spinal cord edema in cats. Effects of steroids on potassium depletion. J. Neurosurg. 1974, 40, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Battey, T.W.; Karki, M.; Singhal, A.B.; Wu, O.; Sadaghiani, S.; Campbell, B.; Davis, S.M.; Donnan, G.; Sheth, K.N.; Kimberly, W.T. Brain Edema Predicts Outcome After Nonlacunar Ischemic Stroke. Stroke 2014, 45, 3643–3648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, B.; Aston, J.; Dines, M.; Caraman, E.; Yacyshyn, M.; McCarthy, M.; Olson, J.E. Early Brain Edema is a Predictor of In-Hospital Mortality in Traumatic Brain Injury. J. Emerg. Med. 2017, 53, 18–29. [Google Scholar] [CrossRef]
- Leonard, A.V.; Thornton, E.; Vink, R. The Relative Contribution of Edema and Hemorrhage to Raised Intrathecal Pressure after Traumatic Spinal Cord Injury. J. Neurotrauma 2015, 32, 397–402. [Google Scholar] [CrossRef]
- Baethmann, A.; Maier-Hauff, K.; Schürer, L.; Lange, M.; Guggenbichler, C.; Vogt, W.; Jacob, K.; Kempski, O. Release of glutamate and of free fatty acids in vasogenic brain edema. J. Neurosurg. 1989, 70, 578–591. [Google Scholar] [CrossRef] [Green Version]
- Lok, J.; Wang, X.-S.; Xing, C.-H.; Maki, T.-K.; Wu, L.-M.; Guo, S.-Z.; Noviski, N.; Arai, K.; Whalen, M.J.; Lo, E.H.; et al. Targeting the Neurovascular Unit in Brain Trauma. CNS Neurosci. Ther. 2014, 21, 304–308. [Google Scholar] [CrossRef]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology 2018, 145, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, I.; Iwasaki, Y.; Isu, T.; Akino, M.; Abe, H. Significance of spinal cord swelling in the prognosis of acute cervical spinal cord injury. Spinal Cord 1989, 27, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Miyanji, F.; Furlan, J.C.; Aarabi, B.; Arnold, P.M.; Fehlings, M.G.; Pierce, J.L.; Donahue, J.H.; Nacey, N.C.; Quirk, C.R.; Perry, M.T.; et al. Acute Cervical Traumatic Spinal Cord Injury: MR Imaging Findings Correlated with Neurologic Outcome—Prospective Study with 100 Consecutive Patients. Radiology 2007, 243, 820–827. [Google Scholar] [CrossRef]
- Parashari, U.C.; Khanduri, S.; Bhadury, S.; Kohli, N.; Parihar, A.; Singh, R.; Srivastava, R.; Upadhyay, D. Diagnostic and prognostic role of MRI in spinal trauma, its comparison and correlation with clinical profile and neurological outcome, according to ASIA impairment scale. J. Craniovertebr. Junction Spine 2011, 2, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zusman, B.E.; Kochanek, P.M.; Jha, R.M. Cerebral Edema in Traumatic Brain Injury: A Historical Framework for Current Therapy. Curr. Treat. Options Neurol. 2020, 22, 1–28. [Google Scholar] [CrossRef]
- Badaut, J.; Plesnila, N. (Eds.) Brain Edema: From Molecular Mechanisms to Clinical Practice; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Klatzo, I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987, 72, 236–239. [Google Scholar] [CrossRef]
- Miller, J.D. The management of cerebral oedema. Br. J. Hosp. Med. 1979, 2, 152–166. [Google Scholar]
- Bell, B.A. A History of the Study of Cerebral Edema. Neurosurgery 1983, 13, 724–728. [Google Scholar] [CrossRef]
- Milhorat, T.H. Classification of the cerebral edemas with reference to hydrocephalus and pseudotumor cerebri. Child’s Nerv. Syst. 1992, 8, 301–306. [Google Scholar] [CrossRef]
- Fishman, R.A. Brain Edema. N. Engl. J. Med. 1975, 293, 706–711. [Google Scholar] [CrossRef]
- Marmarou, A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 2007, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iencean, S. Brain edema—A new classification. Med. Hypotheses 2003, 61, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Constantini, S. Ionic and water shifts in injured central nervous tissue. In The Neuro-Biology of Central Nervous System Trauma; Salzman, S.K., Faden, A.I., Eds.; Oxford University Press: New York, NY, USA, 1994; pp. 123–130. [Google Scholar]
- Rungta, R.; Choi, H.B.; Tyson, J.R.; Malik, A.; Dissing-Olesen, L.; Lin, P.J.; Cain, S.M.; Cullis, P.R.; Snutch, T.P.; MacVicar, B.A. The Cellular Mechanisms of Neuronal Swelling Underlying Cytotoxic Edema. Cell 2015, 161, 610–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimelberg, H.K. Current concepts of brain edema. J. Neurosurg. 1995, 83, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Kent, T.A.; Chen, M.; Tarasov, K.V.; Gerzanich, V. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol. 2007, 6, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Qin, Z.; Yin, X. Classification mechanism and clinical analysis of perihematomal edema in intrac-erebral hemorrhage. Brain Hemorrhages 2020, 1, 141–145. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.; Daud, M.F.; Idris, J.; Ng, A.; Naicker, A.S.; Ismail, O.; Kumar, R.A.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Pan, Q.-L.; Lin, F.-X.; Liu, N.; Chen, R.-C. The role of aquaporin 4 (AQP4) in spinal cord injury. Biomed. Pharmacother. 2021, 145, 112384. [Google Scholar] [CrossRef]
- Obenaus, A.; Badaut, J. Role of the non-invasive imaging techniques in monitoring and understanding the evolution of brain edema. J. Neurosci. Res. 2021, 100, 1191–1200. [Google Scholar] [CrossRef]
- Yang, C.; He, T.; Wang, Q.; Wang, G.; Ma, J.; Chen, Z.; Li, Q.; Wang, L.; Quan, Z. Elevated intraspinal pressure drives edema progression after acute compression spinal cord injury in rabbits. Exp. Neurol. 2022, 357, 114206. [Google Scholar] [CrossRef]
- Leinonen, V.; Vanninen, R.; Rauramaa, T. Raised intracranial pressure and brain edema. Handb. Clin. Neurol. 2018, 145, 25–37. [Google Scholar] [CrossRef]
- Chen, S.; Gallagher, M.J.; Papadopoulos, M.C.; Saadoun, S. Non-linear Dynamical Analysis of Intraspinal Pressure Signal Predicts Outcome After Spinal Cord Injury. Front. Neurol. 2018, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S. Pathophysiology of the blood—Spinal cord barrier in traumatic injury. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: Cambridge, MA, USA, 2004; pp. 437–518. [Google Scholar] [CrossRef]
- Tomko, P.; Farkaš, D.; Čížková, D.; Vanický, I. Longitudinal enlargement of the lesion after spinal cord injury in the rat: A consequence of malignant edema? Spinal Cord 2016, 55, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Leypold, B.; Flanders, A.; Burns, A. The Early Evolution of Spinal Cord Lesions on MR Imaging following Traumatic Spinal Cord Injury. Am. J. Neuroradiol. 2008, 29, 1012–1016. [Google Scholar] [CrossRef] [Green Version]
- Saadoun, S.; Papadopoulos, M. Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010, 168, 1036–1046. [Google Scholar] [CrossRef]
- Grassner, L.; Grillhösl, A.; Griessenauer, C.J.; Thomé, C.; Bühren, V.; Strowitzki, M.; Winkler, P.A. Spinal Meninges and Their Role in Spinal Cord Injury: A Neuroanatomical Review. J. Neurotrauma 2018, 35, 403–410. [Google Scholar] [CrossRef]
- Maikos, J.T.; Qian, Z.; Metaxas, D.; Shreiber, D.I.; Lucas, E.; Whyte, T.; Liu, J.; Russell, C.; Tetzlaff, W.; Cripton, P.A.; et al. Finite Element Analysis of Spinal Cord Injury in the Rat. J. Neurotrauma 2008, 25, 795–816. [Google Scholar] [CrossRef]
- Maikos, J.T.; Elias, R.A.; Shreiber, D. Mechanical Properties of Dura Mater from the Rat Brain and Spinal Cord. J. Neurotrauma 2008, 25, 38–51. [Google Scholar] [CrossRef]
- Sakka, L.; Gabrillargues, J.; Coll, G. Anatomy of the Spinal Meninges. Open Neurosurg. 2016, 12, 168–188. [Google Scholar] [CrossRef]
- Chopra, N.; Menounos, S.; Choi, J.P.; Hansbro, P.M.; Diwan, A.D.; Das, A. Blood-Spinal Cord Barrier: Its Role in Spinal Disorders and Emerging Therapeutic Strategies. NeuroSci 2021, 3, 1–27. [Google Scholar] [CrossRef]
- Winkler, E.A.; Sengillo, J.D.; Bell, R.D.; Wang, J.; Zlokovic, B.V. Blood–Spinal Cord Barrier Pericyte Reductions Contribute to Increased Capillary Permeability. J. Cereb. Blood Flow Metab. 2012, 32, 1841–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prockop, L.D.; Naidu, K.A.; Binard, J.E.; Ransohoff, J. Selective Permeability of [3H]-D-Mannitol and [l4C]-Carboxyl-lnulin Across the Blood-Brain Barrier and Blood-Spinal Cord Barrier in the Rabbit. J. Spinal Cord Med. 1995, 18, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and Clinical Implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Schnell, L.; Fearn, S.; Klassen, H.; Schwab, M.E.; Perry, V.H. Acute inflammatory responses to mechanical lesions in the CNS: Differences between brain and spinal cord. Eur. J. Neurosci. 1999, 11, 3648–3658. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Olivas, A.D.; Noble-Haeusslein, L.J. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin. Neurosci. Res. 2006, 6, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Rust, R.; Kaiser, J. Insights into the Dual Role of Inflammation after Spinal Cord Injury. J. Neurosci. 2017, 37, 4658–4660. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.; Lu, B.; Caron, M.; Caporicci-Dinucci, N.; Hatrock, D.; Petrecca, K.; Bourque, G.; Stratton, J.A. Single Cell Transcriptomics of Ependymal Cells Across Age, Region and Species Reveals Cilia-Related and Metal Ion Regulatory Roles as Major Conserved Ependymal Cell Functions. Front. Cell. Neurosci. 2021, 15, 703951. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef] [Green Version]
- Winkler, E.A.; Minter, D.; Yue, J.; Manley, G.T. Cerebral Edema in Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 473–488. [Google Scholar] [CrossRef]
- Wichmann, T.O.; Kasch, H.; Dyrskog, S.; Høy, K.; Møller, B.K.; Krog, J.; Hviid, C.V.B.; Hoffmann, H.J.; Rasmussen, M.M. The inflammatory response and blood-spinal cord barrier integrity in traumatic spinal cord injury: A prospective pilot study. Acta Neurochir. 2022, 164, 3143–3153. [Google Scholar] [CrossRef]
- Lee, K.; Na, W.; Lee, J.Y.; Na, J.; Cho, H.; Wu, H.; Yune, T.Y.; Kim, W.-S.; Ju, B.-G. Molecular mechanism of Jmjd3-mediated interleukin-6 gene regulation in endothelial cells underlying spinal cord injury. J. Neurochem. 2012, 122, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, M.; Ma, X.; Feng, H.; Song, J.; Lv, C.; He, Y. Inhibition of HMGB1 reduces rat spinal cord astrocytic swelling and AQP4 expression after oxygen-glucose deprivation and reoxygenation via TLR4 and NF-κB signaling in an IL-6-dependent manner. J. Neuroinflamm. 2017, 14, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.S.; Vannemreddy, P.; Patnaik, R.; Mohanty, S. Histamine receptors influence blood-spinal cord barrier permeability, edema formation, and spinal cord blood flow following trauma to the rat spinal cord. Acta Neurochir. 2006, 96, 316–321. [Google Scholar] [CrossRef]
- Conner, A.C.; Bill, R.M.; Conner, M.T. An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol. Membr. Biol. 2012, 30, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.M.; Lee, H.; Clark, Z.; Miranpuri, G.S.; Nacht, C.; Patel, K.; Liu, L.; Joslin, J.; Kintner, D.; Resnick, D.K. Pathogenesis of spinal cord injury induced edema and neuropathic pain: Expression of multiple isoforms of wnk1. Ann. Neurosci. 2014, 21, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Simard, J.M.; Chen, M.; Tarasov, K.V.; Bhatta, S.; Ivanova, S.; Melnitchenko, L.; Tsymbalyuk, N.; West, G.A.; Gerzanich, V. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006, 12, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.D.; Gerzanich, V.; Geng, Z.; Simard, J.M. Glibenclamide Reduces Hippocampal Injury and Preserves Rapid Spatial Learning in a Model of Traumatic Brain Injury. J. Neuropathol. Exp. Neurol. 2010, 69, 1177–1190. [Google Scholar] [CrossRef] [Green Version]
- Simard, J.M.; Kilbourne, M.; Tsymbalyuk, O.; Tosun, C.; Caridi, J.; Ivanova, S.; Keledjian, K.; Bochicchio, G.; Gerzanich, V. Key Role of Sulfonylurea Receptor 1 in Progressive Secondary Hemorrhage after Brain Contusion. J. Neurotrauma 2009, 26, 2257–2267. [Google Scholar] [CrossRef]
- Halsey, A.M.; Conner, A.C.; Bill, R.M.; Logan, A.; Ahmed, Z. Aquaporins and Their Regulation after Spinal Cord Injury. Cells 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Törnroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2021, 145, 64–75. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Wang, X.; Li, W.; Chen, J.; Sun, H. Pretreatment with AQP4 and NKCC1 Inhibitors Concurrently Attenuated Spinal Cord Edema and Tissue Damage after Spinal Cord Injury in Rats. Front. Physiol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Deng, J.; Xu, X.; Cao, F.; Lu, K.; Li, D.; Cheng, X.; Wang, X.; Zhao, Y. Aquaporin-4 deletion ameliorates hypoglycemia-induced BBB permeability by inhibiting inflammatory responses. J. Neuroinflamm. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mao, J.; Wang, T.; Fu, X. Downregulation of Aquaporin-4 Protects Brain Against Hypoxia Ischemia via Anti-inflammatory Mechanism. Mol. Neurobiol. 2016, 54, 6426–6435. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.-N.; Zhou, X.-Y.; Zhang, L.-X.; Chen, G.-X.; Wang, T.-H.; Xia, Q.-J.; Liang, N.; Zhang, X. The Dual Role of AQP4 in Cytotoxic and Vasogenic Edema Following Spinal Cord Contusion and Its Possible Association With Energy Metabolism via COX5A. Front. Neurosci. 2019, 13, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; Macdonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799.e19. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef]
- Tourdias, T.; Mori, N.; Dragonu, I.; Cassagno, N.; Boiziau, C.; Aussudre, J.; Brochet, B.; Moonen, C.; Petry, K.G.; Dousset, V. Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J. Neuroinflamm. 2011, 8, 143. [Google Scholar] [CrossRef]
- Fukuda, A.; Pop, V.; Spagnoli, D.; Ashwal, S.; Obenaus, A.; Badaut, J. Delayed increase of astrocytic aquaporin 4 after juvenile traumatic brain injury: Possible role in edema resolution? Neuroscience 2012, 222, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Hsu, M.; Seldin, M.; Verkman, A.S.; Scharfman, H.E.; Binder, D.K. Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann. Neurol. 2010, 67, 794–801. [Google Scholar] [CrossRef]
- Thrane, A.S.; Rappold, P.M.; Fujita, T.; Torres, A.; Bekar, L.K.; Takano, T.; Peng, W.; Wang, F.; Thrane, V.R.; Enger, R.; et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca 2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. USA 2010, 108, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.-N.; Sun, X.-L.; Gao, L.; Fan, Y.; Ding, J.-H.; Hu, G. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol. Cell Neurosci. 2007, 34, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Illarionova, N.; Gunnarson, E.; Li, Y.; Brismar, H.; Bondar, A.; Zelenin, S.; Aperia, A. Functional and molecular interactions between aquaporins and Na,K-ATPase. Neuroscience 2010, 168, 915–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, G.-H.; Baethmann, A.; Kempski, O. Mechanisms of glial swelling induced by glutamate. Can. J. Physiol. Pharmacol. 1992, 70, S334–S343. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Olsson, Y.; Dey, P. Early accumulation of serotonin in rat spinal cord subjected to traumatic injury. Relation to edema and blood flow changes. Neuroscience 1990, 36, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Collard, C.D.; Park, K.A.; Montalto, M.C.; Alapati, S.; Buras, J.A.; Stahl, G.L.; Colgan, S.P. Neutrophil-derived Glutamate Regulates Vascular Endothelial Barrier Function. J. Biol. Chem. 2002, 277, 14801–14811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [Green Version]
- Sharif-Alhoseini, M.; Khormali, M.; Rezaei, M.; Safdarian, M.; Hajighadery, A.; Khalatbari, M.M.; Meknatkhah, S.; Rezvan, M.; Chalangari, M.; Derakhshan, P.; et al. Animal models of spinal cord injury: A systematic review. Spinal Cord 2017, 55, 714–721. [Google Scholar] [CrossRef]
- Masterman, E.; Ahmed, Z. Experimental Treatments for Oedema in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Cells 2021, 10, 2682. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathogenesis of Brain Edema and Investigation into Anti-Edema Drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Nakamura, T.; Kishigami, Y.; Endo, K.; Azuma, T.; Fujikawa, T.; Tsutsumi, S.; Shimizu, Y. New canine spinal cord injury model free from laminectomy. Brain Res. Protoc. 2005, 14, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-H.; Quan, Z.-X.; Wang, Q.; Wang, G.-J.; He, T.; Chen, Z.-Y.; Li, Q.-C.; Yang, J. Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target. Neural Regen. Res. 2022, 17, 1703. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.C.; Noonan, V.K.; Cadotte, D.W.; Fehlings, M.G.; Speidel, J.; Mattucci, S.; Liu, J.; Kwon, B.K.; Tetzlaff, W.; Oxland, T.R.; et al. Timing of Decompressive Surgery of Spinal Cord after Traumatic Spinal Cord Injury: An Evidence-Based Examination of Pre-Clinical and Clinical Studies. J. Neurotrauma 2011, 28, 1371–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarabi, B.; Olexa, J.; Chryssikos, T.; Galvagno, S.M.; Hersh, D.S.; Wessell, A.; Sansur, C.; Schwartzbauer, G.; Crandall, K.; Shanmuganathan, K.; et al. Extent of Spinal Cord Decompression in Motor Complete (American Spinal Injury Association Impairment Scale Grades A and B) Traumatic Spinal Cord Injury Patients: Post-Operative Magnetic Resonance Imaging Analysis of Standard Operative Approaches. J. Neurotrauma 2019, 36, 862–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badhiwala, J.H.; Wilson, J.R.; Witiw, C.D.; Harrop, J.S.; Vaccaro, A.R.; Aarabi, B.; Grossman, R.G.; Geisler, F.H.; Fehlings, M.G. The influence of timing of surgical decompression for acute spinal cord injury: A pooled analysis of individual patient data. Lancet Neurol. 2020, 20, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.C.; Stewart, W.B. Effect of trauma dose on spinal cord edema. J. Neurosurg. 1981, 54, 802–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, M.; Feigin, I. Cerebral oedema and the water content of normal white matter. J. Neurol. Neurosurg. Psychiatry 1966, 29, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Faas, F.H.; Ommaya, A.K. Brain Tissue Electrolytes and Water Content in Experimental Concussion in the Monkey. J. Neurosurg. 1968, 28, 137–144. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Brain Water Content: A Misunderstood Measurement? Transl. Stroke Res. 2012, 3, 263–265. [Google Scholar] [CrossRef] [Green Version]
- Yashon, D.; Bingham, W.G.; Faddoul, E.M.; Hunt, W.E. Edema of the spinal cord following experimental impact trauma. J. Neurosurg. 1973, 38, 693–697. [Google Scholar] [CrossRef]
- Lemke, M.; Demediuk, P.; McIntosh, T.; Vink, R.; Faden, A. Alterations in tissue Mg++, Na+ and spinal cord edema following impact trauma in rats. Biochem. Biophys. Res. Commun. 1987, 147, 1170–1175. [Google Scholar] [CrossRef]
- Demediuk, P.; Lemke, M.; Faden, A.I. Spinal cord edema and changes in tissue content of Na+, K+, and Mg2+ after impact trauma in rats. Adv. Neurol. 1990, 52, 225–232. [Google Scholar] [PubMed]
- Lemke, M.; Faden, A.I. Edema Development and Ion Changes in Rat Spinal Cord After Impact Trauma: Injury Dose—Response Studies. J. Neurotrauma 1990, 7, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Liu, M.; Moriyama, M.; Ramakrishnan, R.; Forbush, B.; Reddy, P.V.B.; Norenberg, M.D. Na-K-Cl Cotransporter-1 in the Mechanism of Ammonia-induced Astrocyte Swelling. J. Biol. Chem. 2008, 283, 33874–33882. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, A.R.; Norenberg, M.D. The Na-K-Cl Co-transporter in astrocyte swelling. Metab. Brain Dis. 2010, 25, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, I.R. Vasogenic edema following acute and chronic spinal cord compression in the dog. J. Neurosurg. 1975, 42, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, C.; Yonan, J.; Batarseh, R.; Chaar, R.; Jonak, C.R.; Ge, S.; Binder, D.; Rodgers, V.G.J. Implantable Osmotic Transport Device Can Reduce Edema After Severe Contusion Spinal Cord Injury. Front. Bioeng. Biotechnol. 2020, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Weirich, S.D.; Cotler, H.B.; Narayana, P.A.; Hazle, J.D.; Jackson, E.F.; Coupe, K.J.; Mcdonald, C.L.; Langford, L.A.; Harris, J.H. Histopathologic Correlation of Magnetic Resonance Imaging Signal Patterns in a Spinal Cord Injury Model. Spine 1990, 15, 630–638. [Google Scholar] [CrossRef]
- Bilgen, M.; Abbe, R.; Liu, S.J.; Narayana, P.A. Spatial and temporal evolution of hemorrhage in the hyperacute phase of experimental spinal cord injury: In vivo magnetic resonance imaging. Magn. Reson. Med. 2000, 43, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-L.; Li, J.-J.; Zhang, X.; Liu, C.-B.; Yang, D.-G.; Qin, C.; Dong, X.-C.; Li, D.-P.; Zhang, C.; Guo, Y.; et al. Dynamic changes in intramedullary pressure 72 hours after spinal cord injury. Neural Regen. Res. 2019, 14, 886–895. [Google Scholar] [CrossRef]
- Kahle, K.T.; Simard, J.M.; Staley, K.J.; Nahed, B.V.; Jones, P.S.; Sun, D. Molecular Mechanisms of Ischemic Cerebral Edema: Role of Electroneutral Ion Transport. Physiology 2009, 24, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7, 84S–94S. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Park, C.; Zhang, H.; Rahimpour, S.; Murphy, K.R.; Goodwin, C.R.; Karikari, I.O.; Than, K.D.; Shaffrey, C.I.; Foster, N.; et al. Management of Acute Traumatic Spinal Cord Injury: A Review of the Literature. Front. Surg. 2021, 8, 698736. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. Management of Spine Injury Clinical Practice Guidelines; ACS; American College of Surgeons: Chicago, IL, USA, 2022. [Google Scholar]
- Lietke, S.; Zausinger, S.; Patzig, M.; Holtmanspötter, M.; Kunz, M. CT-Based Classification of Acute Cerebral Edema: Association with Intracranial Pressure and Outcome. J. Neuroimag. 2020, 30, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.S.; Mangan, J.J.; Galetta, M.S.; Boody, B.; Bronson, W.; Segar, A.; Kepler, C.K.; Kurd, M.F.; Hilibrand, A.S.; Vaccaro, A.R.; et al. Update on Spinal Cord Injury Management. Clin. Spine Surg. Spine Publ. 2020, 33, 258–264. [Google Scholar] [CrossRef]
- Gadot, R.; Smith, D.N.; Prablek, M.; Grochmal, J.K.; Fuentes, A.; Ropper, A.E. Established and Emerging Therapies in Acute Spinal Cord Injury. Neurospine 2022, 19, 283–296. [Google Scholar] [CrossRef]
- Xu, D.; Yang, L.; Li, Y.; Sun, Y. Clinical study of ganglioside (GM) combined with methylprednisolone (MP) for early acute spinal injury. Pak. J. Pharm. Sci. 2015, 28, 701–704. [Google Scholar]
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; Hurlbert, R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135, 1224–1236. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, N.; Nakashima, H.; Fehlings, M.G. Riluzole as a Neuroprotective Drug for Spinal Cord Injury: From Bench to Bedside. Molecules 2015, 20, 7775–7789. [Google Scholar] [CrossRef]
- Nguyen, A.; Chow, D.S.; Wu, L.; Teng, Y.A.; Sarkar, M.; Toups, E.G. Longitudinal impact of acute spinal cord injury on clinical pharmacokinetics of riluzole, a potential neuroprotective agent. J. Clin. Pharmacol. 2021, 61, 1232–1242. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.L.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord 2015, 54, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Derakhshanrad, N.; Saberi, H.; Yekaninejad, M.S.; Joghataei, M.T.; Sheikhrezaei, A. Granulocyte-colony stimulating factor administration for neurological improvement in patients with postrehabilitation chronic incomplete traumatic spinal cord injuries: A double-blind randomized controlled clinical trial. J. Neurosurg. Spine 2018, 29, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Hanaoka, H.; Fujii, Y.; Hanawa, M.; Kawasaki, Y.; Ozawa, Y.; Fujiwara, T.; Furuya, T.; Ijima, Y.; Saito, J.; et al. Randomized trial of granulocyte colony-stimulating factor for spinal cord injury. Brain 2021, 144, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, Z.; Zhang, Q.; Dai, J.; Kong, J.; Fan, Z.; Li, G. Inhibition of ERK1/2 phosphorylation attenuates spinal cord injury induced astrocyte activation and inflammation through negatively regulating aquaporin-4 in rats. Brain Res. Bull. 2021, 170, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Feng, L.; Muresanu, D.F.; Castellani, R.J.; Sharma, A. Neuroprotective effects of a potent bradykinin B2 receptor antagonist HOE-140 on microvascular permeability, blood flow disturbances, edema formation, cell injury and nitric oxide synthase upregulation following trauma to the spinal cord. Int. Rev. Neurobiol. 2019, 146, 103–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yang, J.; Liu, Y.; Zhou, D.; Hou, M.; Xiang, L. Anti-edema effect of melatonin on spinal cord injury in rats. Biomed. Pap. 2015, 159, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Feng, L.; Muresanu, D.F.; Huang, H.; Menon, P.K.; Sahib, S.; Tian, Z.R.; Lafuente, J.V.; Buzoianu, A.D.; Castellani, R.J.; et al. Topical application of CNTF, GDNF and BDNF in combination attenuates blood-spinal cord barrier permeability, edema formation, hemeoxygenase-2 upregulation, and cord pathology. Prog. Brain Res. 2021, 266, 357–376. [Google Scholar] [CrossRef]
- Li, J.; Jia, Z.; Xu, W.; Guo, W.; Zhang, M.; Bi, J.; Cao, Y.; Fan, Z.; Li, G. TGN-020 alleviates edema and inhibits astrocyte activation and glial scar formation after spinal cord compression injury in rats. Life Sci. 2019, 222, 148–157. [Google Scholar] [CrossRef]
- Cai, J.; Kong, J.; Ma, S.; Ban, Y.; Li, J.; Fan, Z. Upregulation of TRPC6 inhibits astrocyte activation and proliferation after spinal cord injury in rats by suppressing AQP4 expression. Brain Res. Bull. 2022, 190, 12–21. [Google Scholar] [CrossRef]
- Hashemizadeh, S.; Gharaylou, Z.; Hosseindoost, S.; Sardari, M.; Omidi, A.; Ravandi, H.H.; Hadjighassem, M. Long-term administration of bumetanide improve functional recovery after spinal cord injury in rats. Front. Pharmacol. 2022, 13, 932487. [Google Scholar] [CrossRef]
- Li, H.-X.; Cui, J.; Fan, J.-S.; Tong, J.-Z. An observation of the clinical efficacy of combining Riluzole with mannitol and hyperbaric oxygen in treating acute spinal cord injury. Pak. J. Med. Sci. 2021, 37, 320–324. [Google Scholar] [CrossRef]

| Basis of Classification | Edema Categories | Description |
|---|---|---|
| Morpho-histology | Edema | Molt and soft tissue |
| Swelling 1 | Dry and tough tissue | |
| Origins and localizations | Cytotoxic | Intracellular water content |
| Vasogenic | Extracellular water content | |
| Etiologies | Cytotoxic | Cell pumps dysfunction |
| Vasogenic | Disruption of blood barrier | |
| Hydrostatic | Vessel wall imbalance | |
| Interstitial | Ependymal imbalance | |
| Hypoosmotic | Low plasma osmolality | |
| Mechanisms | Cytotoxic Vasogenic Ionic | Cell pumps disfunction Disruption of blood barrier Through trans-endothelium |
| Interdependency | Complex conditions | Combination of nested and interdependent mechanisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seblani, M.; Decherchi, P.; Brezun, J.-M. Edema after CNS Trauma: A Focus on Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 7159. https://doi.org/10.3390/ijms24087159
Seblani M, Decherchi P, Brezun J-M. Edema after CNS Trauma: A Focus on Spinal Cord Injury. International Journal of Molecular Sciences. 2023; 24(8):7159. https://doi.org/10.3390/ijms24087159
Chicago/Turabian StyleSeblani, Mostafa, Patrick Decherchi, and Jean-Michel Brezun. 2023. "Edema after CNS Trauma: A Focus on Spinal Cord Injury" International Journal of Molecular Sciences 24, no. 8: 7159. https://doi.org/10.3390/ijms24087159
APA StyleSeblani, M., Decherchi, P., & Brezun, J. -M. (2023). Edema after CNS Trauma: A Focus on Spinal Cord Injury. International Journal of Molecular Sciences, 24(8), 7159. https://doi.org/10.3390/ijms24087159









