Wound Dressing Modifications for Accelerated Healing of Infected Wounds
Abstract
:1. Introduction
2. The Effect of Bacterial Infection on the Wound-Healing Process
2.1. Hemostasis Phase
2.2. Inflammation Phase
2.3. Proliferation Phase
2.4. Remodeling Phase
2.5. Stages of Wound Infection
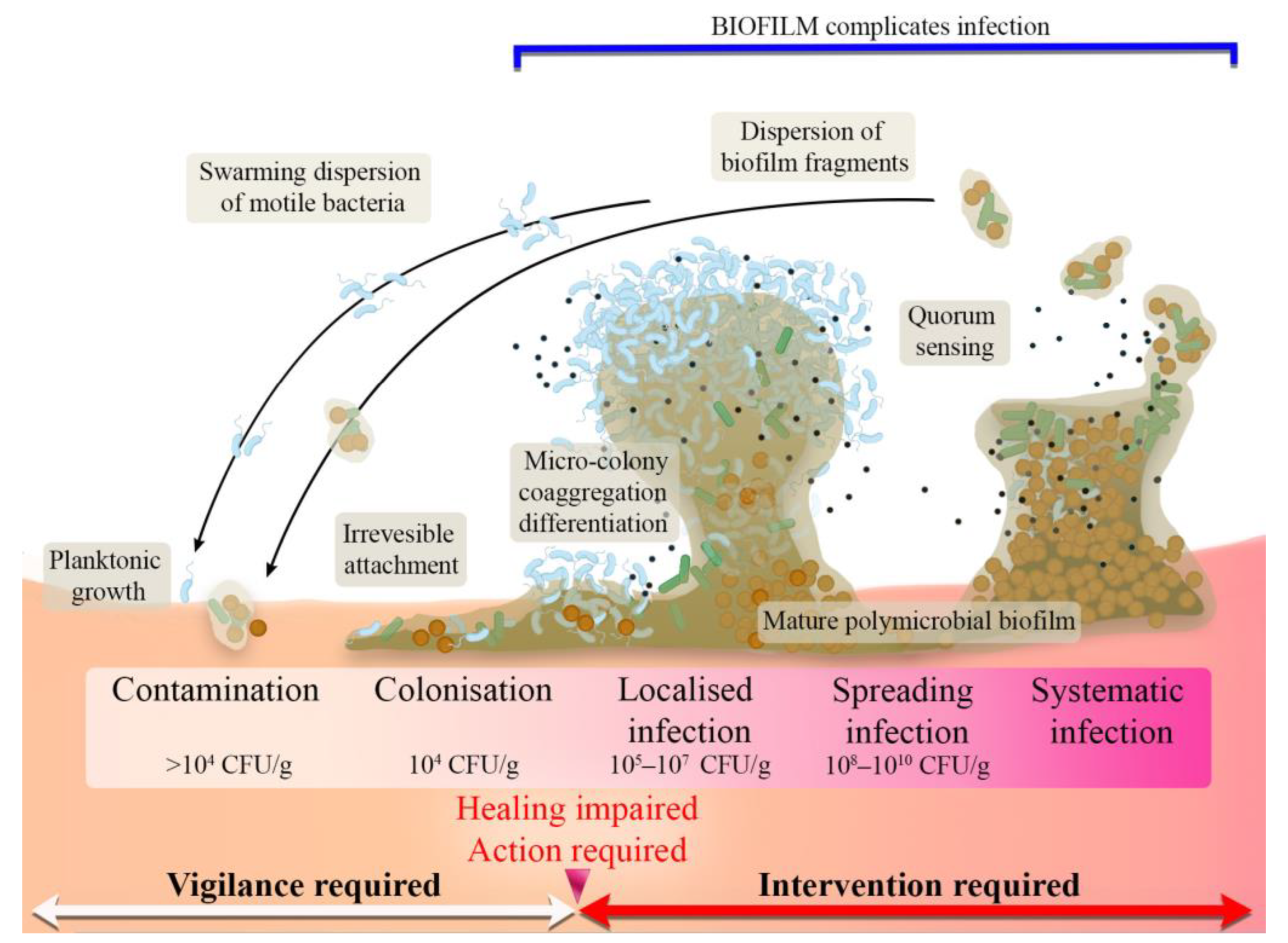
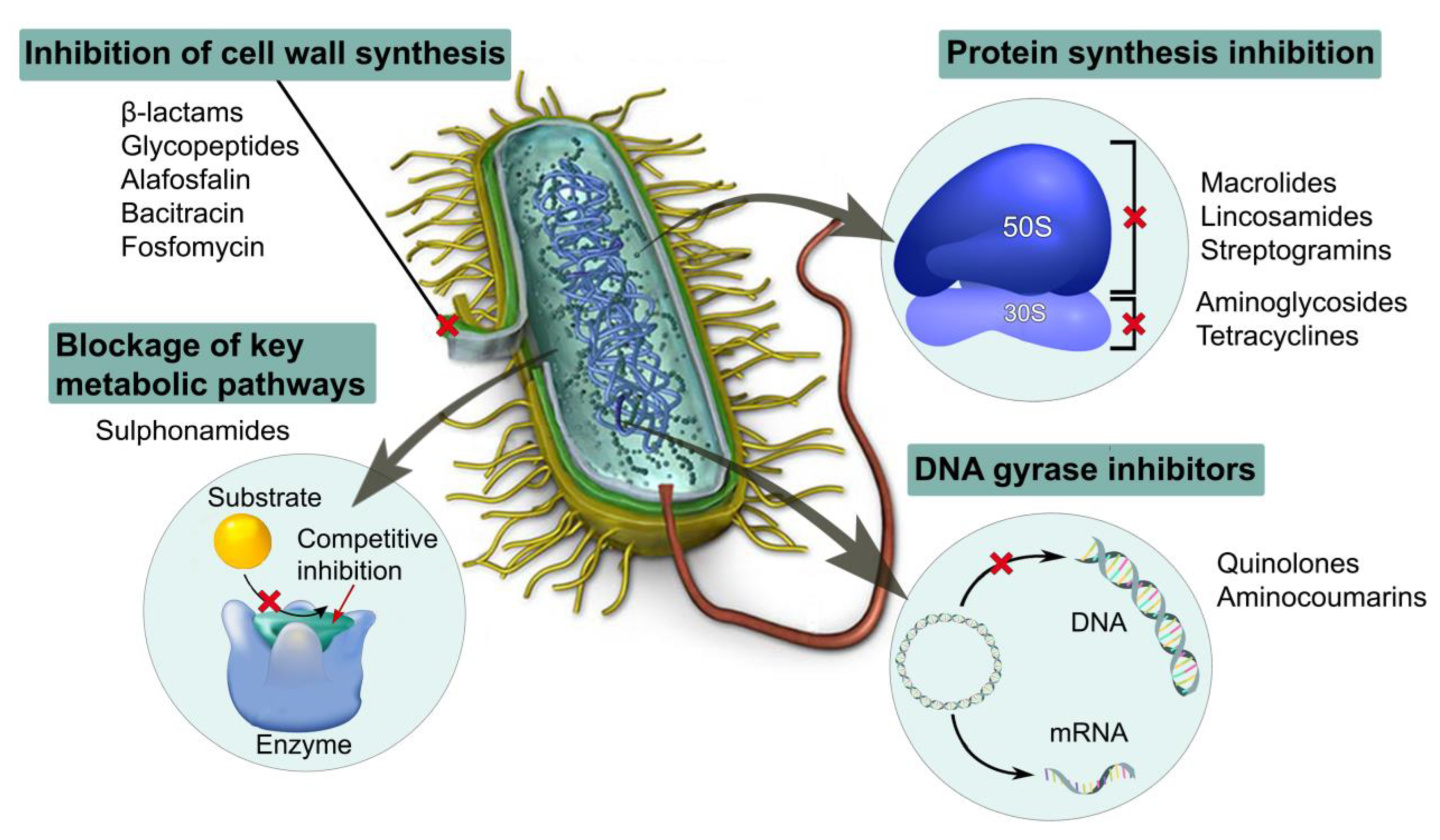
3. Antimicrobial Biomaterials
3.1. Antibiotic-Loaded Dressing Materials
3.2. Nanoparticle-Enriched Dressing Materials
3.2.1. Metallic Nanoparticles
3.2.2. Non-metallic Nanoparticles
3.3. Cationic Organic Agent-Loaded Dressing Materials
3.3.1. Antimicrobial Peptides
3.3.2. Cationic Organic Polymers
3.4. Natural-Compound-Loaded Biomaterials
3.4.1. Essential-Oil-Enriched Dressing Materials
3.4.2. Polyphenol-Enriched Dressing Materials
3.4.3. Curcumin-Enriched Dressing Materials
4. Clinical Use of Antimicrobial Dressings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A Medicinal Plant Used in Skin Wound Healing. Tissue Eng. Part B Rev. 2020, 27, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [Green Version]
- Ridhanya, K.; Rajakumari. Skin wound healing: An update on the current knowledge and concepts. Res. J. Pharm. Technol. 2019, 12, 1448–1452. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Woodmansey, E.J.; Roberts, C.D. Appropriate use of dressings containing nanocrystalline silver to support antimicrobial stewardship in wounds. Int. Wound J. 2018, 15, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological control of inflammation in wound healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M. The physiology of wound healing. J. Wound Care 2000, 9, 299–300. [Google Scholar] [CrossRef]
- Zarbock, A.; Polanowska-Grabowska, R.K.; Ley, K. Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Reviews. 2007, 21, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Wong, B.J.F.; Crumley, R.L. Nerve wound healing: An overview. Otolaryngol. Clin. N. Am. 1995, 28, 881–895. [Google Scholar] [CrossRef]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef]
- Robson, M.C.; Stenberg, B.D.; Heggers, J.P. Wound Healing Alterations Caused by Infection. Clin. Plast. Surg. 1990, 17, 485–492. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Prasetyono, T.O.H. General concept of wound healing, revisited. Med J. Indones. 2009, 18, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Power, C.; Wang, J.H.; Sookhai, S.; Street, J.T.; Redmond, H.P. Bacterial wall products induce downregulation of vascular endothelial growth factor receptors on endothelial cells via a CD14-dependent mechanism: Implications for surgical wound healing. J. Surg. Res. 2001, 101, 138–145. [Google Scholar] [CrossRef]
- Goldman, R. Growth factors and chronic wound healing: Past, present, and future. Adv. Ski. Wound Care 2004, 17, 24–35. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12–34. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.; Baheiraei, N.; Imani, R.; Khodaei, M.; Alizadeh, A.; Rabiee, N.; Moazzeni, S.M. A review of accelerated wound healing approaches: Biomaterial- assisted tissue remodeling. J. Mater. Sci. Mater. Med. 2019, 30, 120. [Google Scholar] [CrossRef]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Impediments to wound healing. Am. J. Surg. 1998, 176, 39S–47S. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Swanson, T.; Angel, D. International Wound Infection Institue: Wound Infection in Clinical Practice Update. Aust. Nurs. Midwifery J. 2017, 24, 33. [Google Scholar]
- Swanson, T.; Grothier, L.; Schultz, G. Wound Infection Made Easy. Wounds International. 2014. Available online: www.woundsinternational.com (accessed on 6 April 2023).
- Haesler, E.; Ousey, K. Clinical practice Evolution of the wound infection continuum. Wounds Int. 2018, 9, 6–10. [Google Scholar]
- Farhan, N.; Jeffery, S. Diagnosing burn wounds infection: The practice gap & advances with moleculight bacterial imaging. Diagnostics. 2021, 11, 268. [Google Scholar] [CrossRef]
- Rujitanaroj, P.-O.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Li, X.; Bai, H.; Yang, Y.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular Antibacterial Materials for Combatting Antibiotic Resistance. Adv. Mater. 2019, 31, 1805092. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, R. Introduction to Antibacterial Biomaterials. Biomater. Sci. 2020, 8, 6812–6813. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chemie Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Kazimierczak, P.; Belcarz, A.; Wilczynska, A.; Vivcharenko, V.; Pajchel, L.; Adaszek, L.; Przekora, A. Biocompatible curdlan-based biomaterials loaded with gentamicin and Zn-doped nano-hydroxyapatite as promising dressing materials for the treatment of infected wounds and prevention of surgical site infections. Biomater. Adv. 2022, 139, 213006. [Google Scholar] [CrossRef]
- Vijayakrishna, K.; Patil, S.; Shaji, L.K.; Panicker, R.R. Gentamicin Loaded PLGA based Biodegradable Material for Controlled Drug Delivery. ChemistrySelect 2019, 4, 8172–8177. [Google Scholar] [CrossRef]
- Gemeinder, J.L.P.; de Barros, N.R.; Pegorin, G.S.; de Singulani, J.L.; Borges, F.A.; Arco, M.C.G.D.; Giannini, M.J.S.M.; Almeida, A.M.F.; de Salvador, S.L.S.; Herculano, R.D. Gentamicin encapsulated within a biopolymer for the treatment of Staphylococcus aureus and Escherichia coli infected skin ulcers. J. Biomater. Sci. Polym. Ed. 2021, 32, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Saha, K.; Sarkar, P.; Chattopadhyay, D. Antibactericidal nanoclay-based biomaterial for sustained delivery of tetracycline hydrochloride. Bull. Mater. Sci. 2020, 43, 248. [Google Scholar] [CrossRef]
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.E.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial nanofibers of pullulan/tetracycline-cyclodextrin inclusion complexes for Fast-Disintegrating oral drug delivery. J. Colloid Interface Sci. 2022, 610, 321–333. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- Elashnikov, R.; Rimpelová, S.; Lyutakov, O.; Pavlíčková, V.S.; Khrystonko, O.; Kolská, Z.; Švorčík, V. Ciprofloxacin-Loaded Poly(N-isopropylacrylamide- co-acrylamide)/Polycaprolactone Nanofibers as Dual Thermo- and pH-Responsive Antibacterial Materials. ACS Appl. Bio Mater. 2022, 5, 1700–1709. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Q.; Kharaghani, D.; Sanaullah; Shahzad, A.; Saito, Y.; Yamamoto, T.; Ogasawara, H.; Kim, I.S. Fabrication of antibacterial electrospun cellulose acetate/ silver-sulfadiazine nanofibers composites for wound dressings applications. Polym. Test. 2019, 74, 39–44. [Google Scholar] [CrossRef]
- Yassue-Cordeiro, P.H.; Zandonai, C.H.; Genesi, B.P.; Lopes, P.S.; Sanchez-Lopez, E.; Garcia, M.L.; Fernandes-Machado, N.R.C.; Severino, P.; Souto, E.B.; da Silva, C.F. Development of chitosan/silver sulfadiazine/zeolite composite films for wound dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, M.; Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Abdi, S.; Moravvej, H.; Vossoughi, M. A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 564, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Hashmi, M.; Khan, M.Q.; Kharaghani, D.; Saito, Y.; Yamamoto, T.; Kim, I.S. Silver sulfadiazine loaded zein nanofiber mats as a novel wound dressing. RSC Adv. 2019, 9, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Phaechamud, T.; Issarayungyuen, P.; Pichayakorn, W. Gentamicin sulfate-loaded porous natural rubber films for wound dressing. Int. J. Biol. Macromol. 2016, 85, 634–644. [Google Scholar] [CrossRef]
- Anbazhagan, S.; Thangavelu, K.P. Application of tetracycline hydrochloride loaded-fungal chitosan and Aloe vera extract based composite sponges for wound dressing. J. Adv. Res. 2018, 14, 63–71. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Naeimi, M.; Tajedin, R.; Farahmandfar, F.; Naeimi, M.; Monajjemi, M. Preparation and characterization of vancomycin-loaded chitosan/PVA/PEG hydrogels for wound dressing. Mater. Res. Express 2020, 7, 095401. [Google Scholar] [CrossRef]
- Özkahraman, B.; Özbaş, Z.; Bayrak, G.; Tamahkar, E.; Perçin, I.; Kılıç Süloğlu, A.; Boran, F. Characterization and antibacterial activity of gelatin–gellan gum bilayer wound dressing. Int. J. Polym. Mater. 2021, 71, 1240–1251. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Green, B.N.; Johnson, C.D.; Egan, J.T.; Rosenthal, M.; Griffith, E.A.; Evans, M.W. Methicillin-resistant Staphylococcus aureus: An overview for manual therapists. J. Chiropr. Med. 2012, 11, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Ranghar, S.; Sirohi, P.; Verma, P.; Agarwal, V. Nanoparticle-based drug delivery systems: Promising approaches against infections. Brazilian Arch. Biol. Technol. 2014, 57, 209–222. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjug. Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.G. A novel strategy for antimicrobial agents: Silver nanoparticles. Metal Nanopart. Pharma. 2017, 139–153. [Google Scholar] [CrossRef]
- Vyavahare, S.; Padole, N.; Avari, J. A REVIEW: SILVER NANOPARTICLES IN WOUND HEALING. Eur. J. Pharm. Med. Res. 2021, 8, 212–218. [Google Scholar]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polym. Test. 2019, 79, 106022. [Google Scholar] [CrossRef]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan–Ag nanoparticles and chitosan–Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial Lignin-Based Polyurethane/Ag Composite Foams for Improving Wound Healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Abu Ghith, D.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Int. J. Pharm. 2019, 565, 174–186. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcucho, J.; Narváez, D.M.; Castro-Mayorga, J.L. Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus aureus. Nanomaterials 2020, 10, 1692. [Google Scholar] [CrossRef]
- Karuppannan, S.K.; Ramalingam, R.; Mohamed Khalith, S.B.; Musthafa, S.A.; Dowlath, M.J.H.; Munuswamy-Ramanujam, G.; Arunachalam, K.D. Copper oxide nanoparticles infused electrospun polycaprolactone/gelatin scaffold as an antibacterial wound dressing. Mater. Lett. 2021, 294, 129787. [Google Scholar] [CrossRef]
- Paydayesh, A.; Heleil, L.; Dadkhah, A.S. Preparation and application of poly (hydroxyl ethyl methacrylate) nanocomposite hydrogels containing iron oxide nanoparticles as wound dressing. Orig. Res. Artic. Polym. Polym. Compos. 2019, 30, 09673911211063106. [Google Scholar] [CrossRef]
- Chircov, C.; Bejenaru, I.T.; Nicoară, A.I.; Bîrcă, A.C.; Oprea, O.C.; Tihăuan, B. Chitosan-Dextran-Glycerol Hydrogels Loaded with Iron Oxide Nanoparticles for Wound Dressing Applications. Pharmaceutics 2022, 14, 2620. [Google Scholar] [CrossRef]
- Arab, M.; Jallab, M.; Ghaffari, M.; Moghbelli, E.; Saeb, M.R. Synthesis, rheological characterization, and antibacterial activity of polyvinyl alcohol (PVA)/zinc oxide nanoparticles wound dressing, achieved under electron beam irradiation. Iran. Polym. J. 2021, 30, 1019–1028. [Google Scholar] [CrossRef]
- Majumder, S.; Dahiya, U.R.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Antibacterial activity of gold nanorods against staphylococcus aureus and propionibacterium acnes: Misinterpretations and artifacts. Int. J. Nanomed. 2017, 12, 7311–7322. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Iwanaga, A.; Shirosaki, Y.; Kawashita, M. In situ synthesis of magnetic iron oxide nanoparticles in chitosan hydrogels as a reaction field: Effect of cross-linking density. Colloids Surfaces B Biointerfaces 2019, 179, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Song, S.C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef]
- Panwar, H.; Sharma, C.; Lichtfouse, E. (Eds.) Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2020; Volume 1, pp. 168–170. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.-Based Complement. Altern. Med 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Vaez, A.; Samadian, H.; Sahrapeyma, H.; Mirzaii, M.; Ghorbani, S.; Goodarzi, A. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018, 117, 601–609. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shabani Shayeh, J. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Jian, Z.; Wang, H.; Liu, M.; Chen, S.; Wang, Z.; Qian, W.; Luo, G.; Xia, H. Polyurethane-modified graphene oxide composite bilayer wound dressing with long-lasting antibacterial effect. Mater. Sci. Eng. C 2020, 111, 110833. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Q.; Xu, Z.; Wang, Y.; Zhu, B.; Fan, L.; Gao, L. Aloe-Emodin/Carbon Nanoparticle Hybrid Gels with Light-Induced and Long-Term Antibacterial Activity. ACS Biomater. Sci. Eng. 2018, 4, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Yadegari, A.; Tayebi, L. Wound dressing application of pH-sensitive carbon dots/chitosan hydrogel. RSC Adv. 2017, 7, 10638–10649. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Owh, C.; Chee, P.L.; Leow, W.R.; Liu, X.; Wu, Y.L.; Guo, P.; Loh, X.J.; Chen, X. Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 2018, 47, 6917–6929. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surfaces B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Wu, T.; Wang, W.; Li, B.; Wang, M.; Chen, L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, Z.; Hu, J.; Liu, Y. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater. Sci. Eng. C 2021, 128, 112319. [Google Scholar] [CrossRef]
- Amariei, G.; Kokol, V.; Boltes, K.; Letón, P.; Rosal, R. Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications. RSC Adv. 2018, 8, 28013–28023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshar, A.; Yuca, E.; Wisdom, C.; Alenezi, H.; Ahmed, J.; Tamerler, C.; Edirisinghe, M. Next-generation Antimicrobial Peptides (AMPs) incorporated nanofibre wound dressings. Med. Devices Sensors 2021, 4, e10144. [Google Scholar] [CrossRef]
- Chizari, M.; Khosravimelal, S.; Tebyaniyan, H.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Fabrication of an Antimicrobial Peptide-Loaded Silk Fibroin/Gelatin Bilayer Sponge to Apply as a Wound Dressing; An In Vitro Study. Int. J. Pept. Res. Ther. 2022, 28, 18. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, M.; Luo, H.; Niu, L.; Feng, Y.; Li, M. Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function. Materials 2020, 13, 285. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Feng, X.; Xu, R.; Dong, S.; Wu, M.; Zheng, X.; Lu, W.; Li, B. A Handy Skin Wound Dressing Prepared by Alginate and Cationic Nanofibrillated Cellulose Derived from Solid Residues of Herbs. BioResources 2021, 16, 5926–5946. [Google Scholar] [CrossRef]
- Yang, X.; Sha, D.; Xu, J.; Niu, N.; Shi, K.; Pan, Y.; Yu, C.; Wei, H.; Wang, B.; Ji, X. Preparation of cationic polyelectrolyte grafted polyvinyl alcohol-formaldehyde macroporous hydrogels and their antibacterial properties. New J. Chem. 2019, 43, 14961–14971. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Elesawy, B.H.; Elfasakhany, A.; Badruddin, I.A.; Kamangar, S. Wound dressings coated with silver nanoparticles and essential oil of Labdanum. Appl. Nanosci. 2021, 13, 1345–1354. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pereira dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to be Used in Wound Dressing Applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Tonelli, A.E.; Hudson, S.M. Preparation and characterization of chitosan–Alginate polyelectrolyte complexes loaded with antibacterial thyme oil nanoemulsions. Appl. Sci. 2019, 9, 3933. [Google Scholar] [CrossRef] [Green Version]
- Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Gradisteanu Pircalabioru, G.; Raiciu, A.D.; Bita, A.I.; Antoniac, A.; Antoniac, V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials 2022, 15, 6923. [Google Scholar] [CrossRef]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.A.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J 2018, 5, 6–14. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Li, H.; Shen, S.; Yu, K.; Wang, H.; Fu, J. Construction of porous structure-based carboxymethyl chitosan/sodium alginate/tea polyphenols for wound dressing. Int. J. Biol. Macromol. 2023, 233, 123404. [Google Scholar] [CrossRef]
- Zeng, Z.; Guo, C.; Lu, D.; Geng, Z.; Pei, D.; Yu, S. Polyphenol–Metal Functionalized Hydrogel Dressing with Sustained Release, Antibacterial, and Antioxidant Properties for the Potential Treatment of Chronic Wounds. Macromol. Mater. Eng. 2022, 307, 2200262. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, Y.; Wei, Y.; Wang, Y.; Jin, Z.; Ma, G.; Jiang, Y.; Zhang, W.; Hu, Z. Facile preparation of polyphenol-crosslinked chitosan-based hydrogels for cutaneous wound repair. Int. J. Biol. Macromol. 2023, 228, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Cheng, C.; Peng, X.; Xi, L.; Wan, C.; Shi, S.; Wang, Y.; Yu, X. An agar-polyvinyl alcohol hydrogel loaded with tannic acid with efficient hemostatic and antibacterial capacity for wound dressing. Food Funct. 2022, 13, 9622–9634. [Google Scholar] [CrossRef] [PubMed]
- Wutticharoenmongkol, P.; Hannirojram, P.; Nuthong, P. Gallic acid-loaded electrospun cellulose acetate nanofibers as potential wound dressing materials. Polym. Adv. Technol. 2019, 30, 1135–1147. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Gómez, E.; Cejudo-Bastante, C.; Casas, L.; Montes, A.; Mantell, C.; de la Ossa-Fernández, E.J.M.; Pereyra, C. Development of functionalized alginate dressing with mango polyphenols by supercritical technique to be employed as an antidiabetic transdermal system. J. Supercrit. Fluids 2021, 175, 105274. [Google Scholar] [CrossRef]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Yazdanpanah, Z.; Soltani, Y.A.; Sardroud, H.A.; Nasirtabrizi, M.H.; Chen, X. Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: Wound dressing application with anti-bacterial and low cell toxicity properties. J. Biomater. Sci. Polym. Ed. 2020, 31, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Manna, P.J.; Mitra, T.; Pramanik, N.; Kavitha, V.; Gnanamani, A.; Kundu, P.P. Potential use of curcumin loaded carboxymethylated guar gum grafted gelatin film for biomedical applications. Int. J. Biol. Macromol. 2015, 75, 437–446. [Google Scholar] [CrossRef]
- Tong, W.Y.; bin Abdullah, A.Y.K.; binti Rozman, N.A.S.; bin Wahid, M.I.A.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Tan, W.N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbu, G.D.; Anusuya, T. Investigation on Curcumin nanocomposite for wound dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, N.; Natarajan, T.S.; Rajiv, S. Preparation and characterization of electrospun curcumin loaded poly(2-hydroxyethyl methacrylate) nanofiber-A biomaterial for multidrug resistant organisms. J. Biomed. Mater. Res. Part A 2015, 103, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Arpana, C.; Balashanmugam, P.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surfaces B Biointerfaces 2019, 182, 110339. [Google Scholar] [CrossRef]
- Luxminarayan, L.; Neha, S.; Amit, V.; Khinchi, M.P. The Stages of Drug Discovery and Development Process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67. [Google Scholar]
- Gottrup, F.; Cullen, B.M.; Karlsmark, T.; Bischoff-Mikkelsen, M.; Nisbet, L.; Gibson, M.C. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 2013, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, F.; Jiang, W.; Zhao, A. Nanosilver Dressing in Treating Deep II Degree Burn Wound Infection in Patients with Clinical Studies. Comput. Math. Methods Med. 2021, 2021, 3171547. [Google Scholar] [CrossRef]
- Wang, C.H.; Cherng, J.H.; Liu, C.C.; Fang, T.J.; Hong, Z.J.; Chang, S.J.; Fan, G.Y.; Hsu, S.D. Procoagulant and antimicrobial effects of chitosan in wound healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Kressmann, B.; Malacarne, S.; Toumanova, A.; Jaafar, J.; Lew, D.; Lipsky, B.A. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC Infect. Dis. 2018, 18, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uçkay, I.; Kressmann, B.; Di Tommaso, S.; Portela, M.; Alwan, H.; Vuagnat, H.; Maître, S.; Paoli, C.; Lipsky, B.A. A randomized controlled trial of the safety and efficacy of a topical gentamicin–collagen sponge in diabetic patients with a mild foot ulcer infection. SAGE Open Med. 2018, 6, 2050312118773950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibbald, R.G.; Coutts, P.; Woo, K.Y. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing-clinical trial results. Adv. Skin Wound Care 2011, 24, 78–84. [Google Scholar] [CrossRef] [PubMed]
| Phase of Wound Healing | Effect of Bacterial Contamination | Effect of Bacterial Infection | Ref. |
|---|---|---|---|
| Homeostasis | Unaffected initial wound closure | Impaired healing due to the release of endotoxins, exotoxins, and tissue-destroying enzymes | [16] |
| Inflammation | Enhanced inflammatory cell accumulation and bactericidal and chemotactic activity | Reduced effectiveness of the complement cascade by depletion of different factors; Increased production of proteolytic enzymes, antimicrobial proteins, and reactive oxygen species; Damage to the host tissue due to increased production of microbicidal molecules synthesized by neutrophils; Prolonged inflammatory state that results in high levels of pro-inflammatory mediators and impaired repair; Disbalance in MMPs and their inhibitor concentrations, contributing to wound chronicity | [17,18,19,20,22] |
| Proliferation | Unaffected epithelialization and granulation processes | Suppression of endothelial cell migration and proliferation; Biofilm formation due to considerable bacterial colonization over 105 and consequently decreased epithelialization | [11,25,29] |
| Remodeling | Unaffected tensile strength of the skin as a result of normal healing | Hindered fibroblast replication, and thus limited type I collagen production; Increased production of collagen-digesting enzymes due to the presence of endotoxins | [20,34] |
| Antibiotic | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Gentamicin | 0.2% wt% | Sponge | Curdlan/agarose | Staphylococcus aureus; Pseudomonas aeruginosa | [48] |
| Gentamicin | 0–10 wt% | Nanofibers | Chitosan/alginate | Staphylococcus aureus; Escherichia coli | [60] |
| Gentamicin | 15 wt% | Film | Natural rubber/triethyl citrate/xanthan gum | Staphylococcus aureus; Pseudomonas aeruginosa | [61] |
| Tetracycline hydrochloride | 0.5 wt% | Sponge | Chitosan, chitosan/aloe vera | Bacillus subtilis; Staphylococus aureus; Escherchia coli; Klebsiella pnemoniae | [62] |
| Tetracycline hydrochloride | 0.5% w/v | Nanofibers | Polyvinyl alcohol/chitosan | Staphylococcus aureus; Staphylococcus epidermidis; Escherichia coli | [63] |
| Ciprofloxacin | 10 wt% | Nanofibers | Poly(N-isopropyl-acrylamide-co-acrylamide)/ polycaprolactone | Staphylococcus epidermidis; Escherichia coli | [54] |
| Ciprofloxacin | 0.1 wt% | Film | Chitosan/cellulose | Pseudomonas aeruginosa; Staphylococcus aureus | [55] |
| Silver sulfadiazine | 0.125–0.5 wt% | Nanofibers | Cellulose acetate/silver-sulfadiazine | Escherichia coli; Bacillus subtilis | [56] |
| Silver sulfadiazine/zeolite complex | 5 wt% | Film | Chitosan/zeolite | Escherichia coli; Staphylococcus aureus; Pseudomonas aeruginosa; Candida albicans | [57] |
| Silver sulfadiazine | 0.3–0.6 wt% | Nanofibers | Zein | Escherichia coli; Bacillus subtilis | [59] |
| Vancomycin | Not provided | Hydrogel | Chitosan/polyvinyl alcohol/polyethylene glycol | Staphylococcus aureus | [64] |
| Ampicillin | 2–6 wt% | Hydrogel | Gelatin/gellan gum | Escherichia coli; Staphylococcus aureus | [65] |
| Metallic Nanoparticles | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Ag | 0.2–0.7 wt% | Nanofibers | Polyvinyl alcohol/ polyvinylpyrrolidone/pectin/mafenide acetate | Escherichia coli; Staphylococcus aureus; Pseudomonas aeruginosa | [74] |
| Ag | Not provided | Sponge | Chitosan | Escherichia coli; Staphylococcus aureus; Pseudomonas aeruginosa | [75] |
| Ag | <0.1 wt% | Foam | Lignin-based/polyurethane | Escherichia coli; Staphylococcus aureus | [76] |
| Au | Not provided | Hydrogel | Polyacrylic acid/polyallylamine hydrochloride/poly ethylene glycol | Staphylococcus aureus; Pseudomonas aeruginosa | [77] |
| Au | ≈4 × 10−4 wt% | Gel | Pluronic®F127/hydroxypropyl methylcellulose | Staphylococcus aureus | [78] |
| CuO | 0.05–0.1 wt% | Film | Polycaprolactone | Methicillin-resistant Staphylococcus aureus | [79] |
| CuO | 1 wt% | Nanofibers | Polycaprolactone/gelatin | Escherichia coli; Pseudomonas aeruginosa; Methicillin-resistant Staphylococcus aureus; Staphylococcus aureus | [80] |
| Fe3O4 | 5–15 wt% | Hydrogel | Poly(hydroxyl ethyl methacrylate) | Escherichia coli; Staphylococcus aureus | [81] |
| Fe3O4 | 1–10 wt% | Hydrogel | Chitosan/dextran/glycerol | Staphylococcus aureus; Pseudomonas aeruginosa; Candida albicans | [82] |
| ZnO | 0.05–0.2 wt% | Hydrogel | Polyvinyl alcohol | Bacillus subtilis | [83] |
| ZnO | 10 wt% | Hydrogel | Silk woven fabric/ammonium persulphate/N,N′-bismethylacrylamide | Escherichia coli | [84] |
| Organic Nanoparticles | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Insulin-loaded chitosan | Not provided | Nanofibers | Poly(ε-caprolactone)/collagen | Not provided | [93] |
| Quercetin-loaded graphene oxide | Not provided | Nanofibers | Poly(ε-caprolactone)/quercetin | Staphylococcus aureus | [94] |
| Graphene oxide/grafted graphene oxide | 0.1–1 wt% | Film | Thermoplastic polyurethane | Staphylococcus aureus; Escherichia coli | [95] |
| Aloe-Emodin/Carbon | Not provided | Hydrogel | Polyethylene glycol | Staphylococcus aureus; Escherichia coli | [96] |
| Carbon dots | 0.25–2 wt% | Hydrogel | Chitosan | Staphylococcus aureus | [97] |
| Antibacterial Peptides | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Tet213 | 0.05% w/v | Foam | Alginate/hyaluronic acid/collagen | Escherichia coli; Staphylococcus aureus | [104] |
| ε-polylysine | 24.6% wt% 27.9% wt% | Nanofiber | Hyaluronic acid | Escherichia coli; Staphylococcus aureus | [105] |
| Lysozyme/nisin | 1.14 × 10−5–8.97 × 10−6 mmol/mg | Fibrous material | Polyvinyl alcohol/polyacrylic acid | Staphylococcus aureus | [106] |
| GH12-COOH-M2/ AMP2 | 3.5–17.5% w/v | Nanofiber | Polyethylene oxide | Staphylococcus epidermidis | [107] |
| CM11 | 0.8, 1.6, and 3.2% w/v | Sponge | Silk fibroin/gelatin | Staphylococcus aureus; Escherichia coli; Pseudomonas aeruginosa | [108] |
| Piscidin-1 | 0.4, 0.8, and 1.6% w/v | Hydrogel | Chitosan | Acinetobacter baumannii | [109] |
| Cationic Polymers | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Polydiallyl dimethyl ammonium chloride brushes grafted from bacterial cellulose nanofibers | 5, 10, and 15 wt% | Hydrogel | Polydopamine/polyacrylamide | Escherichia coli; Staphylococcus aureus | [110] |
| Poly(hexamethylene biguanide) hydrochloride | 0.5–10 wt% | Sponge | Silk fibroin | Escherichia coli; Staphylococcus aureus | [111] |
| Cationic nanofibrillated cellulose | 1.4 wt% | Hydrogel | Sodium alginate | Bacillus subtilis; Escherichia coli | [112] |
| [2-(methacryloyloxy)ethyl]trimethyl ammonium chloride | 5–80 wt% | Hydrogel | Polyvinyl alcohol-formaldehyde | Escherichia coli; Staphylococcus aureus; Pseudomonas aeruginosa | [113] |
| Essential Oils or Their Compounds | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Clove, oregano, and tea tree essential oils | Not provided | Hydrogel membrane | Polyvinyl alcohol/starch/glutaraldehyde (cross-linker) | Escherichia coli; Staphylococcus aureus | [118] |
| Satureja mutica, Oliveria decumbens essential oils | 10 wt% | Nanofibers | Chitosan/polyvinyl alcohol (the core) and poly-vinylpyrrolidone/maltodextrin (the shell) | Pseudomonas aeruginosa; Escherichia coli; Staphylococcus aureus; Candida dubliniensis; Candida albicans | [119] |
| Thyme essential oil | 1–3% v/v | Film | Chitosan/alginate | Escherichia coli; Staphylococcus aureus | [120] |
| Fennel, pine, peppermint, and thyme essential oils | 12% | Film | Polyvinyl alcohol/polyvinyl pyrrolidone | Staphylococcus aureus; Enterococcus faecalis; Escherichia coli; Pseudomonas aeruginosa; Candida albicans | [121] |
| Chamomile blue, cinnamon, lavender, tea tree, peppermint, eucalyptus, lemongrass, and lemon essential oils | 16, 50, and 66 wt% | Film | Sodium alginate | Escherichia coli; Candida albicans | [115] |
| Cistus ladanifer essential oils | Not provided | Wound fabric modified by dip coating method | Sodium alginate/silver nanoparticles | Escherichia coli; Klebsiella pneumoniae; Staphylococcus aureus; Bacillus subtilis; Aspergillus niger | [114] |
| Cinnamaldehyde | 0.8 wt% | Nanofibers | Chitosan | Staphylococcus aureus | [122] |
| Polyphenols | Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|---|
| Tea polyphenols | 0.5 and 1 wt% | Foam | Carboxymethyl chitosan/sodium alginate | Staphylococcus aureus; Escherichia coli | [124] |
| Epigallocatechin-3-gallate | 2 wt% | Hydrogel | Silk fibroin/kappa-carrageenan | Escherichia coli; Staphylococcus aureus | [125] |
| Tannic acid, oligomeric proanthocyanidins (−)-epigallocatechin-3-O-gallate | Not provided | Hydrogel | Carboxymethyl chitosan/phenylboronic acid | Staphylococcus aureus; Escherichia coli | [126] |
| Tannic acid | 1 wt% | Nanofibers | Chitosan/pullulan | Escherichia coli | [127] |
| Tannic acid | 1–10% w/v | Hydrogel | Agar/polyvinyl alcohol | Staphylococcus aureus; Escherichia coli | [128] |
| Gallic acid | 20 and 40 wt% | Nanofibers | Cellulose acetate | Staphylococcus aureus | [129] |
| Mango leaf extract (main components: gallic acid, iriflophenone 3-C-β-D-glucoside, iriflophenone 3-C-(2-O-p-hydroxybenzoyl)-β-D-glucoside, and mangiferin) | 5% v/v | Fibrous material | Alginate | Staphylococcus aureus | [130] |
| Curcumin Concentration | Type of Wound Dressing | Biomaterial Composition | Tested Microorganism | Ref. |
|---|---|---|---|---|
| 7 and 13 wt% | Hydrogel film | Gelatin/ionically modified bacterial cellulose | Staphylococcus aureus; Escherichia coli | [133] |
| 1% w/v | Film | Carboxylmethyl guar gum/gelatin | Escherichia coli; Enterobacter aerogenes; Vibrio vulnificus; Pseudomonas aeruginosa; Bacillus cereus; Bacillus subtilis; Lysinibacillus; Staphylococcus aureus | [134] |
| Not provided | Film | Nanocellulose fibers/polyvinyl alcohol | Bacillus cereus; Bacillus coagulans; Streptococcus sp.; methicilin-resistant Staphylococcus aureus; Escherichia coli; Proteus mirabilis; Yersinia sp.; Pseudomonas aeruginosa; Candida albicans; Candida utilis | [135] |
| 10 wt% | Fibrous materials | Cellulose acetate/polyvinylpyrrolidone | Staphylococcus aureus | [136] |
| 0.025–0.1 wt% | Cotton cloth | Cotton cloth/polyvinyl alcohol | Escherichia coli; Bacillus subtilis; Staphylococcus aureus; Proteus vulgaris; Enterococcusi faecalis; Staphylococcus epidermis; Klebsiella pneumoniae; Enterobacter aerogenes; Pseudomonas mendocina; Coliform | [137] |
| Not provided | Fibrous material | Polycaprolactone/quaternary ammonium salt-modified montmorillonite | Escherichia coli; Staphylococcus aureus | [132] |
| 3 and 5 wt% | Nanofiber material | Poly(2-hydroxyethyl methacrylate | Methicillin-resistant staphylococcus aureus; non-methicillin-resistant Staphylococcus aureus; extended-spectrum β-lactamse Escherichia coli and Klebsiella pneumonia; non-extended-spectrum β-lactamse Escherichia coli and Klebsiella pneumonia | [138] |
| Not provided | Transdermal patch | Polyvinyl alcohol/chitosan | Escherichia coli, Pseudomonas aeruginosa; Staphylococcus aureus; Bacillus subtilis | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivcharenko, V.; Trzaskowska, M.; Przekora, A. Wound Dressing Modifications for Accelerated Healing of Infected Wounds. Int. J. Mol. Sci. 2023, 24, 7193. https://doi.org/10.3390/ijms24087193
Vivcharenko V, Trzaskowska M, Przekora A. Wound Dressing Modifications for Accelerated Healing of Infected Wounds. International Journal of Molecular Sciences. 2023; 24(8):7193. https://doi.org/10.3390/ijms24087193
Chicago/Turabian StyleVivcharenko, Vladyslav, Marta Trzaskowska, and Agata Przekora. 2023. "Wound Dressing Modifications for Accelerated Healing of Infected Wounds" International Journal of Molecular Sciences 24, no. 8: 7193. https://doi.org/10.3390/ijms24087193
APA StyleVivcharenko, V., Trzaskowska, M., & Przekora, A. (2023). Wound Dressing Modifications for Accelerated Healing of Infected Wounds. International Journal of Molecular Sciences, 24(8), 7193. https://doi.org/10.3390/ijms24087193






