Development of a Novel, Automated High-Throughput Device for Performing the Comet Assay
Abstract
1. Introduction
2. Results
2.1. Initial Testing of the AHT Device
2.2. Qualitative Comparison of Comets Generated by the AHT Device and the HT Assay
2.3. Correlation between Comets Generated by the AHT Device and the HT Assay
3. Discussion
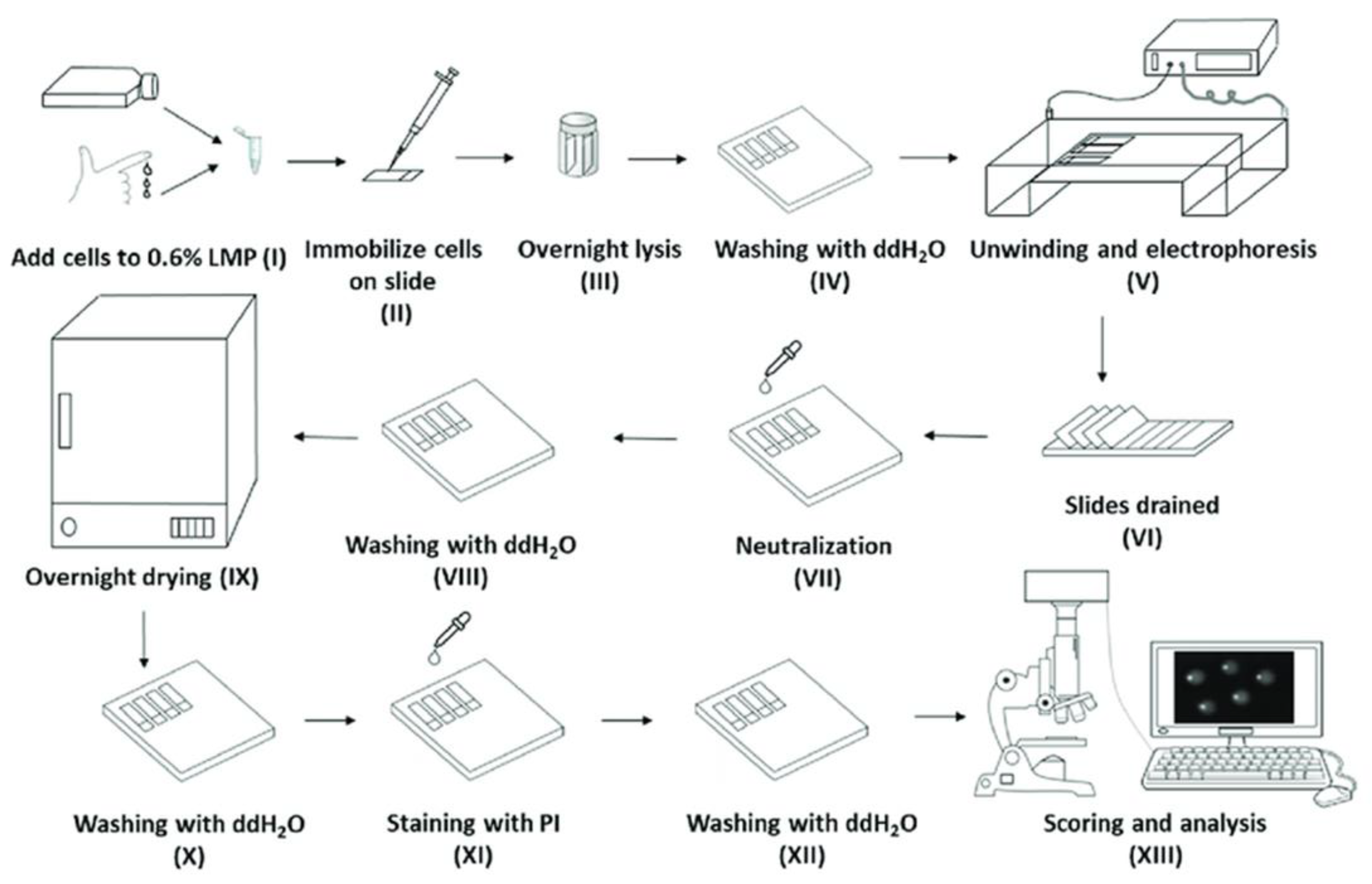
4. Materials and Methods
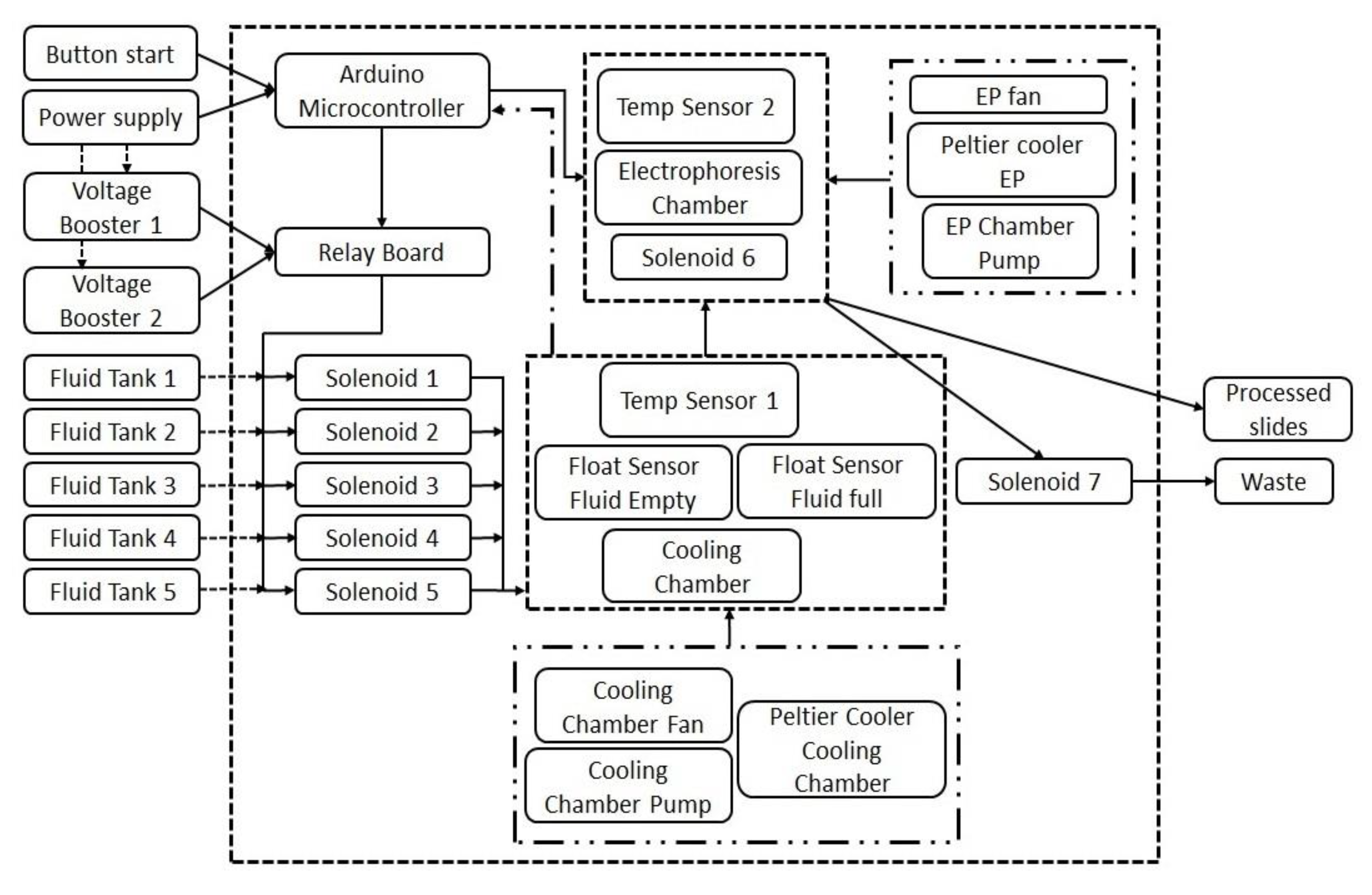
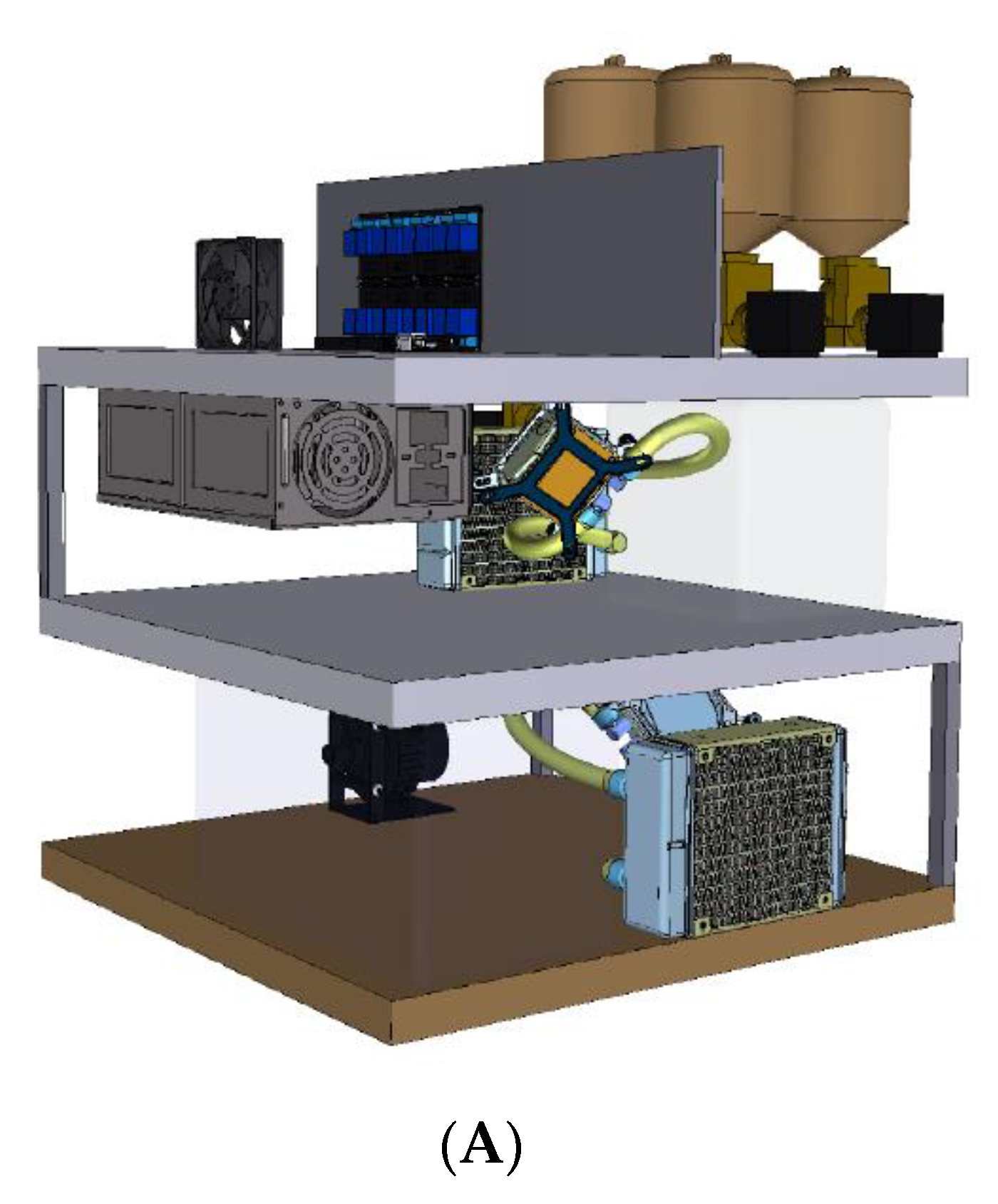
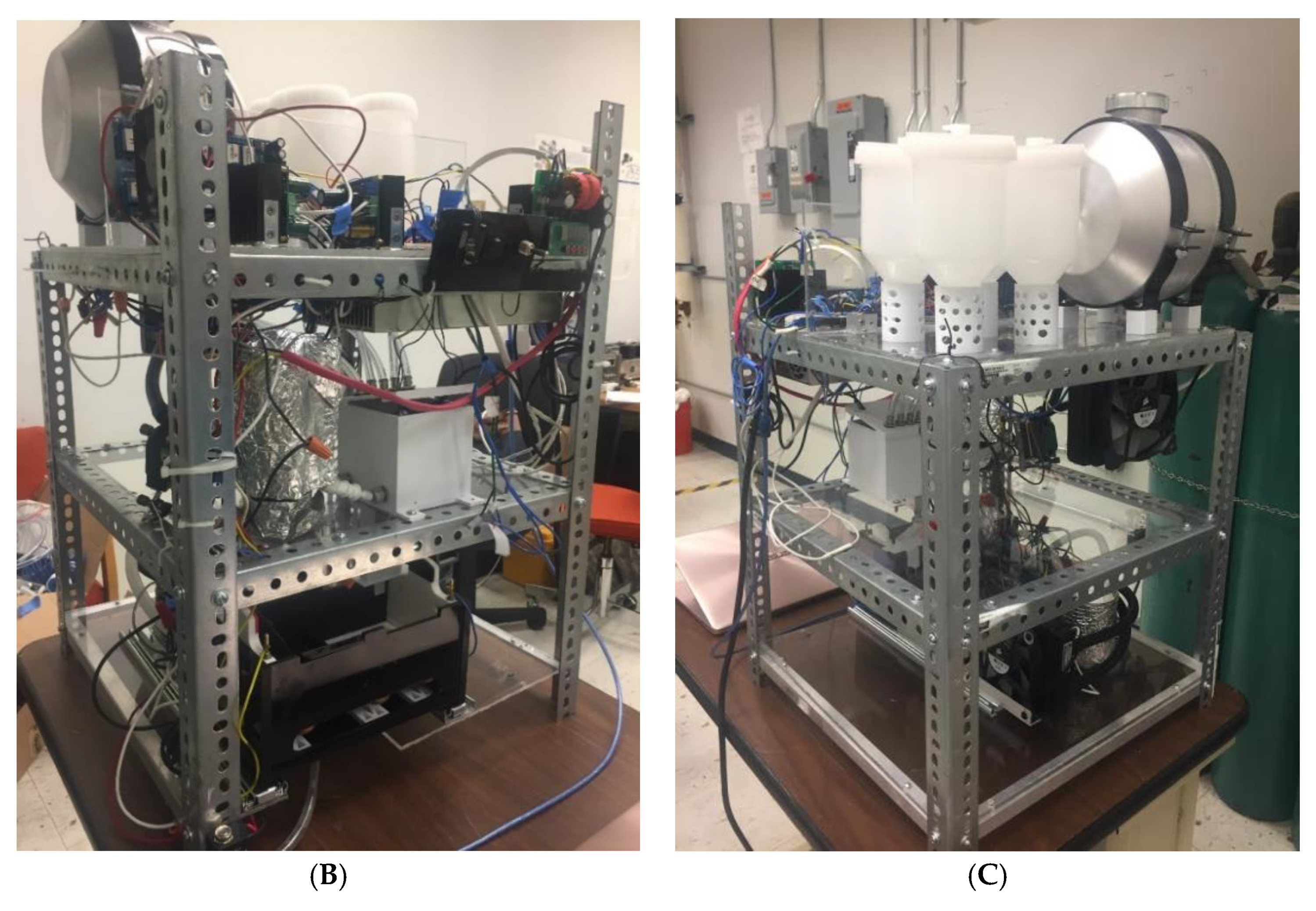
4.1. Overview of the Automated High-Throughput (AHT) Comet Assay Device
4.2. Technical Details of the AHT Device
4.3. Comparison of the Performance of our Established High-Throughput (HT) Comet Assay and an AHT Device
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caburet, S.; Conti, C.; Bensimon, A. Combing the genome for genomic instability. Trends Biotechnol. 2002, 20, 344–350. [Google Scholar] [CrossRef]
- Wu, H.; Roks, A.J. Genomic instability and vascular aging: A focus on nucleotide excision repair. Trends Cardiovasc. Med. 2014, 24, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kapoor, A.; Gu, Y.; Chow, M.J.; Peng, J.; Zhao, K.; Tang, D. Contributions of DNA Damage to Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, T.; Suart, C.E.; Hung, C.L.K.; Graham, K.J.; Barba Bazan, C.A.; Truant, R. DNA Damage Repair in Huntington’s Disease and Other Neurodegenerative Diseases. Neurotherapeutics 2019, 16, 948–956. [Google Scholar] [CrossRef]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair. Mol. Biotechnol. 2004, 26, 249. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.; Rojas, E. Environmental and occupational biomonitoring using the Comet assay. Mutat. Res. 2009, 681, 93–109. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Witte, I.; Plappert, U.; de Wall, H.; Hartmann, A. Genetic toxicity assessment: Employing the best science for human safety evaluation part III: The comet assay as an alternative to in vitro clastogenicity tests for early drug candidate selection. Toxicol. Sci. 2007, 97, 21–26. [Google Scholar] [CrossRef]
- McKenna, D.J.; McKeown, S.R.; McKelvey-Martin, V.J. Potential use of the comet assay in the clinical management of cancer. Mutagenesis 2008, 23, 183–190. [Google Scholar] [CrossRef]
- Forchhammer, L.; Ersson, C.; Loft, S.; Möller, L.; Godschalk, R.W.; van Schooten, F.J.; Jones, G.D.; Higgins, J.A.; Cooke, M.; Mistry, V.; et al. Inter-laboratory variation in DNA damage using a standard comet assay protocol. Mutagenesis 2012, 27, 665–672. [Google Scholar] [CrossRef]
- Godschalk, R.W.L.; Ersson, C.; Stępnik, M.; Ferlińska, M.; Palus, J.; Teixeira, J.P.; Costa, S.; Jones, G.D.D.; Higgins, J.A.; Kain, J.; et al. Variation of DNA damage levels in peripheral blood mononuclear cells isolated in different laboratories. Mutagenesis 2014, 29, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ersson, C.; Møller, P.; Forchhammer, L.; Loft, S.; Azqueta, A.; Godschalk, R.W.; van Schooten, F.J.; Jones, G.D.; Higgins, J.A.; Cooke, M.S.; et al. An ECVAG inter-laboratory validation study of the comet assay: Inter-laboratory and intra-laboratory variations of DNA strand breaks and FPG-sensitive sites in human mononuclear cells. Mutagenesis 2013, 28, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Al-Salmani, K.; Abbas, H.H.; Schulpen, S.; Karbaschi, M.; Abdalla, I.; Bowman, K.J.; So, K.K.; Evans, M.D.; Jones, G.D.; Godschalk, R.W.; et al. Simplified method for the collection, storage, and comet assay analysis of DNA damage in whole blood. Free Radic. Biol. Med. 2011, 51, 719–725. [Google Scholar] [CrossRef]
- Møller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milić, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Costa Pereira, C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for describing comet assay procedures and results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Karbaschi, M.; Abdulwahed, A.; Quinete, N.S.; Evans, M.D.; Cooke, M.S. A High-Throughput Comet Assay Approach for Assessing Cellular DNA Damage. J. Vis. Exp. 2022, 183, e63559. [Google Scholar] [CrossRef]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Stang, A.; Witte, I. Performance of the comet assay in a high-throughput version. Mutat. Res. 2009, 675, 5–10. [Google Scholar] [CrossRef]
- Wood, D.K.; Weingeist, D.M.; Bhatia, S.N.; Engelward, B.P. Single cell trapping and DNA damage analysis using microwell arrays. Proc. Natl. Acad. Sci. USA 2010, 107, 10008–10013. [Google Scholar] [CrossRef]
- Ge, J.; Chow, D.N.; Fessler, J.L.; Weingeist, D.M.; Wood, D.K.; Engelward, B.P. Micropatterned comet assay enables high throughput and sensitive DNA damage quantification. Mutagenesis 2014, 30, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gutzkow, K.B.; Langleite, T.M.; Meier, S.; Graupner, A.; Collins, A.R.; Brunborg, G. High-throughput comet assay using 96 minigels. Mutagenesis 2013, 28, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Karbaschi, M.; Cooke, M.S. Novel method for the high-throughput processing of slides for the comet assay. Sci. Rep. 2014, 4, 7200. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Prasongtanakij, S.; Wood, D.K.; Weingeist, D.M.; Fessler, J.; Navasummrit, P.; Ruchirawat, M.; Engelward, B.P. CometChip: A High-throughput 96-Well Platform for Measuring DNA Damage in Microarrayed Human Cells. JoVE 2014, 92, e50607. [Google Scholar] [CrossRef]
- Ji, Y.; Virzi, J.; Abdalla, N.; Cooke, M.S. Further Improvements to the Comet Assay. Oxidative Stress Group, Department of Molecular Biosciences, University of South Florida, Tampa, FL, USA. 2023; manuscript in preparation. [Google Scholar]
- Sinha, A.; Saleh, A.; Endersby, R.; Yuan, S.H.; Chokshi, C.R.; Brown, K.R.; Kuzio, B.; Kauppinen, T.; Singh, S.K.; Baker, S.J.; et al. RAD51-Mediated DNA Homologous Recombination Is Independent of PTEN Mutational Status. Cancers 2020, 12, 3178. [Google Scholar] [CrossRef]
- Cooke, M.S.; Karbaschi, M. High throughput approach for processing slides for all variations of the single cell gel electrophoresis assay. U.S. Patent US10401323, 3 September 2019. [Google Scholar]
- Cooke, M.S.; Shah, P.; Karbaschi, M.; Bhansali, S. Devices and methods for high-throughput assay. U.S. Patent US9897572, 20 February 2018. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbaschi, M.; Ji, Y.; Mujawar, M.A.; Mendoza, M.; Marquez, J.S.; Sonawane, A.; Shah, P.; Ross, C.; Bhansali, S.; Cooke, M.S. Development of a Novel, Automated High-Throughput Device for Performing the Comet Assay. Int. J. Mol. Sci. 2023, 24, 7187. https://doi.org/10.3390/ijms24087187
Karbaschi M, Ji Y, Mujawar MA, Mendoza M, Marquez JS, Sonawane A, Shah P, Ross C, Bhansali S, Cooke MS. Development of a Novel, Automated High-Throughput Device for Performing the Comet Assay. International Journal of Molecular Sciences. 2023; 24(8):7187. https://doi.org/10.3390/ijms24087187
Chicago/Turabian StyleKarbaschi, Mahsa, Yunhee Ji, Mubarak A. Mujawar, Mario Mendoza, Juan S. Marquez, Apurva Sonawane, Pratikkumar Shah, Chris Ross, Shekhar Bhansali, and Marcus S. Cooke. 2023. "Development of a Novel, Automated High-Throughput Device for Performing the Comet Assay" International Journal of Molecular Sciences 24, no. 8: 7187. https://doi.org/10.3390/ijms24087187
APA StyleKarbaschi, M., Ji, Y., Mujawar, M. A., Mendoza, M., Marquez, J. S., Sonawane, A., Shah, P., Ross, C., Bhansali, S., & Cooke, M. S. (2023). Development of a Novel, Automated High-Throughput Device for Performing the Comet Assay. International Journal of Molecular Sciences, 24(8), 7187. https://doi.org/10.3390/ijms24087187






