Abstract
Despite its increasing application in pursing potential ligands, the capacity of receptor affinity chromatography is greatly challenged as most current research studies lack a comprehensive characterization of the ligand–receptor interaction, particularly when simultaneously determining their binding thermodynamics and kinetics. This work developed an immobilized M3 muscarinic receptor (M3R) affinity column by fixing M3R on amino polystyrene microspheres via the interaction of a 6-chlorohexanoic acid linker with haloalkane dehalogenase. The efficiency of the immobilized M3R was tested by characterizing the binding thermodynamics and kinetics of three known drugs to immobilized M3R using a frontal analysis and the peak profiling method, as well as by analyzing the bioactive compounds in Daturae Flos (DF) extract. The data showed that the immobilized M3R demonstrated good specificity, stability, and competence for analyzing drug–protein interactions. The association constants of (−)-scopolamine hydrochloride, atropine sulfate, and pilocarpine to M3R were determined to be (2.39 ± 0.03) × 104, (3.71 ± 0.03) × 104, and (2.73 ± 0.04) × 104 M−1, respectively, with dissociation rate constants of 27.47 ± 0.65, 14.28 ± 0.17, and 10.70 ± 0.35 min−1, respectively. Hyoscyamine and scopolamine were verified as the bioactive compounds that bind to M3R in the DF extract. Our results suggest that the immobilized M3R method was capable of determining drug–protein binding parameters and probing specific ligands in a natural plant, thus enhancing the effectiveness of receptor affinity chromatography in diverse stages of drug discovery.
1. Introduction
A receptor is a functional protein that mediates signal transduction in cells. After a drug enters the body, it forms a stable complex with a receptor through affinity and then initiates the downstream signal transduction pathway to exert its efficacy. The binding of a ligand to a receptor is a molecular recognition process that relies on van der Waals forces, hydrogen bonds, and/or ionic bonds. The recognition behavior of a receptor to a drug is important for elucidating the mechanism of the action of drugs, discovering new drug targets, guiding clinical drug use, and developing innovative drugs [1,2].
Based on the theory of receptors, the study of a drug and its target receptors in vitro plays a key role in the assessment of pharmacodynamics. The measurement of drug–receptor associations can reveal the relevant coupling principle, afford a valuable theory for drug development [3]. Similarly, the binding interactions provide avenues for assays of bioactive compounds from complex matrices because of the specificity of a receptor to its ligands [4,5]. Thus, technologies that enable the rapid detection of drug–receptor associations and bioactive components in a complex mixture have caught the attention of chemists, biologists, and pharmacists.
Several modern techniques have been used to examine drug–protein interactions, including surface plasmon resonance [6], graph neural networks [7], multispectroscopic analysis [8], molecular docking and molecular dynamics simulations [9,10], and chromatographic methods [11]. Among these methodologies, quantitative affinity chromatography is extremely powerful. This method combines chromatographic separation and the specific binding capacity of the receptor protein [11,12], the advantages of which not only provide good specificity but also address the principle of drug–receptor interactions efficiently [13].
Despite the considerable advancement of G-protein-coupled receptor ligand research for drug discovery, the development of a muscarinic acetylcholine receptor (mAChR) binding system remains a challenge. There are five subisoforms of mAChRs (M1–M5) that cause differential physiological reactions [14,15]. Among them, the M3 muscarinic receptor (M3R) has been found to be expressed in the smooth muscle, bronchus, brain, and glands [16,17]. Such a widespread distribution makes this receptor a wonderful therapeutic target against multiple disorders, such as stomachache, asthma, and ulcerative colitis [18]. Thus far, the ligands of M3R have mostly been developed as synthetic orthostatic antagonists, such as scopolamine, atropine, and tiotropium [19]. They can bind to the corresponding receptor via sites of the endogenous neurotransmitter acetylcholine, consequently prohibiting the subsequent signaling of acetylcholine that causes certain disorders [20]. Although these drugs are widely employed in practice, the development of selective M3R antagonists is still limited due to the lack of a highly selective way to design or assay the ligands for the receptor.
In this study, we constructed a haloalkane dehalogenase (Halo)-tagged M3R in Escherichia coli. The immobilized Halo-tagged M3R was achieved by mixing cellular lysates and 6-chlorocaproic-acid-modified amino microspheres. The efficiency of the stable-phase labeling with the immobilized Halo-tagged M3R was examined via a receptor–drug association analysis by detecting the binding parameters of three known drugs to the receptor. Additionally, the active ingredients that couple to M3R in Daturae Flos (DF) with high specificity were identified. The results indicated that the immobilized M3R can be used to estimate drug–receptor associations and to determine active ingredients in a natural plant, demonstrating high repeatability, good stability, and precision.
2. Results and Discussion
2.1. Expression of Halo-Tagged M3R
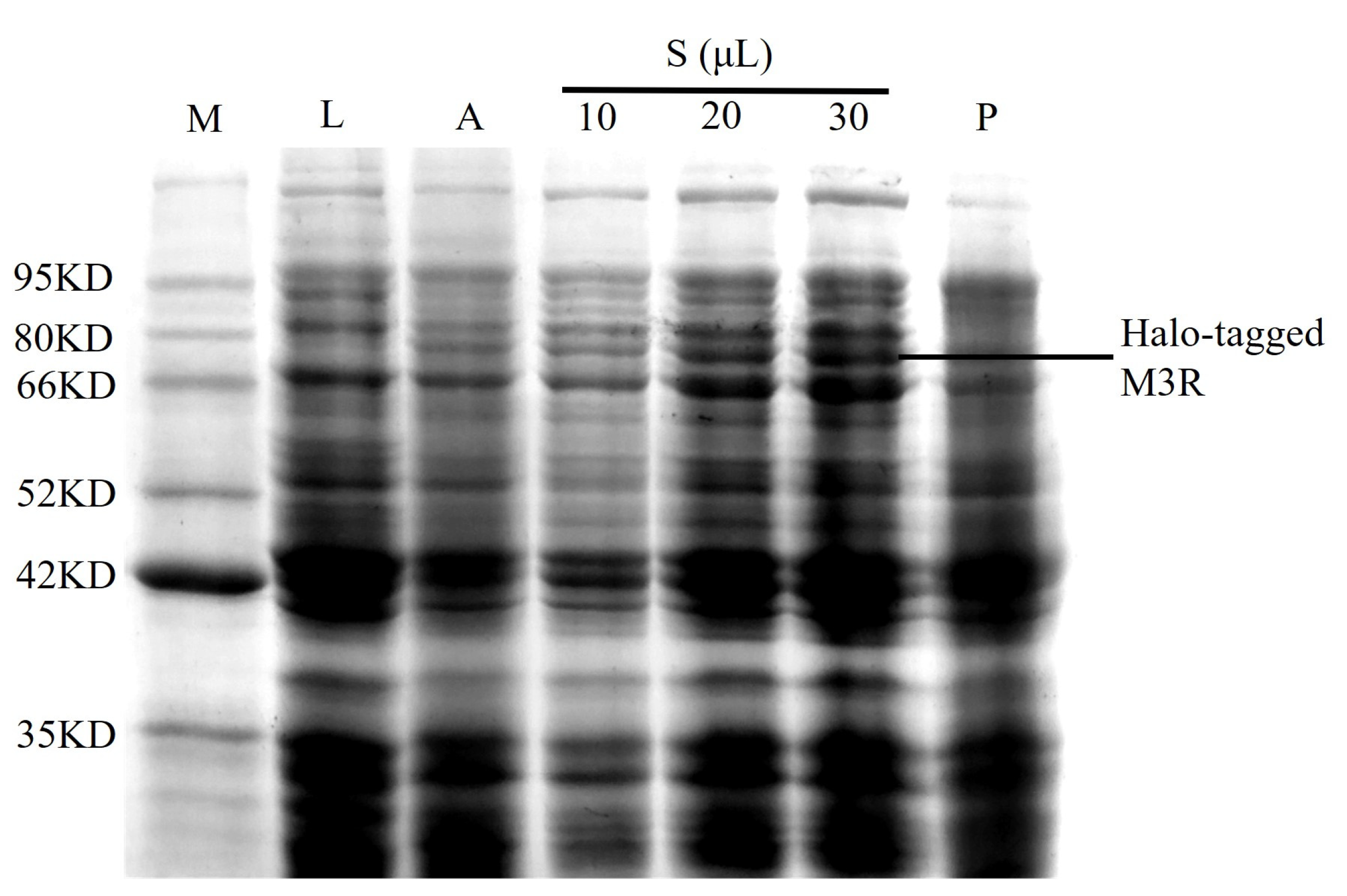
The M3R expression in E. coli lysates was analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis. A sharp band (73.4 kDa) was observed between 66.0 and 80.0 kDa in the autoinduction media sample; however, it was not found in the negative control lysate (Figure 1). Based on the theoretical calculations of M3R (40.4 kDa) and the Halo-tag (33.0 kDa), we considered that this band was the expressed Halo-tagged M3R. in addition to the increase of loaded sample from 10 μL to 30 μL, the band gradually became stronger. Such bands appeared mainly in the supernatant rather than the precipitate, implying that the expressed receptor is soluble because it mainly appeared in the aqueous supernatant but not in the sedimentary fraction.
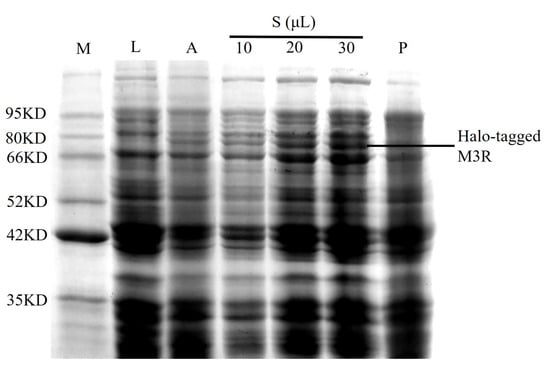
Figure 1.
Identification of M3R in E. coli expressing M3R. Protein marker (M), 10 μL of Luria-Bertani medium (L), 10 μL of autoinduction medium (A), 10 μL, 20 μL, and 30 μL of cell lysate supernatant (S), and 10 μL of cell lysate precipitate (P) were loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel and stained with Coomassie blue. The corresponding molecular weights of the protein marker are indicated on the left side of the gel.
2.2. Specificity and Stability Assessments of the M3R-Immobilized Column
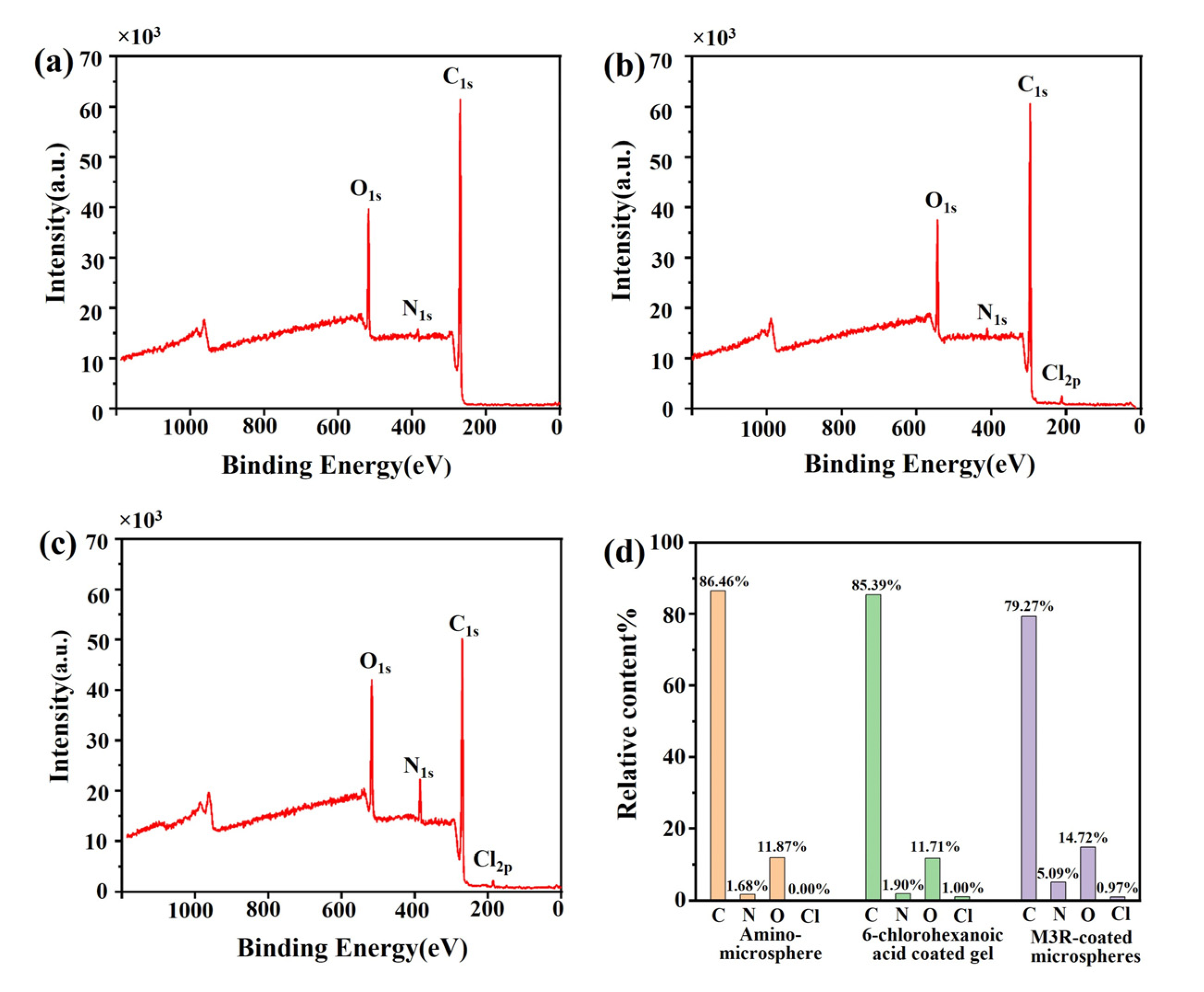
The amount of the immobilized M3R was analyzed by XPS to estimate whether the Halo-tagged M3R was successfully fixed on the microsphere surface. The peaks of the bare microspheres were at 517, 384, and 269 eV, respectively, which completely corresponded to O1s, N1s, and C1s (Figure 2a). In the 6-chlorocaproic-acid-coupled microspheres, compared with the bare microspheres, the relative concentration of the C element was decreased. Due to the successful linkage to 6-chlorocaproic acid, the specific peak of Cl2p occurred at 185 eV (Figure 2b). Due to the enhancement of amino and carboxylic acid groups in M3R, the relative amounts of N and O were tremendously elevated after the microspheres were bound to M3R (Figure 2c). Meanwhile, a further reduced amount of the C element indicated the successful binding of M3R as well. These results suggested that M3R was successfully bound to the microspheres.
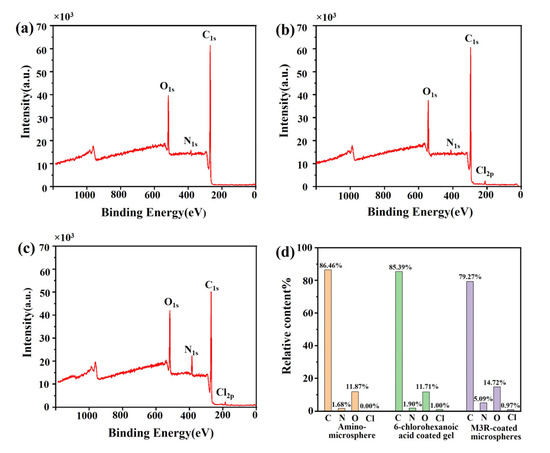
Figure 2.
The element characterization of amino microspheres by X-ray photoelectron spectroscopy. The images show the element characterization of amino microspheres (a), 6-chlorohexanoic-acid-coated microspheres (b), and M3R-coated microspheres (c) by X-ray photoelectron spectroscopy (d). The percentage distribution of the elements C, N, O, and Cl in the amino microspheres, 6-chlorohexanoic-acid-coated amino microspheres, or M3-receptor-coated amino microspheres are calculated.
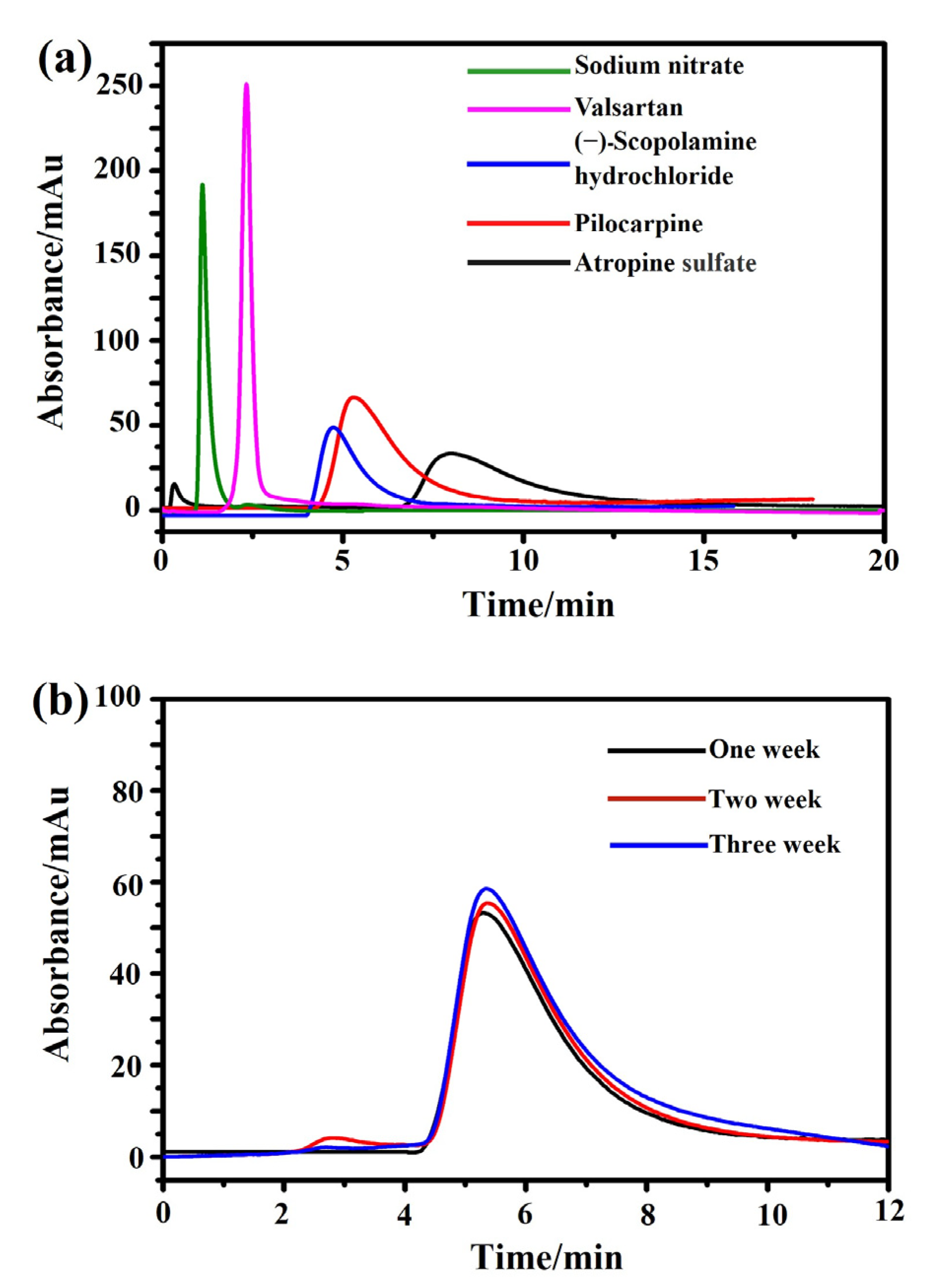
Next, the specificity and stability of the M3R-immobilized column were tested. The reference compound, sodium nitrite, had a retention time of 1.1 min on the M3R-immobilized column. However, as expected, the retention times of (−)-scopolamine hydrochloride, atropine sulfate, and pilocarpine were 4.7, 7.9, and 5.4 min, respectively, which were significantly longer than that of sodium nitrite, suggesting that receptor binding and specific association occurred among the drugs. Valsartan (an antagonist of type I angiotensin II receptor) had a retention time of 2.3 min, showing little difference from the system void time and indicating that no specific binding occurred on the column (Figure 3a). In summary, the retention times of both specific and nonspecific receptor ligands revealed that the M3R-immobilized column demonstrated specificity to bind the receptor’s ligands.
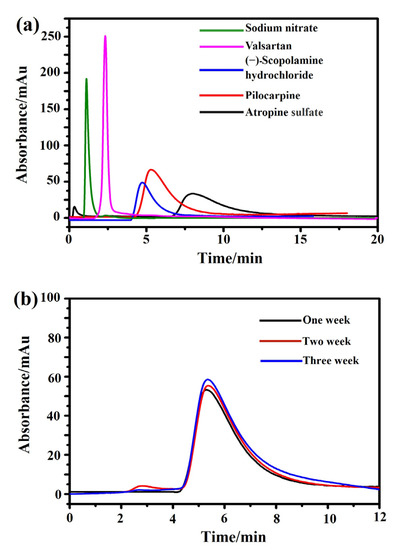
Figure 3.
Characterization of specificity and stability of the immobilized M3R by the ligand-binding assay. (a) The chromatograms of atropine sulfate (brown), pilocarpine (orange), (−)-scopolamine hydrochloride (blue), valsartan (pink), and sodium nitrate (green) on the M3R-immobilized column. (b) The chromatograms of pilocarpine on the M3R-immobilized column in week one (brown), week two (orange), and week three (blue).
Next, the stability of the immobilized M3R was detected via the peak profiles and retention times of pilocarpine over three weeks. No significant alterations of the peak profiles and retention times were found, as indicated by their standard deviations of less than 5.0% (Figure 3b). Based on these results, we deduced that the immobilized M3R was stable for detecting the ligand retention times for 21 days, which is long enough to test drug–receptor interactions and bioactive compounds in herbs.
2.3. M3R-Immobilized Column for Determination of the Binding Coefficient of Drug–Receptor Interactions
2.3.1. Association Constants and Binding Site Numbers
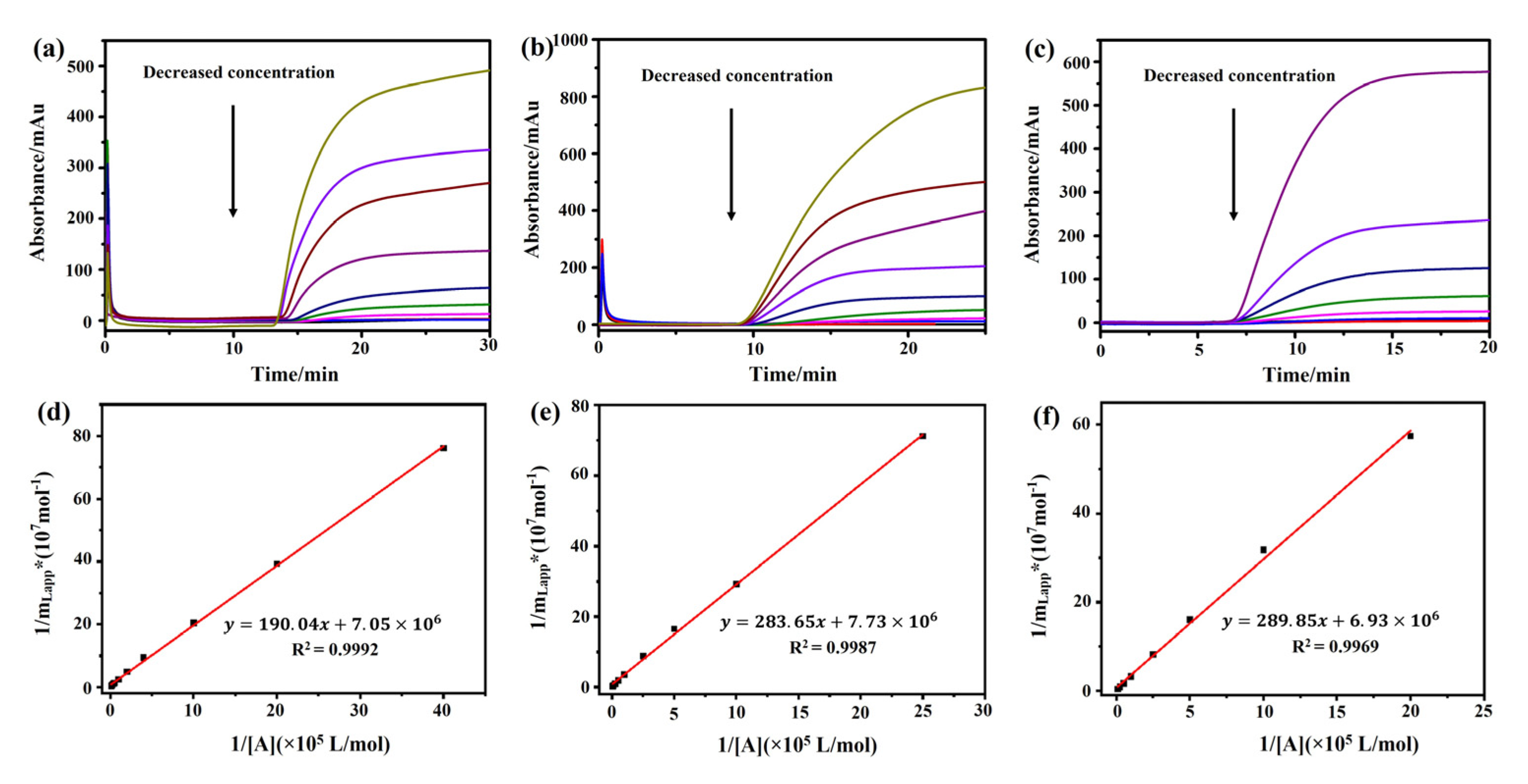
A frontal analysis was conducted with different concentrations of atropine sulfate, (−)-scopolamine hydrochloride, and pilocarpine. We observed that the breakthrough times of these three drugs decreased along with the increase in their concentrations (Figure 4a–c). A linear correlation was observed between 1/mLapp and 1/[A] of the drugs (Figure 4d–f, Table 1). The regression equations of (−)-scopolamine hydrochloride, atropine sulfate, and pilocarpine were y = 289.85x + 6.93 × 106, y = 190.04x + 7.05 × 106, and y = 283.65x + 7.73 × 106, with correlation coefficients (R2) of 0.9992, 0.9969, and 0.9987, respectively. The binding parameters of the three drugs were estimated by Equation (1) and are summarized in Table 1. The frontal analysis showed that the binding constants of (−)-scopolamine hydrochloride, atropine sulfate, and pilocarpine to M3R were (2.39 ± 0.03) × 104, (3.71 ± 0.03) × 104, and (2.73 ± 0.04) × 104 M−1, respectively. The high coefficients of the linear regressions suggested that there was only a single type of binding site for the drugs. The numbers of binding sites for (−)-scopolamine hydrochloride, atropine sulfate, and pilocarpine to M3R were calculated as (1.44 ± 0.19) × 10−7, (1.42 ± 0.45) × 10−7, and (1.29 ± 0.30) × 10−7 M, respectively. Therefore, the order of the M3R binding constants of these three drugs was as follows: atropine sulfate > pilocarpine > (−)-scopolamine hydrochloride.
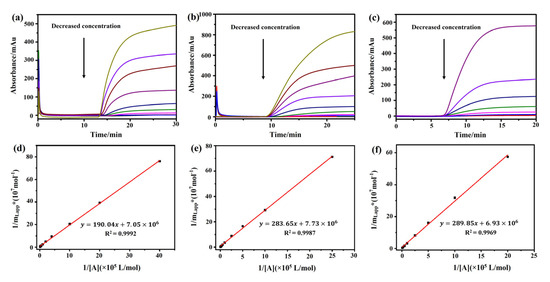
Figure 4.
Analysis of the association constants of atropine sulfate, pilocarpine, and (−)-scopolamine hydrochloride with M3R by frontal affinity chromatography. The breakthrough curves of atropine sulfate (a), pilocarpine (b), and (−)-scopolamine hydrochloride (c) by the M3R-immobilized column. The regression curves of atropine sulfate (d), pilocarpine (e), and (−)-scopolamine hydrochloride (f) obtained by plotting 1/mLapp vs. 1/[A].

Table 1.
The association constants (KA), numbers of binding sites (mL), and dissociation rate constants (kd) of atropine sulfate, pilocarpine, and (−)-scopolamine hydrochloride determined by M3R-immobilized column chromatography.
2.3.2. Dissociation Rate Constants
Peak profile analysis is a powerful method for determining the dynamics of drug–protein interactions. A previous study revealed that the concentration of the injected solute plays a crucial role in the apparent dissociation rate constant (kd) examined by peak profile analysis. The kd value can be accurately detected once the flow rate is high enough to keep the term κ (kinetic factor) constant [21]. Therefore, an optimal flow rate is required to ensure the accurate measurement of kinetic parameters by a peak profile analysis. In this experiment, we chose flow rates of 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8, 3.2, and 3.6 mL/min.
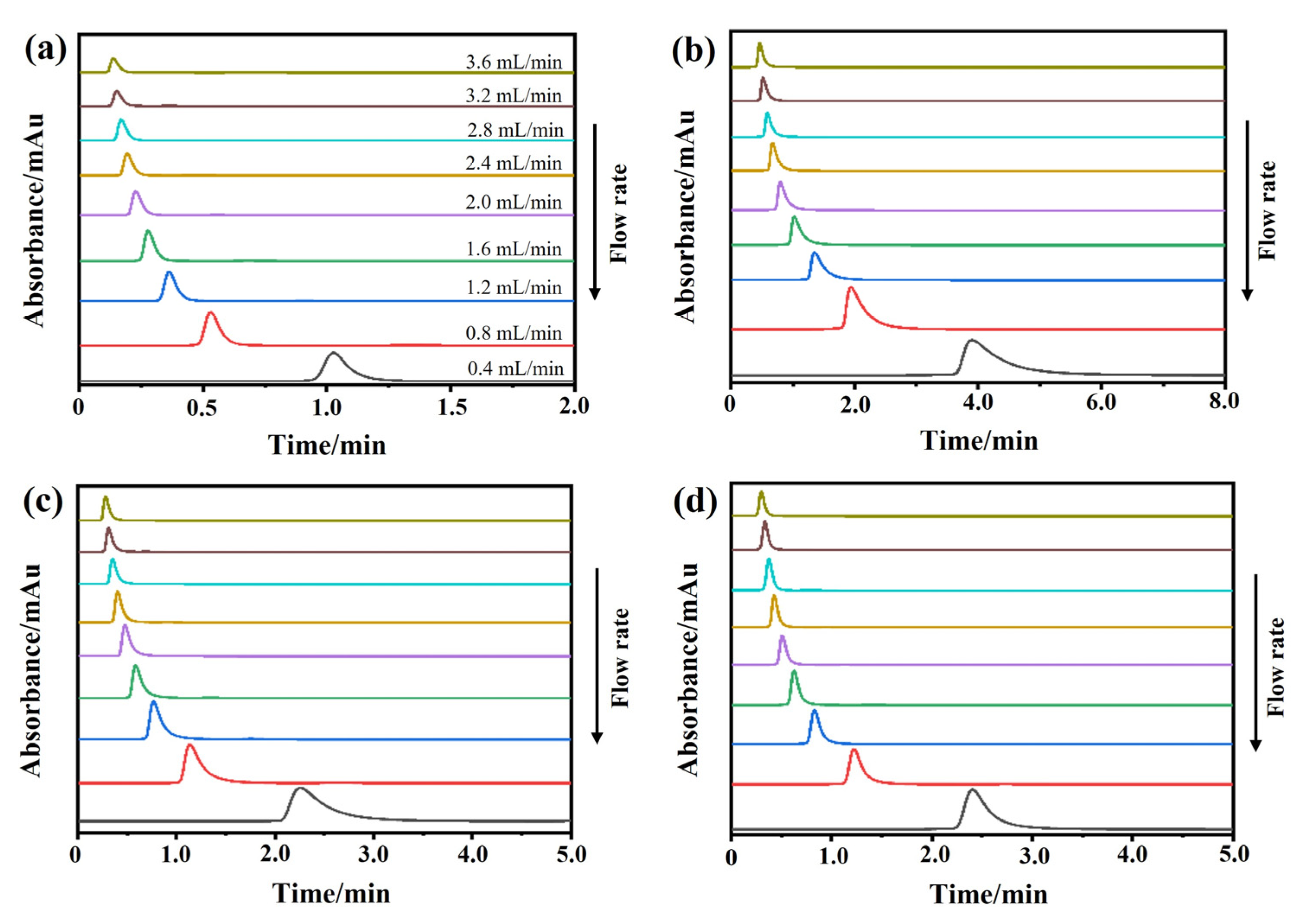
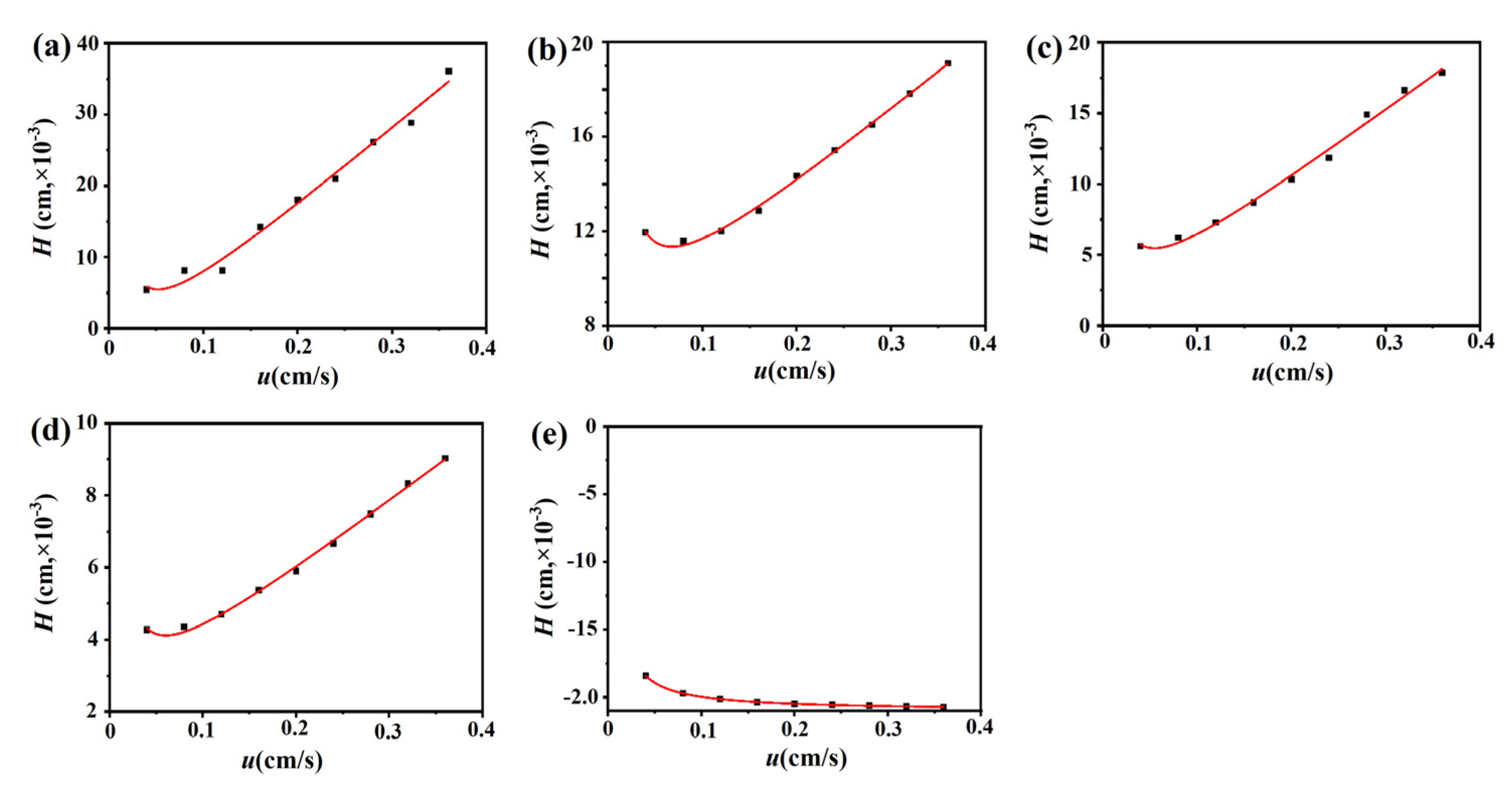
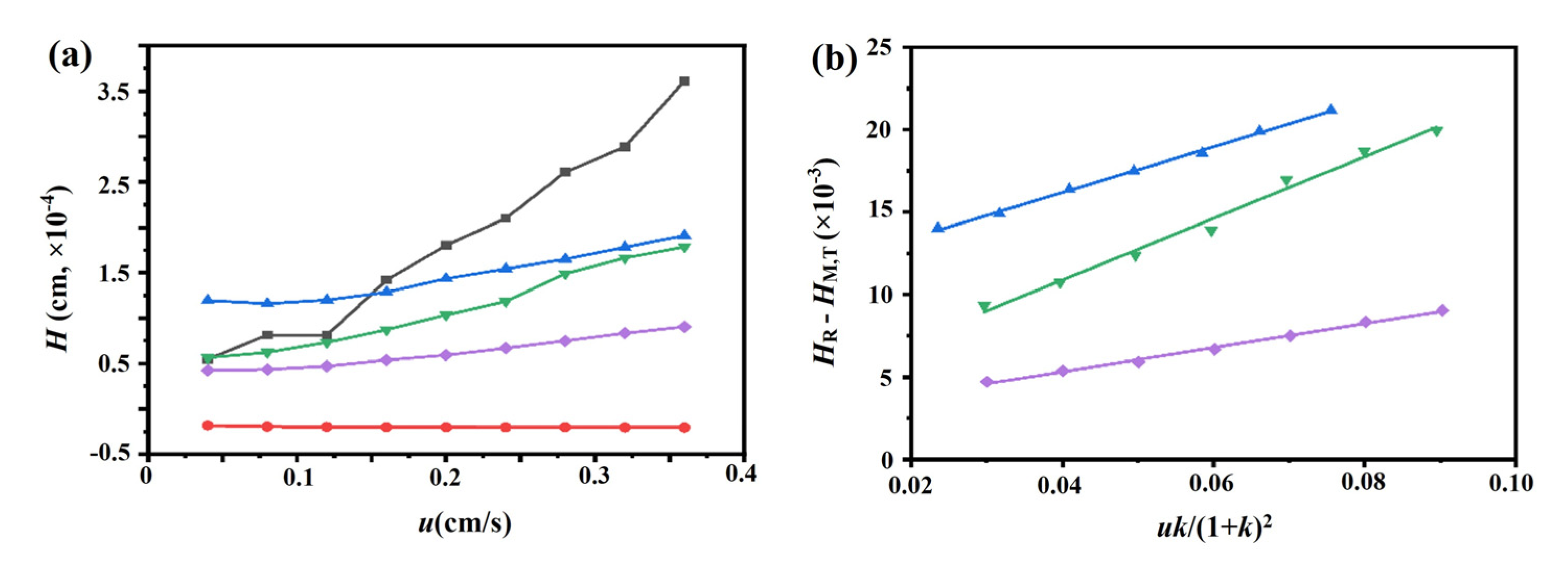
The chromatograms of these three drugs at multiple flow rates are presented in Figure 5a–d. The retention times of (−)-scopolamine hydrochloride, atropine sulfate, and sodium nitrite were reduced as the flow rates were increased. The plate height plots of sodium nitrate and the three drugs on the M3R-immobilized column with multiple flow rates are presented in Figure 6a–d. The linear velocities were 0.04, 0.12, 0.16, 0.20, 0.24, 0.28, 0.32, and 0.36 cm/s, corresponding to flow velocities of 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8, 3.2, and 3.6 mL/min, respectively. We observed that once the flow rate elevation was between 0.8 and 3.6 mL/min (0.08–0.36 cm/s), the HR values of the drugs were obviously enhanced. The linear tendency of HR vs. u suggests that a flow rate of 0.8 mL/min may be the minimal receivable flow velocity to determine the kd value of drugs on the M3R-immobilized column. We also found that sodium nitrate (HM), as a non-retentive analyte, had a significantly greater plate height than the other three drugs. This could be caused by the widespread and slight nonspecific binding to the receptor and the obvious diffusion of sodium nitrate [22,23]. Therefore, a correction was necessary to obtain the actual plate height for the notionally non-retained substance (HM,T). After correction using Equation (3), it was found that the HM,T value almost remained constant at a linear velocity range of 0.08–0.36 cm/s (Figure 6e). After merging the theoretically non-retained substance and the plate height curves of the three drugs, it was more clear that HR was greater than HM,T as the linear velocity was elevated to higher values (Figure 7a). The tendency between HM,T and HR was determined as anticipated by Equation (2). As a result, the typical plots of (HR–HM,T) vs. (uk/(1 + k)2) of the three drugs on the M3R-immobilized column at multiple flow rates are shown in Figure 7b. The linear regression equations for atropine sulfate, pilocarpine, and (−)-scopolamine hydrochloride were as follows: y = 140.0 × 10−3x + 106.8 × 10−4, y = 72.8 × 10−3x + 24.0 × 10−4, and y = 187.0 × 10−3x + 33.4 × 10−4, respectively. The correlation parameters (R2) spanned from 0.9867 to 0.9983. The dissociation rate constants of the immobilized M3R and the three drugs were estimated based on the slopes of the linear curves (summarized in Table 1). Their order was as follows: pilocarpine > atropine sulfate > (−)-scopolamine hydrochloride. Though greater than the data detected by radioligand binding, the kd values revealed a similar ranking order [24] (Table 1).
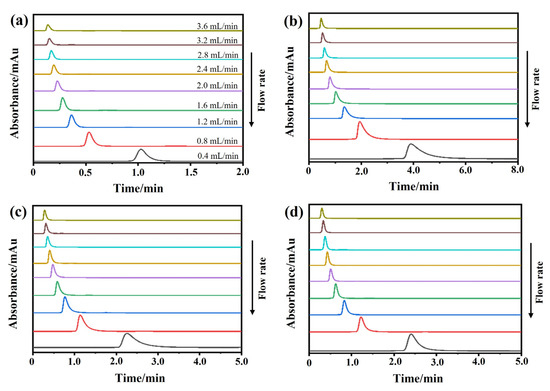
Figure 5.
Representative chromatograms. The representative chromatograms of sodium nitrate (a), atropine sulfate (b), pilocarpine (c), and (−)-scopolamine hydrochloride (d) on the M3R-immobilized column at indicated flow rates.
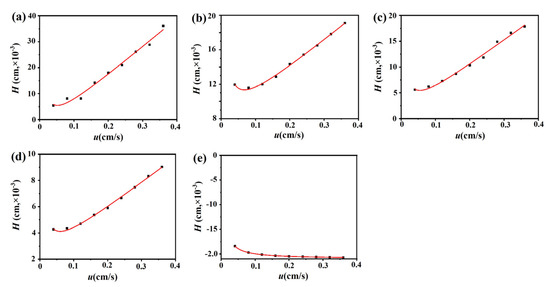
Figure 6.
Fitting plots of the three M3R-bound drugs, sodium nitrate, and the theoretical non-retained substance. Fitting curves of the M3R-bound linear velocity (u) vs. plate height (H) of sodium nitrate (a), atropine sulfate (b), pilocarpine (c), (−)-scopolamine hydrochloride (d), and the theoretical non-retained substance (e).
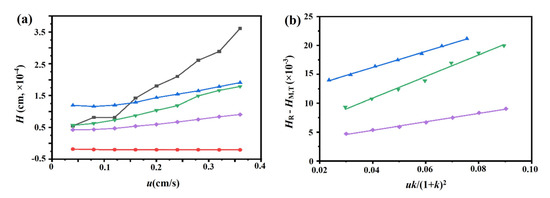
Figure 7.
Typical peak profiles of the three drugs, sodium nitrate, and the theoretical non-retained substance on the M3R-immobilized column. The typical peak profiles of the three drugs, sodium nitrate, and the theoretical non-retained substance on the M3R-immobilized column by a multianalyte approach (a) and fitting plots of (HR−HM) vs. (uk)/(1 + k)2 for the binding of the three drugs to M3R (b). The blue triangle (▲) indicates atropine sulfate, the purple diamond (♦) represents pilocarpine, the green triangle (▼) is (−)-scopolamine hydrochloride, the black square (■) refers to sodium nitrate, and the red circle (●) represents the theoretical unretained substance.
Its ability to be applied at comparably high flow velocities is an asset of the peak profiling approach, which has become an optimal method for drug–protein association analysis in high-throughput screening. It can be applied in weak-to-moderate binding conditions (i.e., KA ≤ 106 M−1) [25]. The above results demonstrated that the association constants of the three drugs to M3R, calculated through frontal analysis, were less than 105 M−1 (Table 1). The peak profiling approach was suitable for detecting the dissociation of the drugs from M3R in our study. Using the M3R affinity chromatography assay, we assessed the integration characteristics and mechanism between the receptor and the drugs, using frontal analysis and the peak profiling method as a mathematical model.
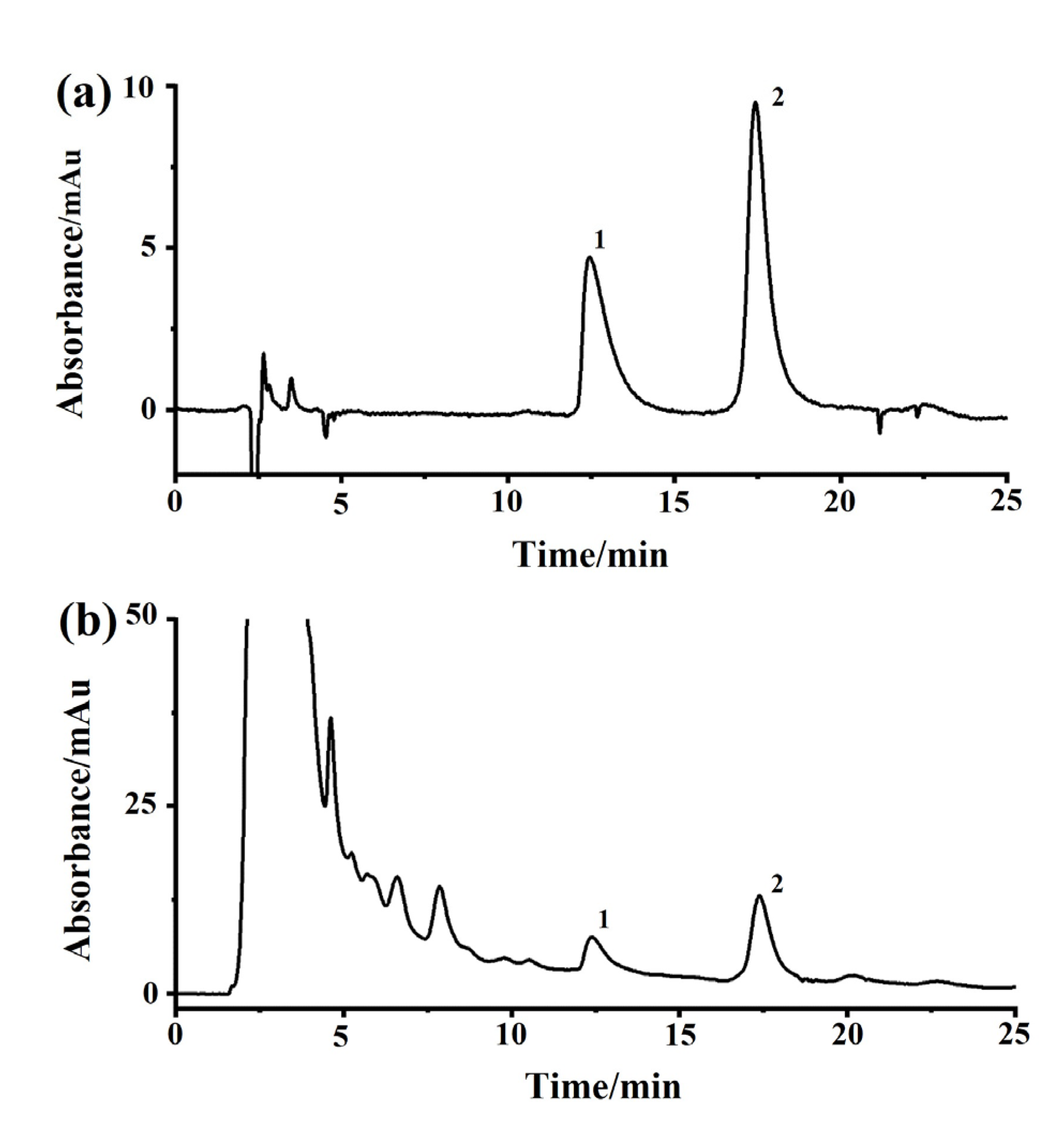
2.4. Identification of Bioactive Compounds in the DF Extract
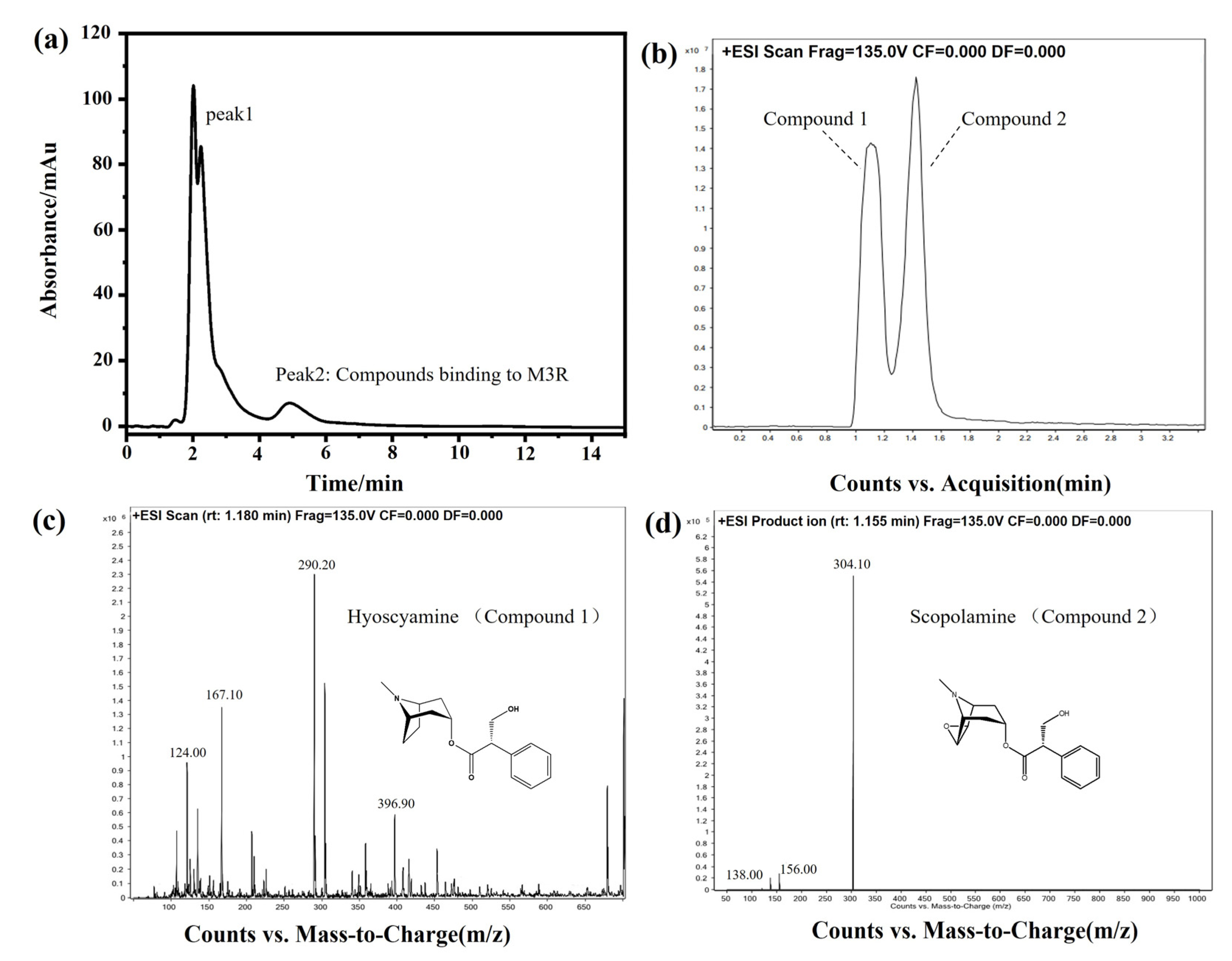
DF is the dried flower of Datura metel L. As an herbal medicine, DF has been applied to treat asthma, relieve pain, and smooth muscle spasms in traditional Chinese medicine clinical practice. Based on the pharmacological mechanism, we hypothesized that some bioactive components of DF can bind to M3R. Next, DF extracts were analyzed by the M3R-immobilized chromatographic column. Indeed, certain compounds did bind to the M3R-immobilized column. Two intense peaks of the chromatogram were observed (Figure 8a). Concerning two of the peaks, peak one was deemed a general mixture without any specific affinity to the immobilized M3R because the retention time was close to the void time of the column. Peak two had a longer retention time than the void time, indicating that the mixture coupled to M3R was coeluted. The following HPLC-Q-TOF-MS assay suggested that the eluted mixture was composed of two components, with the highest intensities at 290.20 ([M+H]+) and 304.10 ([M+H]+) corresponding to molecular weights of 289.20 and 303.10, respectively (Figure 8b–d). The daughter ions of these two ions (m/z) were 167.10 ([M–C8H14N]+) and 124.00 ([M–C9H8O3]+) as well as 156.00 ([M–C9H8O2]+) and 138.00 ([M–C9H8O2-OH]+). These compounds were verified as hyoscyamine and scopolamine [26,27].
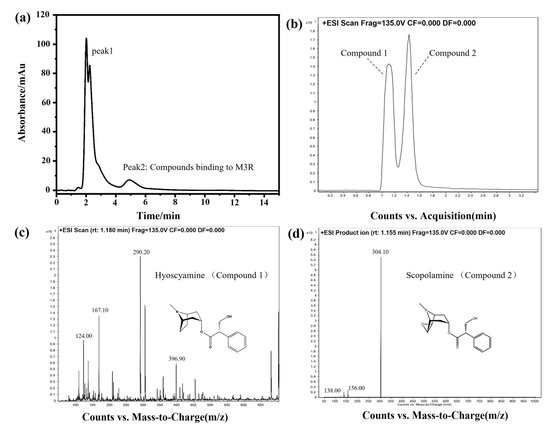
Figure 8.
Identification of bioactive compounds of Daturae Flos extract using the M3R-immobilized column. (a) Representative chromatogram of the Daturae Flos extract on the M3R-immobilized column. Peak 2 was attributed to the compounds in the extract that specifically bind to M3R since the retention time was longer than the void time. (b) The total ion current of peak 2 in the chromatogram on the reversed-phase XDB-C18 column (2.1 mm × 150 mm, 3.5 μm) coupled with an electrospray-MS/MS system. (c) Compound 1 was identified as hyoscyamine by HPLC-MS/MS. (d) Compound 2 was identified as scopolamine by HPLC-MS/MS.
A previous report indicated that the main active components in DF are tropane alkaloids, such as scopolamine, hyoscyamine, anisodine, anisodamine, et al. [28]. Our results showing that hyoscyamine and scopolamine were derived from DF were consistent with these findings. Hyoscyamine and scopolamine are also well-known agonists of mAChRs. These results demonstrate that the bioactive compounds of DF for treating asthma, relieving pain, and smoothing muscle spasms could be compounds such as hyoscyamine and scopolamine. The mechanisms underlying the anti-asthmatic and spasmolytic activity of hyoscyamine and scopolamine depend on the inhibition of M3R in effector cells as well as blocking the transmission of acetylcholine to relax the smooth muscle of the bronchus or other tissues. In summary, these results verified the accuracy of the method for analyzing the M3R-binding bioactive components in complex systems. Herein, we did not determine other compounds of the FD extract, including anisodamine and anisodine, using the M3R affinity column. Such results could be caused due to the low affinity of the subtype-selective ligands of M3R to the column or too low amounts of these compounds in the sample. The potential communication between M3R and other compounds requires further study.
3. Materials and Methods
3.1. Chemicals and Instruments
Reference standards of atropine sulfate (CAS: 55-48-1) and L-hyoscyamine sulfate (CAS: 620-61-1) were obtained from Xushuo Biotechnology (Shanghai, China). Valsartan (CAS: 137862-53-4) was purchased from Macklin Biochemical (Shanghai, China). Pilocarpine (CAS: 54-71-7) was purchased from MedChemExpress. (−)-Scopolamine hydrochloride (CAS: 55-16-3) was obtained from Aladdin (Shanghai, China). Ampicillin was obtained from Shanghai regal Biology Technology Co., Ltd. (Shanghai, China). Normal macroporous silica gel with a pore size of 300 Å and a particle size of 7.0 μm was obtained from the Shandong Bona Biological Technology Group (Jinan, China). The protein marker was obtained from Bitai Biotechnology (Shanghai, China). Sodium nitrite was obtained from Tianjin Kemio Chemical Reagent Co. Ltd. (Tianjin, China). DF was provided by the Wenzexuan Chinese Herbal Medicine Company. The other chemicals used were analytically pure, unless otherwise specified.
The VG Scientific ESCALAB220i-XL analyzer used for X-ray photoelectron spectroscopy (XPS) detection was from Thermo Scientific (Surrey, UK). The ZZXT-A wrapping machine was obtained from Elite Analytical Instruments (Dalian, China). The chromatographic system consisted of an Agilent 1100 high-performance liquid chromatography (HPLC)–ion trap mass spectrometer and an Agilent 1260 HPLC (Santa Clara, CA, USA).
3.2. Preparation of the M3R-Immobilized Column
The Halo-tag (GenBank accession: ADN27525.1) was synthesized by Sangon Biotech (Shanghai, China). The Halo-tagged M3R was expressed in E. coli BL21 (DE3), according to a previously described method [29]. Briefly, a single positive colony in an ampicillin (100.0 μg/mL)-containing semi-solid medium was picked up and incubated in 50 mL of ampicillin-containing Luria-Bertani medium at 37 °C. Fifteen hours later, the culture was transferred into auto-induction medium and incubated for another 12 h. After centrifugation, the collected cells were suspended in phosphate buffer, disrupted with an ultrasonic cell disruptor, and centrifuged to obtain the supernatant for the immobilization.
The immobilized M3R column was prepared through a bio-orthogonal reaction between haloalkane dehalogenase and chloroalkanes, which was described in a previous report [30]. Briefly, amino microspheres were reacted with γ-(aminopropyl) triethoxysilane and silica gel. The Schiff base method was employed to estimate the amino concentration on the resultant silica gel, and it showed that the concentration was 135.06 μmol/g. Then, 0.6 g of amino microspheres (1.0 equiv.) was soaked in 8.0 mL of 6-chlorocaproic acid (1.2 equiv., 14.94 mg) in N,N-dimethylformamide and reacted with 38.12 mg of 2-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (1.2 equiv.) and 42.77 μL of N,N-diisopropylethylamine (3.0 equiv.) for 4.0 h under stirring at room temperature. Next, the amino microspheres were collected and washed with methanol and phosphate buffer (20 mM, pH 7.4), successively. Finally, the prepared amino microspheres were mixed in 60 mL of a M3R-containing cell lysate for 1 h at 4 °C, filtered, rinsed with phosphate buffer (20 mM, pH 7.4), packed in a stainless steel column (4.6 mm × 30 mm) at a pressure of 300 bar, and stored in a refrigerator at 4 °C until their next use.
3.3. Characterization of the M3R-Immobilized Column
The elements of the microsphere surfaces were examined by XPS. As a stable phase control, both microporous silica gel and 6-chlorohexanoic-acid-coated microspheres were applied to eliminate the impact of the linker on the surface elements. Such alterations were used as probes to test whether the receptor was fixed on the microspheres.
A 5–10 nm layer of gold was covered on three types of dried microspheres under a vacuum. The elements O, C, and N on the surfaces of the microspheres were detected by a monochromatic Al Kα (1486.6 eV, 14.5 kV, 30 mA) in survey scanning mode. The binding energies were adjusted to the C1s peak at 284.8 eV. The atom number per unit on the microsphere surface was set as the photoelectron value. Advantage software (Thermo Scientific) was applied for the XPS analysis. The corresponding atomic sensitivity factor and the characteristic peak area were employed to calculate the relative content of the target element.
3.4. Stability and Specificity Analyses of the M3R-Immobilized Column
The specificity of the M3R-immobilized column was analyzed by detecting the retention times of atropine sulfate, (−)-scopolamine hydrochloride (a muscarinic subtype nonspecific antagonist), pilocarpine (a specific agonist of M3R), and valsartan (an antagonist of type I angiotensin II receptor). Sodium nitrite, which does not specifically bind to protein, was employed to detect the void time of the chromatographic system. A relative standard deviation of >5.0% for the retention times between drugs was stipulated as an acceptable specificity. The detection wavelengths of sodium nitrite, atropine sulfate/pilocarpine, and (−)-scopolamine hydrochloride were set at 254 nm, 217 nm, and 210 nm, respectively. Phosphate buffer (20 mM, pH 7.4) was the mobile phase, and was kept at a flow rate of 0.2 mL/min. The injection volume was 10 μL. The stability of the immobilized M3R was assessed by analyzing the retention times and the peak profiles of (−)-scopolamine hydrochloride for three weeks.
3.5. Receptor–Drug Interaction Analysis
3.5.1. Frontal Analysis
Similar to previous reports, the numbers of the binding sites of the isolated M3R-bound compounds and the affinity constants were detected by frontal analysis [31,32]. This detection was based on the mean shift of the breakthrough curves to the shortest breakthrough times during infusion of the M3R-immobilized column with enhanced concentrations of compounds. Different concentrations of three ligands were supplied into the mobile phases, and the M3R-immobilized column was continuously rinsed until stable absorptions were obtained. The concentrations of atropine sulfate used were 0.25, 0.5, 1.0, 2.5, 5.0, 10.0, 20.0, 40.0, 50.0, and 80.0 μM; those of pilocarpine were 0.4, 1.0, 2.0, 4.0, 10.0, 20.0, 40.0, 100.0, and 160.0 μM; and those of (−)-scopolamine hydrochloride were 0.5, 1.0, 2.0, 4.0, 10.0, 20.0, 40.0, and 100.0 μM. Each ligand concentration was assayed in triplicate. The corresponding breakthrough curves were documented. The binding parameters were estimated via Equation (1) [33]:
where mLapp is the molar concentration of the solute at the inflection point of the breakthrough curve, KA is the drug’s affinity constant to the protein, [A] is the molar concentration of the detection compound in the mobile phase, and mL is the number of binding sites. Based on the Equation (1), the curve of 1/mLapp vs. 1/[A] will have a slope of 1/(mLKA) and a y-intercept of 1/(mL). The affinity constant can be estimated by the ratio of the intercept to the slope. The number of binding sites was calculated by the inverse of the y-intercept.
3.5.2. Peak Profiling Method
We used the peak profiling method to probe the binding kinetics of the drug–receptor interaction. The dissociation rate constant of each drug from the immobilized M3R was tested with this approach. Here, the applied concentrations of atropine sulfate, (−)-scopolamine hydrochloride, pilocarpine, and sodium nitrate were 0.3, 0.3, 0.5, and 0.1 mM, respectively. We used multiple flow rates of 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8, 3.2, and 3.6 mL/min but avoided an extremely high flow rate.
Through peak profiling with multiple flow rates, the apparent dissociation rate constants of the drug–protein interactions were determined by Equation (2) [33,34]:
where HR and HM represent the solution’s total plate height as well as the non-retained substance on the column, respectively, u refers to the linear velocity of the mobile phase, and k indicates the analytic retention factor. The kd value can be estimated through plotting the curve of (HR−HM) vs. (uk)/(1 + k)2, which leads to an inverse linear relationship of the slope and kd.
An amendment of HM (HM,T) was applied to calculate the plate heights for inferential non-retained substances on the M3R-immobilized column by Equation (3) if HM could not be detected directly by the conventional non-retained substances [22,23,35]:
where λ represents the correction coefficient, which can be determined from the B-term proportion for the inferential non-retained substance and that of the usual non-retained substance, BC and AC are the B-term and A-term for the usual non-retained substance, respectively, and Csm,T represents the stagnant mobile phase mass transfer involving the C-term for the inferential non-retained substance.
3.6. Analysis of the M3R-Binding Bioactive Components in the DF Extract
3.6.1. Preparation of the DF Extract
The heating under reflux method was used to make the anhydrous extract of DM. In brief, 10 g of dried DF was soaked in 100 mL of water for 0.5 h, boiled for 2 × 1 h, filtered, and concentrated to 10 mL via rotary evaporation at 60 °C under reduced pressure. A solution of 95% ethanol was applied to carry out alcohol sedimentation to reduce the alcohol content of the concentrated extract to 75%. The liquid supernatant was concentrated at 60 °C to obtain an herbal solution of 1.0 g/mL. As shown in Figure 9, the concentrations of scopolamine and hyoscyamine in the extracted solution were condensed to 0.22% and 0.08% of the raw herb through the processes described in the Chinese Pharmacopoeia (Pharmacopoeia of PR China, 2020).
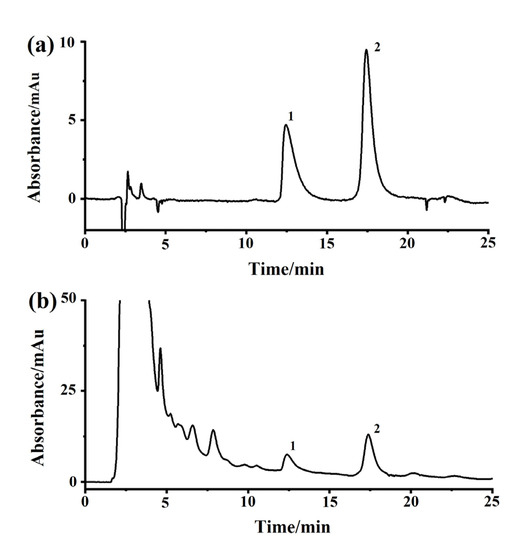
Figure 9.
Representative chromatograms of Daturae Flos extract on a reversed-phase column with a detection wavelength of 216 nm. (a) Reference standards of atropine sulfate and (−)-scopolamine hydrochloride. (b) Extract of Daturae Flos; 1, atropine sulfate; 2, (−)-scopolamine hydrochloride.
3.6.2. Identification of Active Compounds in the DF Extract
The M3R-immobilized column was utilized to analyze the active components in the DF extract with an Agilent 1100 chromatographic system. The mobile phase was 5 mM ammonium acetate buffer, the flow rate was 0.3 mL/min, the sample volume was 10 μL, and the detection wavelength was 254 nm. The chromatographic peak, which had a retention time that was longer than the void time, was collected and considered the M3R-targeted bioactive compound. It was further analyzed using high performance liquid chromatography (HPLC)–mass spectrometry (MS).
An Eclipse XDB-C18 column (2.1 × 150 mm, 3.5 μm) was employed for chromatographic analysis with a 0.1% formic acid solution and methanol (40:60, v/v) as the mobile phase, a flow rate of 0.3 mL/min, and a column temperature of 30 °C. The MS analysis was carried out in both negative and positive modes with a scan range of 100–2000 amu, a nebulizing gas pressure of 30 psi, a flow rate of 8.0 L/min, and a dry gas temperature of 32.5 °C, respectively.
4. Conclusions
Taking M3R as a probe, this work illustrates how the immobilized receptor column could be used to analyze the binding thermodynamics and kinetics of drug–receptor interactions and to screen bioactive compounds from a natural plant that target the receptor. Due to its good specificity and stability, immobilized M3R was applied to determine the binding thermodynamics and kinetics of (−)-scopolamine hydrochloride, atropine sulfate, and pilocarpine to the receptor. Additionally, using the immobilized receptor, hyoscyamine and scopolamine were screened and verified as the bioactive compounds from the DF extract that bind to M3R. By using such a strategy, it is possible to enhance the effectiveness of receptor affinity chromatography in discovering and assessing a ligand. This offers receptor affinity chromatography the capacity to address the issues of current techniques, such as the non-specific adsorption of a drug/protein, the large volume of a sample, a pressure-mediated volume change, or a long incubation time. As such, we concluded that receptor immobilization is a favorable and promising approach for analyzing drug–protein interactions and probing bioactive components from complex matrices, including natural products.
Author Contributions
Methodology, H.F. and X.H.; validation, X.H.; formal analysis, H.F. and Z.Z.; investigation, H.F. and T.W.; data curation, L.W.; writing—original draft preparation, H.F.; writing—review and editing, Y.Z.; supervision, Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by grants from the National Natural Science Foundation of China (82174088) and the Natural Science Basic Research Program of Shaanxi Province (2020JM-437).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the paper.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zhang, X.F.; He, C.L.; Wang, M.; Zhou, Q.T.; Yang, D.H.; Zhu, Y.; Feng, W.B.; Zhang, H.; Dai, A.T.; Chu, X.J.; et al. Structures of the human cholecystokinin receptors bound to agonists and antagonists. Nat. Prod. Rep. 2021, 17, 1230–1237. [Google Scholar] [CrossRef]
- Soriano-Ursua, M.A.; Trujillo-Ferrara, J.; Correa-Basurto, J.; Vilar, S. Recent Structural Advances of beta 1 and beta 2 Adrenoceptors Yield Keys for Ligand Recognition and Drug Design. J. Med. Chem. 2013, 56, 8207–8223. [Google Scholar] [CrossRef] [PubMed]
- Rapposelli, S.; Gaudio, E.; Bertozzi, F.; Gul, S. Editorial: Protein-Protein Interactions: Drug Discovery for the Future. Front. Chem. 2021, 9, 811190. [Google Scholar] [CrossRef]
- Sun, C.R.; Fu, J.D.; He, S.; Pan, Y.J. An integrative approach for the isolation, screening and analysis of antitumor agents by liquid chromatography combined with mass spectrometry. Anal. Chim. Acta 2009, 655, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Lin, H.; Wang, J.C.; Lin, Y.J.; Zhang, T.T.; Jiang, Z.J. Recent advances in bio-affinity chromatography for screening bioactive compounds from natural products. J. Pharm. Biomed. Anal. 2019, 165, 182–197. [Google Scholar] [CrossRef]
- Aristotelous, T.; Hopkins, A.L.; Navratilova, I. Surface plasmon resonance analysis of seven-transmembrane receptors. Methods Enzymol. 2015, 556, 499–525. [Google Scholar]
- Zhang, Z.H.; Chen, L.F.; Zhong, F.S.; Wang, D.Y.; Jiang, J.X.; Zhang, S.L.; Jiang, H.L.; Zheng, M.Y.; Li, X.T. Graph neural network approaches for drug-target interactions. Curr. Opin. Struct. Biol. 2022, 73, 102327. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Alanazi, M.M.; Alsaif, N.A.; Bakheit, A.H.; Zargar, S.; Alsalami, O.M.; Khan, A.A. Interaction characterization of a tyrosine kinase inhibitor erlotinib with a model transport protein in the presence of quercetin: A drug–protein and drug–drug interaction investigation using multi-spectroscopic and computational approaches. Molecules 2022, 27, 1265. [Google Scholar] [CrossRef]
- Najafi, V.; Yoosefian, M.; Hassani, Z. Development of venetoclax performance using its new derivatives on BCL-2 protein inhibition. Cell Biochem. Funct. 2023, 41, 58–66. [Google Scholar] [CrossRef]
- Yoosefian, M.; Moghani, M.Z.; Juan, A. In silico evaluation of atazanavir as a potential HIV main protease inhibitor and its comparison with new designed analogs. Comput. Biol. Med. 2022, 145, 105523. [Google Scholar] [CrossRef]
- Li, Q.; Yin, G.W.; Wang, J.; Li, L.K.; Liang, Q.; Zhao, X.; Chen, Y.Y.; Zheng, X.H.; Zhao, X.F. An emerging paradigm to develop analytical methods based on immobilized transmembrane proteins and its applications in drug discovery. Trac-Trends Anal. Chem. 2022, 157, 116728. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, X.Y.; Zheng, X.X.; Zhao, X.; Wang, T.T.; Liang, Q.; Xiao, C.N.; Wang, J.; Li, Q.; Zhao, X.F. Two-point immobilization of a conformation-specific beta(2)-adrenoceptor for recognizing the receptor agonists or antagonists inspired by binding-induced DNA assembly. Biomater. Sci. 2021, 9, 7934–7943. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ning, X.H.; An, Y.X.; Stanley, B.J.; Liang, Y.; Wang, J.; Zeng, K.Z.; Fei, F.H.; Liu, T.; Sun, H.M.; et al. Reliable analysis of the interaction between specific ligands and immobilized beta-2-adrenoceptor by adsorption energy distribution. Anal. Chem. 2018, 90, 7903–7911. [Google Scholar] [CrossRef] [PubMed]
- Brauner-Osborne, H.; Brann, M.R. Pharmacology of muscarinic acetylcholine receptor subtypes (m1–m5): High throughput assays in mammalian cells. Eur. J. Pharmacol. 1996, 295, 93–102. [Google Scholar] [CrossRef]
- Staus, D.P.; Hu, H.L.; Robertson, M.J.; Kleinhenz, A.L.W.; Wingler, L.M.; Capel, W.D.; Latorraca, N.R.; Lefkowitz, R.J.; Skiniotis, G. Structure of the M2 muscarinic receptor-beta-arrestin complex in a lipid nanodisc. Nature 2020, 579, 297–302. [Google Scholar] [CrossRef]
- Belmonte, K.E. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc. Am. Math. Soc. 2005, 2, 297–304. [Google Scholar] [CrossRef]
- Tanaka, H.; Negoro, K.; Koike, T.; Tsukamoto, I.; Yokoyama, K.; Maeda, J.; Inagaki, Y.; Shimoshige, Y.; Ino, K.; Ishizu, K.; et al. Discovery and structure-activity relationships study of positive allosteric modulators of the M3 muscarinic acetylcholine receptor. Bioorg. Med. Chem. 2020, 28, 115531. [Google Scholar] [CrossRef]
- Fisher, T.J.; Vincent, S.G.; Gomeza, J.; Yamada, M.; Wess, J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004, 18, 711–713. [Google Scholar] [CrossRef]
- Liu, H.T.; Hofmann, J.; Fish, J.; Schaake, B.; Eitel, E.; Bartuschat, A.; Kaindl, J.; Rampp, H.; Banerjee, A.; Hubner, H.; et al. Structure-guided development of selective M3 muscarinic acetylcholine receptor antagonists. Proc. Natl. Acad. Sci. USA 2008, 115, 12046–12050. [Google Scholar] [CrossRef]
- Wess, J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 1996, 10, 69–99. [Google Scholar] [CrossRef]
- Talbert, A.M.; Tranter, G.E.; Holmes, E.; Francis, P.L. Determination of drug–plasma protein binding kinetics and equilibria by chromatographic profiling: Exemplification of the method using L-Tryptophan and Albumin. Anal. Chem. 2002, 74, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Ge, J.W.; Zhang, J.W.; Guo, T.; Chi, L.D.; He, Z.G.; Xu, X.; York, P.; Sun, L.X.; Li, H.Y. Multianalyte determination of the kinetic rate constants of drug-cyclodextrin supermolecules by high performance affinity chromatography. J. Chromatogr. A 2014, 1359, 287–295. [Google Scholar] [CrossRef]
- Zhang, J.W.; Li, H.Y.; Sun, L.X.; Wang, C.F. Determination of the kinetic rate constant of cyclodextrin supramolecular systems by high-performance affinity chromatography. Methods Mol. Biol. 2015, 1286, 309–319. [Google Scholar]
- Sykes, D.A.; Dowling, M.R.; Charlton, S.J. Exploring the mechanism of agonist efficacy: A relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol. Pharmacol. 2009, 76, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Anguizola, J.A.; Jackson, A.J.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Tong, Z.H.; Vargas-Badilla, J.; Yoo, M.J.; Zheng, X.W. Chromatographic analysis of drug interactions in the serum proteoteome. Anal. Methods 2011, 3, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Luo, J.G.; Kong, L.Y. Preparative separation of atropine and scopolamine from Daturae metelis Flos using pH-zone-refining counter-current chromatography with counter-rotation and dual-mode elution procedure. J. Sep. Sci. 2011, 34, 806–811. [Google Scholar] [CrossRef]
- Kuang, H.X.; Yang, B.Y.; Xia, Y.G.; Feng, W.S. Chemical constituents from the flower of Datura metel L. Arch. Pharmacal Res. 2008, 31, 1094–1097. [Google Scholar] [CrossRef]
- Zhu, J.J.; Deng, Y.J.; He, Y.S.; Wang, R.; Kuang, H.X.; Wang, Q.H. Research progress on chemical constituents, pharmacological effect and clinical applications of Daturae Flos. Chin. J. Exp. Tradit. Med. 2021, 27, 201–209. [Google Scholar]
- England, C.G.; Luo, H.M.; Cai, W.B. HaloTag technology: A versatile platform for biomedical applications. Bioconjugate Chem. 2015, 26, 975–986. [Google Scholar] [CrossRef]
- Zeng, K.Z.; Li, Q.; Wang, J.; Yin, G.W.; Zhang, Y.J.; Xiao, C.N.; Fan, T.P.; Zhao, X.F.; Zheng, X.H. One-step methodology for the direct covalent capture of GPCRs from complex matrices onto solid surfaces based on the bioorthogonal reaction between haloalkane dehalogenase and chloroalkanes. Chem. Sci. 2018, 9, 446–456. [Google Scholar] [CrossRef]
- Zhao, X.F.; Lu, H.Y.; Huang, J.J.; Zheng, J.B.; Zheng, X.H.; Zhang, Y.Y. Binding interaction between prazosin and immobilized receptor by frontal analysis. Chromatographia 2012, 75, 411–415. [Google Scholar] [CrossRef]
- Calleri, E.; Temporini, C.; Massolini, G. Frontal affinity chromatography in characterizing immobilized receptors. J. Pharm. Biomed. Anal. 2011, 54, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.E.; Ohnmacht, C.M.; Hage, D.S. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal. Chem. 2009, 81, 4320–4333. [Google Scholar] [CrossRef]
- Chen, J.Z.; Schiel, J.E.; Hage, D.S. Non-competitive peak analysis of drug-protein dissociation by high-performance affinity chromatography. J. Sep. Sci. 2009, 32, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Ge, J.W.; Guo, T.; Yang, S.; He, Z.G.; York, P.; Sun, L.X.; Xu, X.; Zhang, J.W. Determination of the kinetic rate constant of cyclodextrin supramolecular systems by high performance affinity chromatography. J. Chromatogr. A 2013, 1305, 139–148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).