LcWRKY17, a WRKY Transcription Factor from Litsea cubeba, Effectively Promotes Monoterpene Synthesis
Abstract
1. Introduction
2. Results
2.1. Identification and Chromosomal Location of LcWRKYs
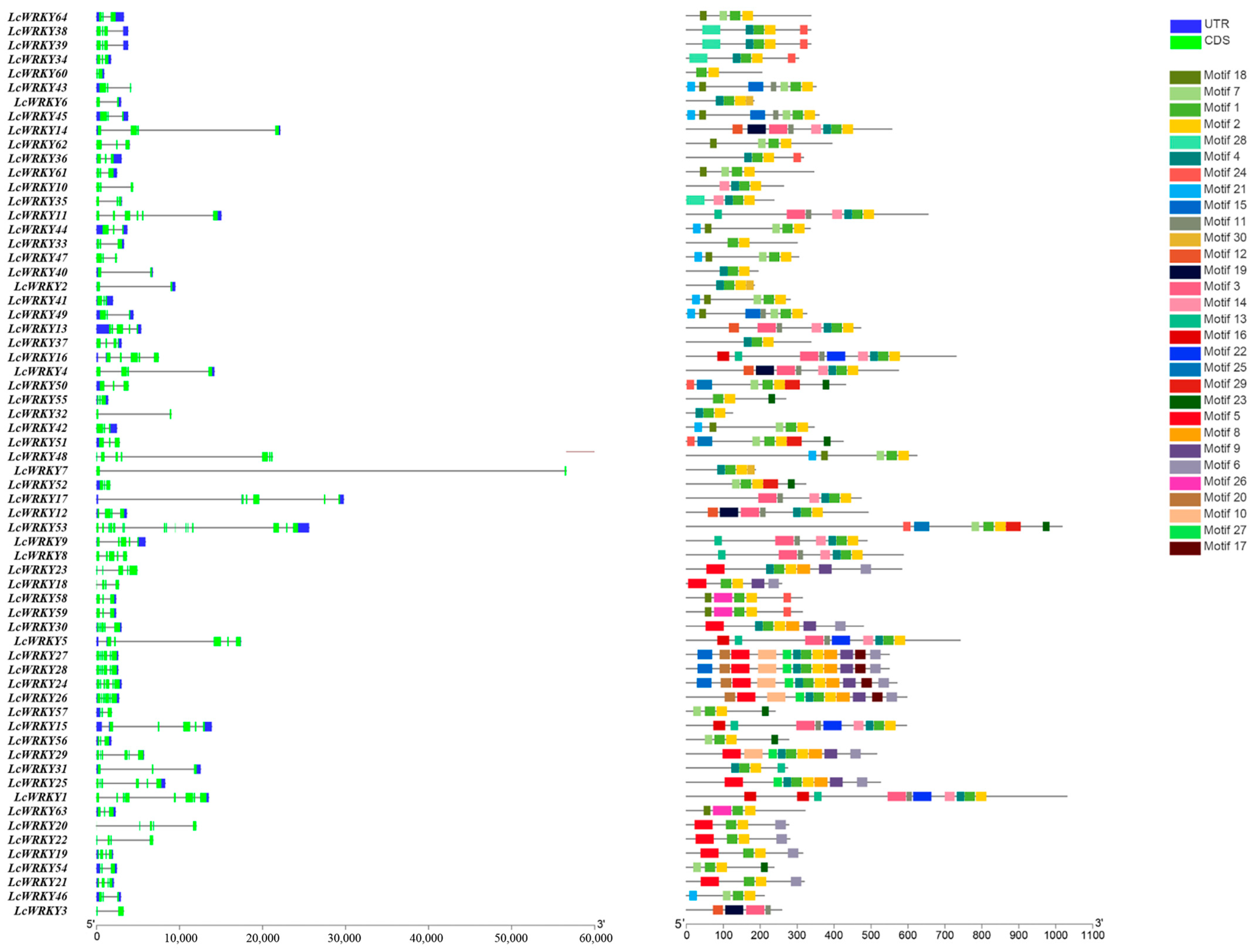
2.2. Motif Analysis and Structural Analysis of LcWRKYs
2.3. Gene Duplication Analysis
2.4. Phylogenetic Analysis of WRKY Phylogeny in L. cubeba
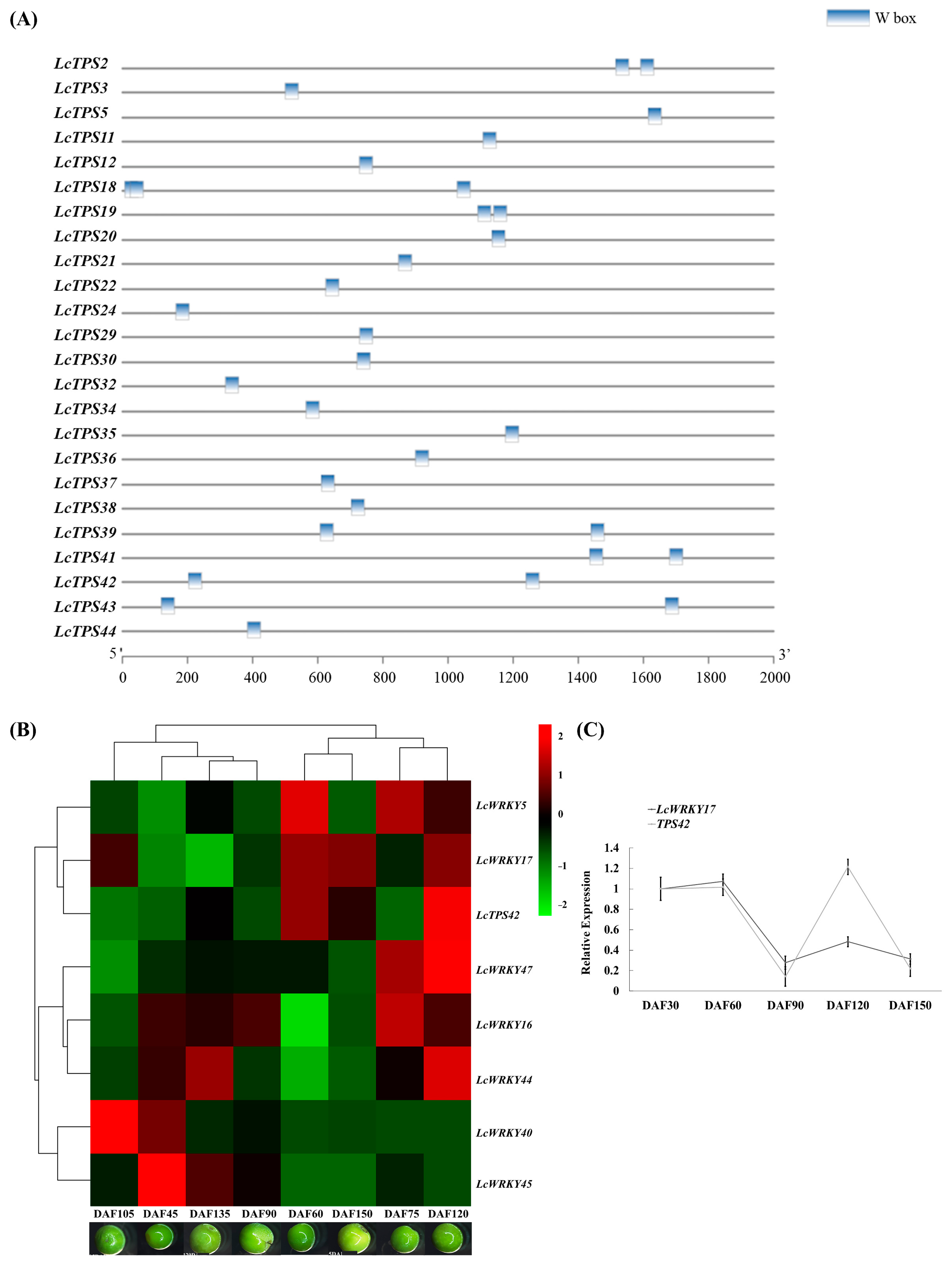
2.5. LcWRKY17, a Group I Protein, Was Co-Expressed with Genes Responsible for Terpene Synthesis
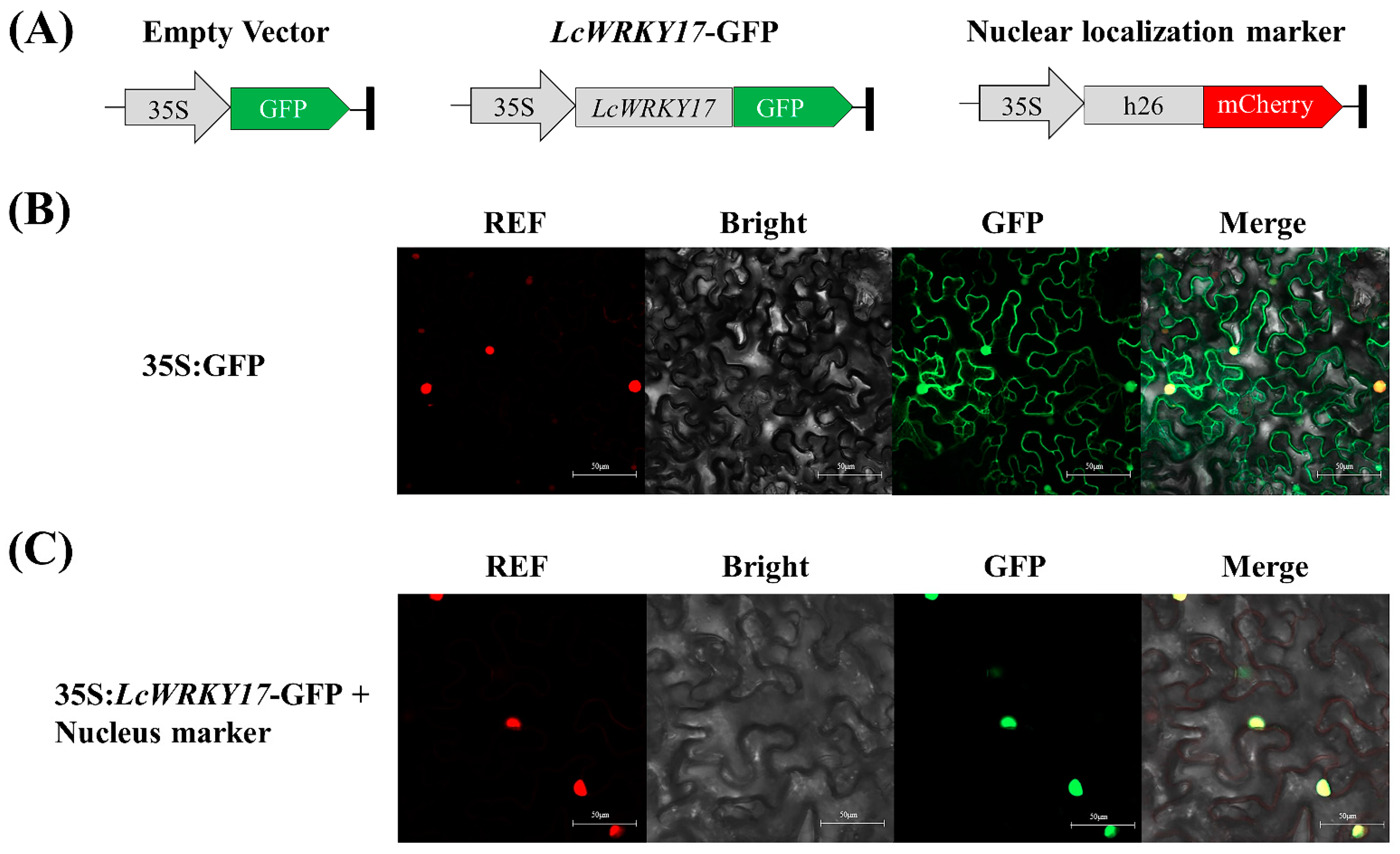
2.6. Subcellular Localization of LcWRKY17
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Transient Overexpression of LcWRKY17 in L. cubeba
2.9. LcWRKY17 Functions by Binding to the LcTPS42 Promoter Binding Element
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Identification of WRKY Genes in L. cubeba
4.3. Chromosomal Location Analysis
4.4. Motif Analysis and Structural Gene Analysis
4.5. Gene Duplication Analysis
4.6. Evolutionary Analysis of the WRKY Gene Family
4.7. Expression Analysis
4.8. Cis-Acting Element Analysis
4.9. LcWRKY17 Gene Cloning
4.10. Subcellular Localization Analysis
4.11. Quantitative Real-Time PCR (qRT-PCR)
4.12. Transient Overexpression of LcWRKY17 in L. cubeba
4.13. Dual-Luciferase and Yeast One-Hybrid (Y1H) Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kumar, P.; Sanyal, R.; Mane, A.B.; Prasanth, D.A.; Patil, M.; Dey, A. Unravelling the regulatory role of miRNAs in secondary metabolite production in medicinal crops. Plant Gene 2021, 27, 100303. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Broun, P.; Somerville, C. Progress in plant metabolic engineering. Proc. Natl. Acad. Sci. USA 2001, 98, 8925–8927. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Robledo-Casados, I.; Sánchez, S. An overview on transcriptional regulators in Streptomyces. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2015, 1849, 1017–1039. [Google Scholar] [CrossRef]
- Wang, Q.; Reddy, V.A.; Panicker, D.; Mao, H.Z.; Kumar, N.; Rajan, C.; Venkatesh, P.N.; Chua, N.H.; Sarojam, R. Metabolic engineering of terpene biosynthesis in plants using a trichome-specific transcription factor MSYABBY5 from spearmint (Mentha spicata). Plant Biotechnol. J. 2016, 14, 1619–1632. [Google Scholar] [CrossRef]
- Paz Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.A.; Saedler, H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987, 6, 3553–3558. [Google Scholar] [CrossRef]
- Bakshi, M.; Oelmüller, R. WRKY transcription factors: Jack of many trades in plants. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef]
- Baillo, E.H.; Hanif, M.S.; Guo, Y.; Zhang, Z.; Xu, P.; Algam, S.A. Genome-wide Identification of WRKY transcription factor family members in sorghum (Sorghum bicolor (L.) moench). PLoS ONE 2020, 15, e236651. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Ling, J.; Jiang, W.; Zhang, Y.; Yu, H.; Mao, Z.; Gu, X.; Huang, S.; Xie, B. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genom. 2011, 12, 471. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Han, J.; Lu, W.; Ren, Z. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef]
- Wu, K.; Guo, Z.; Wang, H.; Li, J. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. MGG 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Ross, C.A.; Liu, Y.; Shen, Q.J. The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 2007, 49, 827–842. [Google Scholar] [CrossRef]
- Ding, W.; Ouyang, Q.; Li, Y.; Shi, T.; Li, L.; Yang, X.; Ji, K.; Wang, L.; Yue, Y. Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiol. 2020, 40, 557–572. [Google Scholar] [CrossRef]
- Rajappa, S.; Krishnamurthy, P.; Kumar, P.P. Regulation of AtKUP2 expression by bHLH and WRKY transcription factors helps to confer increased salt tolerance to Arabidopsis thaliana plants. Front. Plant Sci. 2020, 11, 1311. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Huang, X.; Lyu, L.; Li, W.; Wu, W. Genome-wide identification of WRKY gene family members in black raspberry and their response to abiotic stresses. Sci. Hortic. 2022, 304, 111338. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; You, C.X.; Bi, S.Q.; Wang, X.F.; Hao, Y.J. Md WRKY 40 promotes wounding-induced anthocyanin biosynthesis in association with Md MYB 1 and undergoes Md BT 2-mediated degradation. New Phytol. 2019, 224, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, T.; Shen, Q.; Lu, X.; Pan, Q.; Huang, Y.; Tang, Y.; Fu, X.; Liu, M.; Jiang, W. GLANDULAR TRICHOME-SPECIFIC WRKY 1 promotes artemisinin biosynthesis in Artemisia annua. New Phytol. 2017, 214, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, J.; Gao, C.; Jin, C.; Li, D.; Peng, D.; Du, G.; Li, Y.; Chen, M. Functional characterization of a heterologously expressed Brassica napus WRKY41-1 transcription factor in regulating anthocyanin biosynthesis in Arabidopsis thaliana. Plant Sci. 2018, 268, 47–53. [Google Scholar] [CrossRef]
- Gonzalez, A.; Brown, M.; Hatlestad, G.; Akhavan, N.; Smith, T.; Hembd, A.; Moore, J.; Montes, D.; Mosley, T.; Resendez, J. TTG2 controls the developmental regulation of seed coat tannins in Arabidopsis by regulating vacuolar transport steps in the proanthocyanidin pathway. Dev. Biol. 2016, 419, 54–63. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Wang, S.; Wang, J.; Chen, X. Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kolevski, B.; Smyth, D.R. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 2002, 14, 1359–1375. [Google Scholar] [CrossRef]
- Qu, R.; Cao, Y.; Sun, L.; Wei, L.; Wang, K. Identification and expression analysis of the WRKY gene family in Isatis indigotica. Gene 2021, 783, 145561. [Google Scholar] [CrossRef]
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 2008, 20, 1984–2000. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Gao, M.; Yin, H.; Wu, L.; Wang, Y. Overexpression of geranyl diphosphate synthase small subunit 1 (LcGPPS. SSU1) enhances the monoterpene content and biomass. Ind. Crops Prod. 2020, 143, 111926. [Google Scholar] [CrossRef]
- Sun, X.; Cao, L.; Zhang, S.; Yu, J.; Xu, X.; Xu, C.; Xu, Z.; Qu, C.; Liu, G. Genome-wide analysis of the RGP gene family in Populus trichocarpa and their expression under nitrogen treatment. Gene Expr. Patterns 2020, 38, 119142. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Peng, B.; Hassani, D.; Xie, L.; Liu, H.; Li, Y.; Chen, T.; Liu, P.; Tang, Y.; Li, L. AaWRKY9 contributes to light-and jasmonate-mediated to regulate the biosynthesis of artemisinin in Artemisia annua. New Phytol. 2021, 231, 1858–1874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ren, S.; Liu, X.; Su, L.; Wu, Y.; Zhang, W.; Li, Y.; Jiang, Y.; Wang, H.; Fu, R. SlWRKY35 positively regulates carotenoid biosynthesis by activating the MEP pathway in tomato fruit. New Phytol. 2022, 234, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lin, L.; Chen, Y.; Wang, Y. Digital gene expression profiling to explore differentially expressed genes associated with terpenoid biosynthesis during fruit development in Litsea cubeba. Molecules 2016, 21, 1251. [Google Scholar] [CrossRef]
- Wang, M.; Jiao, Y.; Zhao, Y.; Gao, M.; Wu, L.; Wang, S.; Yang, J.; Wang, J.; Chen, Y.; Wang, Y. Phytohormone and transcriptome of pericarp reveals jasmonate and LcMYC2 are involved in neral and geranial biosynthesis in Litsea cubeba. Ind. Crops Prod. 2022, 177, 114423. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Zhao, Y.; Gao, M.; Wang, J.; Liu, K.; Wang, X.; Wu, L.; Jiao, Y.; Xu, Z. The Litsea genome and the evolution of the laurel family. Nat. Commun. 2020, 11, 1675. [Google Scholar] [CrossRef]
- Kim, C.Y.; Zhang, S. Activation of a mitogen-activated protein kinase cascade induces WRKY family of transcription factors and defense genes in tobacco. Plant J. 2004, 38, 142–151. [Google Scholar] [CrossRef]
- Wang, M.; Vannozzi, A.; Wang, G.; Liang, Y.; Tornielli, G.B.; Zenoni, S.; Cavallini, E.; Pezzotti, M.; Cheng, Z.M. Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Hortic. Res. 2014, 1, 14016. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Chi, Y.; Fan, B.; Chen, Z. Characterization of soybean WRKY gene family and identification of soybean WRKY genes that promote resistance to soybean cyst nematode. Sci. Rep. 2017, 7, 17804. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Gao, M.; Wu, L.; Xiong, S.; Wang, S.; Gao, J.; Zhao, Y.; Wang, Y. Comprehensive identification of bHLH transcription factors in Litsea cubeba reveals candidate gene involved in the monoterpene biosynthesis pathway. Front. Plant Sci. 2022, 13, 1081335. [Google Scholar] [CrossRef]
- Islam, S.; Sajib, S.D.; Jui, Z.S.; Arabia, S.; Islam, T.; Ghosh, A. Genome-wide identification of glutathione S-transferase gene family in pepper, its classification, and expression profiling under different anatomical and environmental conditions. Sci. Rep. 2019, 9, 9101. [Google Scholar] [CrossRef]
- Kayum, M.; Park, J.; Nath, U.K.; Saha, G.; Biswas, M.K.; Kim, H.; Nou, I. Genome-wide characterization and expression profiling of PDI family gene reveals function as abiotic and biotic stress tolerance in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom. 2017, 18, 885. [Google Scholar] [CrossRef]
- Kondrashov, F.A. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc. R. Soc. B Biol. Sci. 2012, 279, 5048–5057. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Fernando, W.G.D. Genomic Variations and Mutational Events Associated with Plant–Pathogen Interactions. Biology 2022, 11, 421. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Chen, Y.; Liu, Y.; Wu, Y.; Ren, S.; Li, L. Identification, evolution and expression analysis of WRKY gene family in Eucommia ulmoides. Genomics 2021, 113, 3294–3309. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Bach, T.J.; Boronat, A.; Caelles, C.; Ferrer, A.; Weber, T.; Wettstein, A. Aspects related to mevalonate biosynthesis in plants. Lipids 1991, 26, 637–648. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, H.; Liu, M.; Chen, B.; Dong, N.; Chang, X.; Wang, J.; Xing, S.; Peng, H.; Zha, L. Transcriptome-wide identification of WRKY transcription factors and their expression profiles in response to methyl jasmonate in Platycodon grandiflorus. Plant Signal. Behav. 2022, 17, 2089473. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, C.; Xu, C.; Sun, J.; Grierson, D.; Zhang, B.; Chen, K. Integration of metabolite profiling and transcriptome analysis reveals genes related to volatile terpenoid metabolism in finger citron (C. medica var. sarcodactylis). Molecules 2019, 24, 2564. [Google Scholar] [CrossRef]
- Han, J.; Wang, H.; Lundgren, A.; Brodelius, P.E. Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochemistry 2014, 102, 89–96. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Yu, L.; Jiang, H.; Guo, Z.; Xu, H.; Jiang, S.; Fang, H.; Zhang, J.; Su, M. MdWRKY11 participates in anthocyanin accumulation in red-fleshed apples by affecting MYB transcription factors and the photoresponse factor MdHY5. J. Agric. Food Chem. 2019, 67, 8783–8793. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Haring, M.A.; Schuurink, R.C. RNA sequencing on Solanum lycopersicum trichomes identifies transcription factors that activate terpene synthase promoters. BMC Genom. 2014, 15, 402. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, M.; Wu, L.; Wu, H.; Chen, Y.; Wang, Y. Expression network of transcription factors in resistant and susceptible tung trees responding to Fusarium wilt disease. Ind. Crops Prod. 2018, 122, 716–725. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Chen, Y.; Gao, M.; Wu, L.; Zhao, Y.; Wang, Y. LcWRKY17, a WRKY Transcription Factor from Litsea cubeba, Effectively Promotes Monoterpene Synthesis. Int. J. Mol. Sci. 2023, 24, 7210. https://doi.org/10.3390/ijms24087210
Gao J, Chen Y, Gao M, Wu L, Zhao Y, Wang Y. LcWRKY17, a WRKY Transcription Factor from Litsea cubeba, Effectively Promotes Monoterpene Synthesis. International Journal of Molecular Sciences. 2023; 24(8):7210. https://doi.org/10.3390/ijms24087210
Chicago/Turabian StyleGao, Jing, Yicun Chen, Ming Gao, Liwen Wu, Yunxiao Zhao, and Yangdong Wang. 2023. "LcWRKY17, a WRKY Transcription Factor from Litsea cubeba, Effectively Promotes Monoterpene Synthesis" International Journal of Molecular Sciences 24, no. 8: 7210. https://doi.org/10.3390/ijms24087210
APA StyleGao, J., Chen, Y., Gao, M., Wu, L., Zhao, Y., & Wang, Y. (2023). LcWRKY17, a WRKY Transcription Factor from Litsea cubeba, Effectively Promotes Monoterpene Synthesis. International Journal of Molecular Sciences, 24(8), 7210. https://doi.org/10.3390/ijms24087210





