Fingerprinting Evaluation and Gut Microbiota Regulation of Polysaccharides from Jujube (Ziziphus jujuba Mill.) Fruit
Abstract
:1. Introduction
2. Results and Discussion
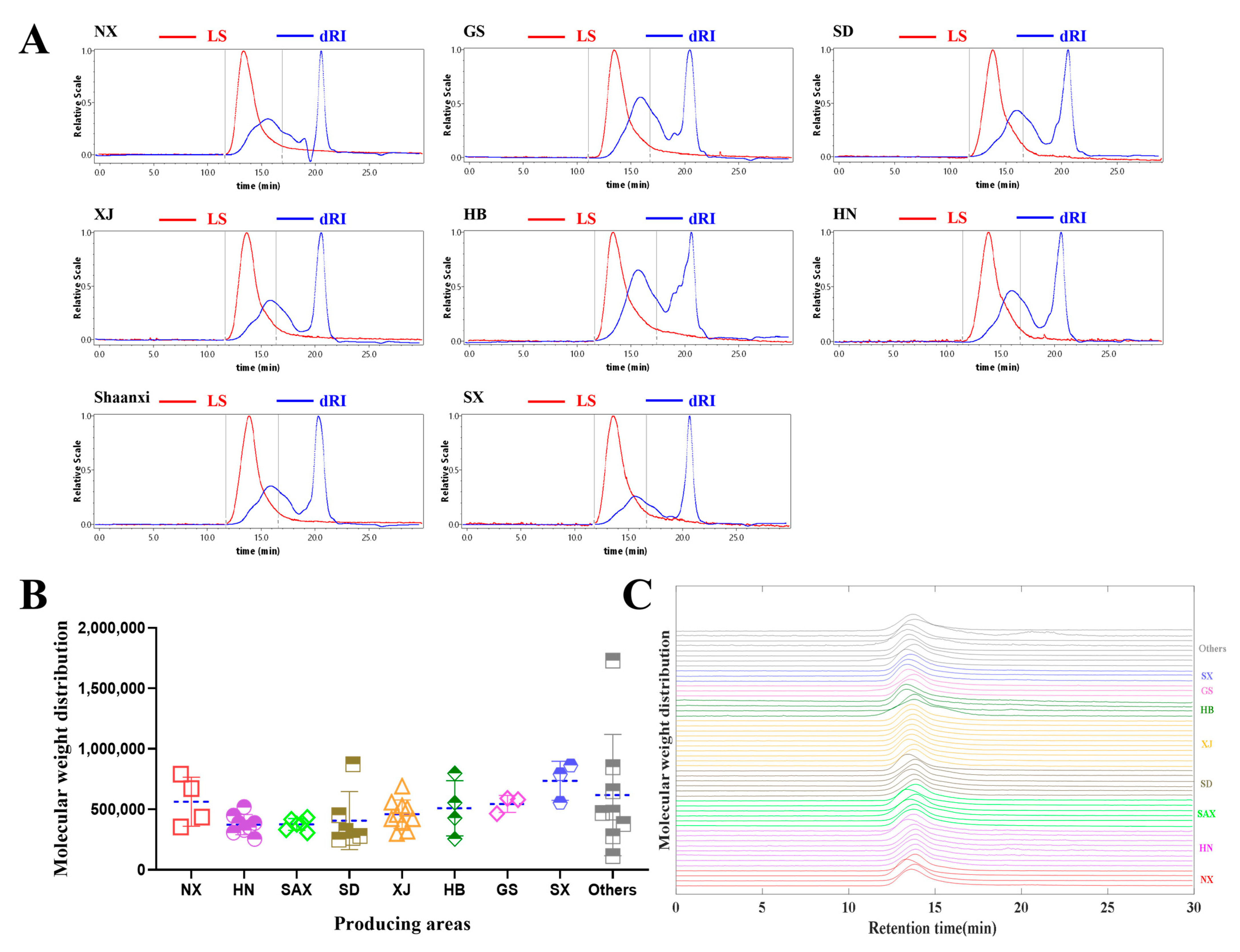
2.1. Determination of Total Polysaccharides Content and MWD of Polysaccharides
2.1.1. Comparison of Total Polysaccharides Content of Jujube Fruits
2.1.2. Comparison of MWD of Polysaccharides from Jujube Fruits
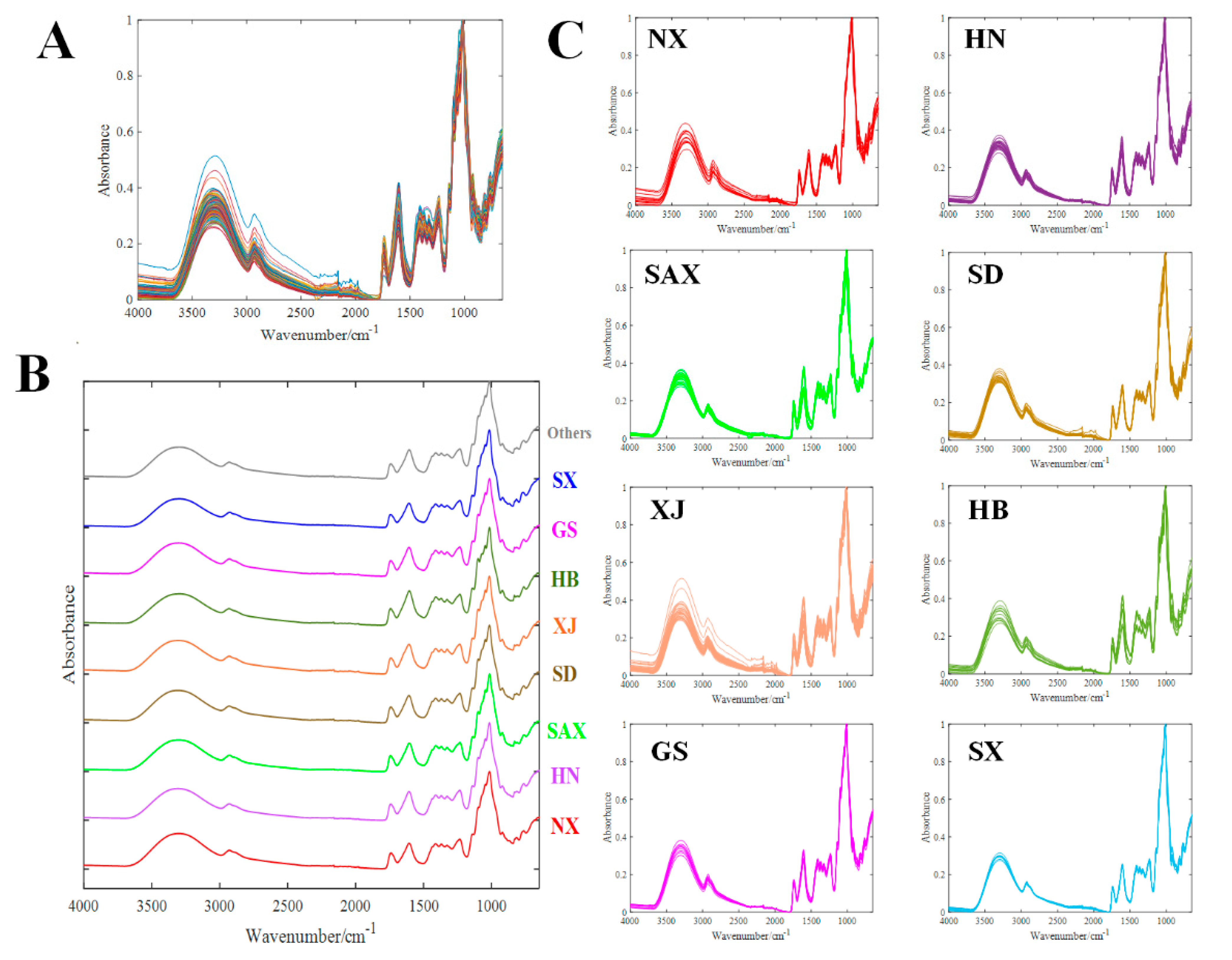
2.2. Polysaccharides Profiling and Fingerprinting Evaluation by FT-IR
2.3. Oligosaccharides Profiling Analysis
2.3.1. Characterization of Oligosaccharides by High-Resolution Mass Spectrometry
2.3.2. Distribution Profile and Fingerprint Evaluation of Oligosaccharides
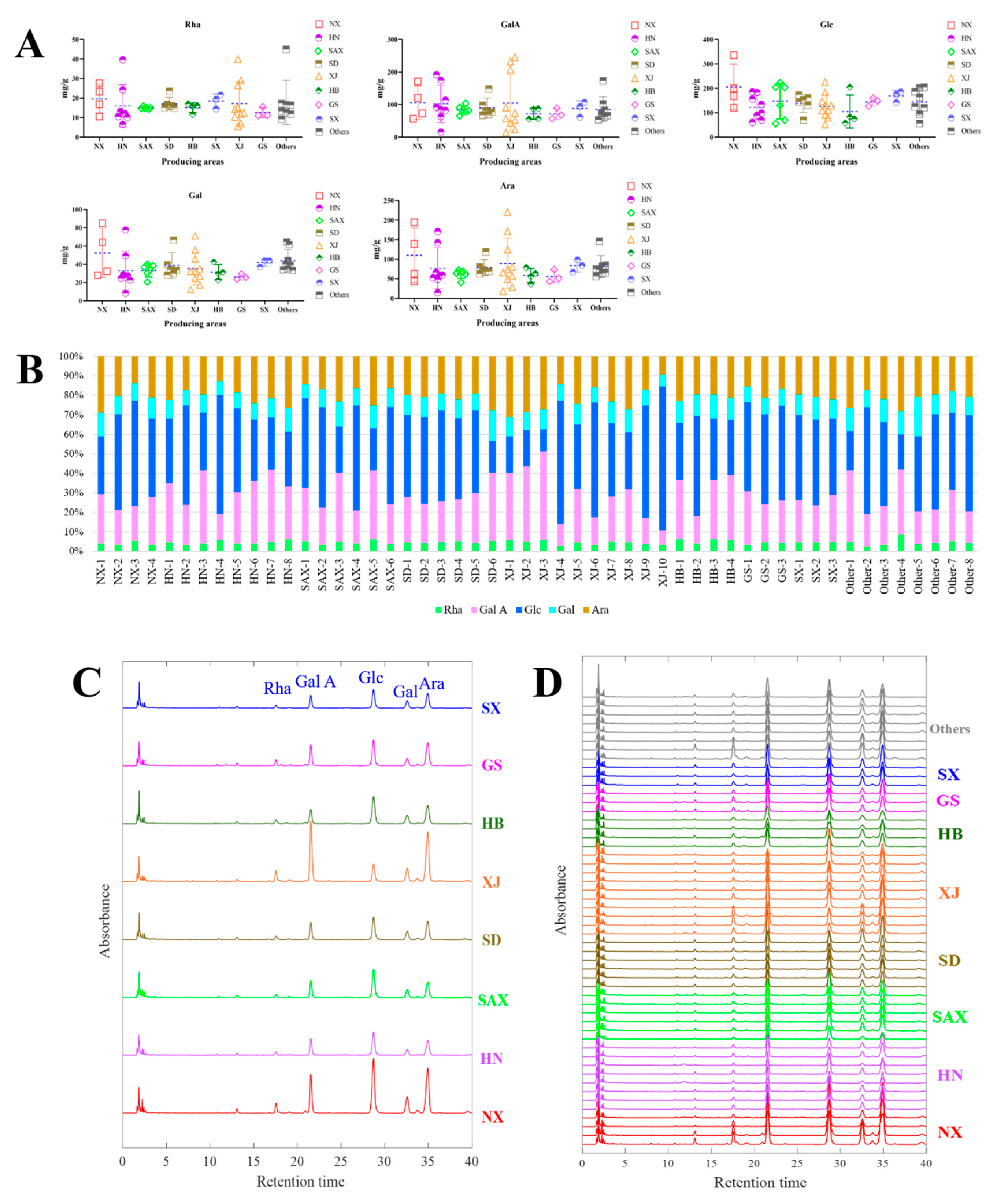
2.4. Monosaccharides Profiling Analysis
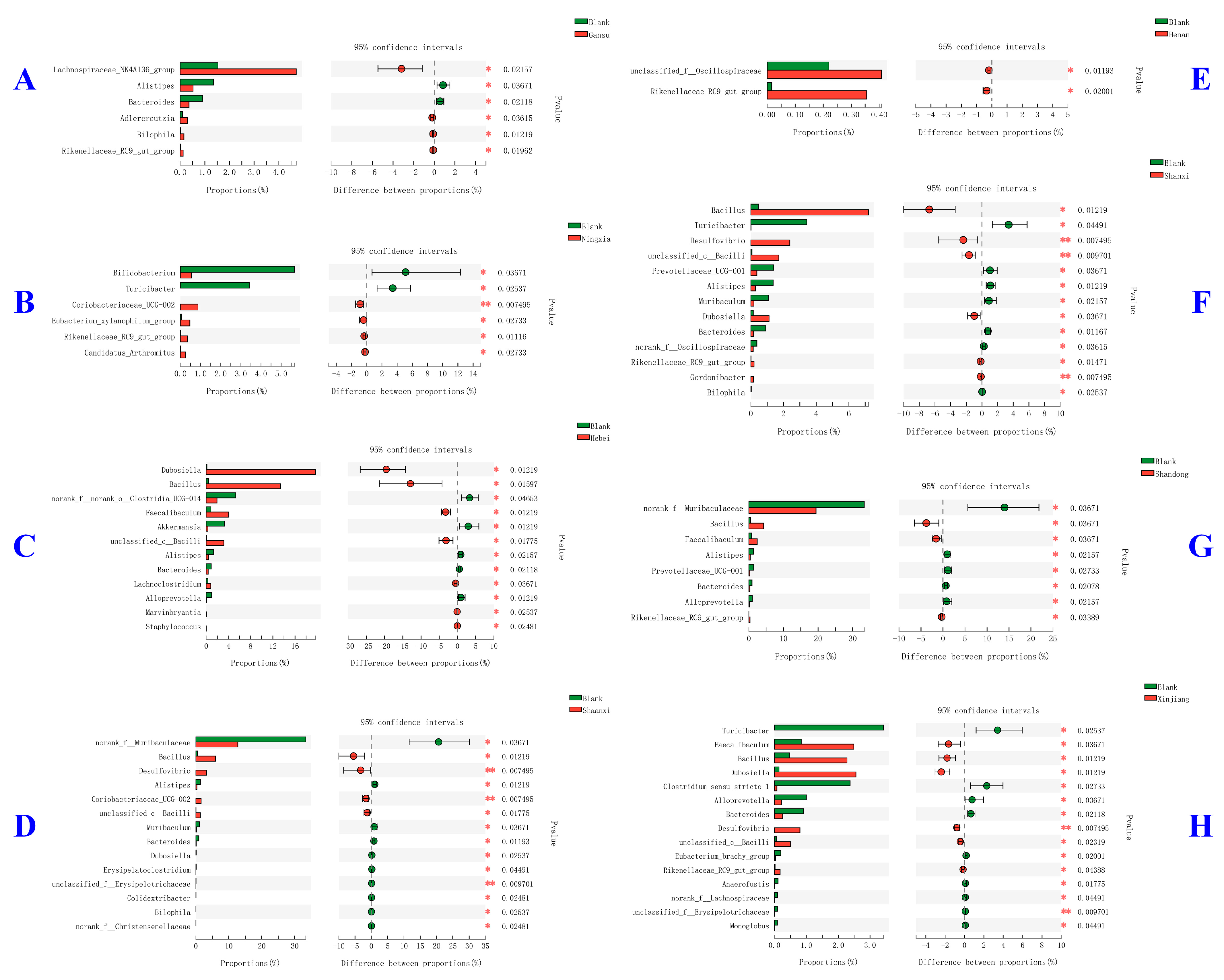
2.5. Modulatory Effects of Different Jujube Fruits Polysaccharides on Mice Gut Microbiota
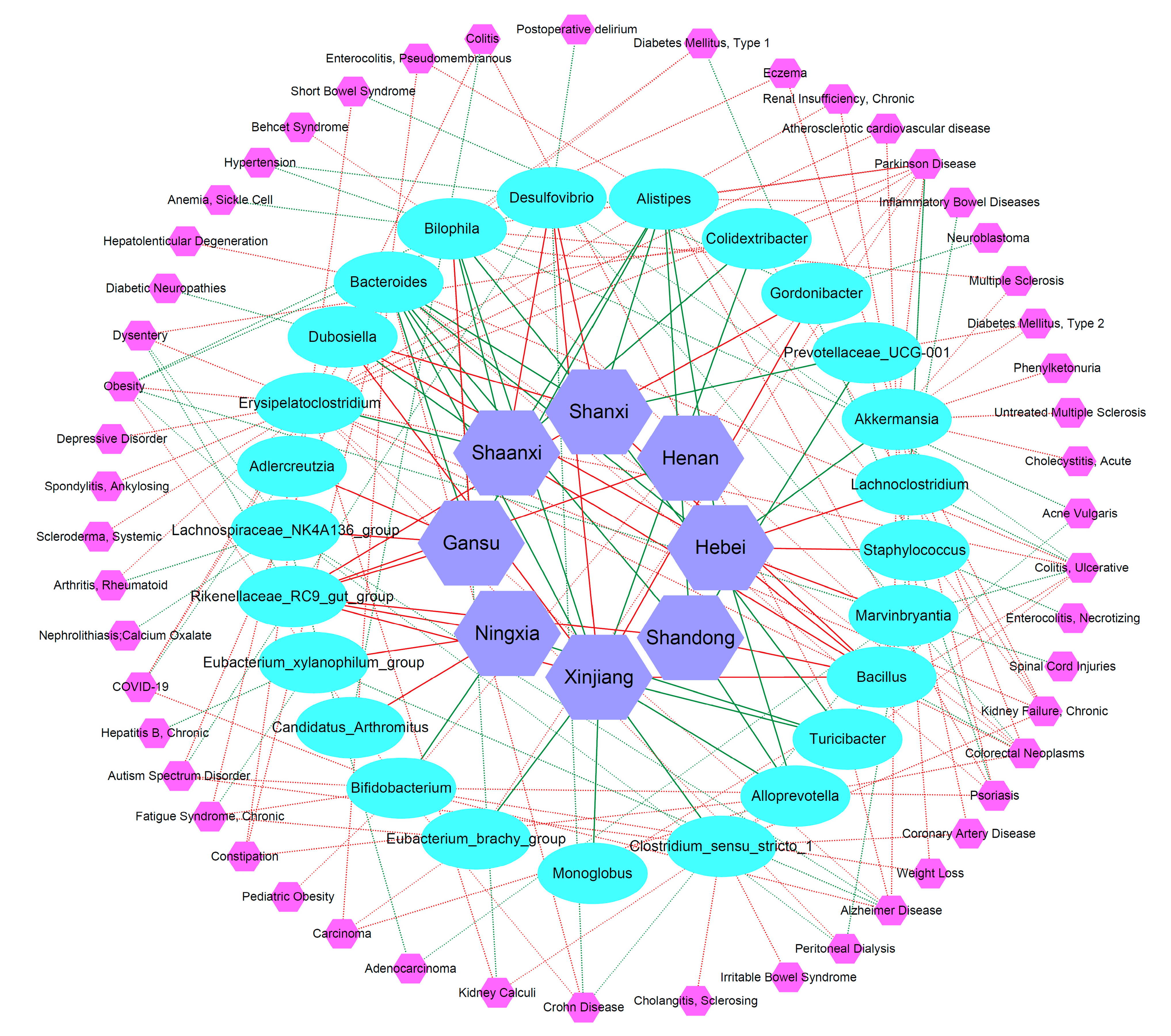
2.6. Network Analysis of Jujube Fruits Polysaccharides-Gut Microbiota-Potential Diseases
3. Materials and Methods
3.1. Materials
3.2. Preparation of Total Polysaccharides, Hydrolyzed Oligosaccharides, and Monosaccharides
3.3. Determination of Total Polysaccharides Content
3.4. Determination of MWD of Polysaccharides by HPGPC-RID-MALLS
3.5. Polysaccharides Profiling by FT-IR Spectroscopic Analysis
3.6. Oligosaccharides Profiling Analysis
3.7. Monosaccharides Profiling Analysis
3.8. Modulatory Effects of Jujube Fruits Polysaccharides on Mice Gut Microbiota
3.8.1. The 16S rRNA Sequencing of Mice Feces
3.8.2. Network Analysis of Jujube Fruits Polysaccharides-Gut Microbiota-Potential Diseases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Shi, Q.; Han, G.; Liu, Y.; Jiang, J.; Jia, Y.; Li, X. Nutrient composition and quality traits of dried jujube fruits in seven producing areas based on metabolomics analysis. Food Chem. 2022, 385, 132627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC-MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Z.; Liu, L.; Wang, L.; Li, X.; Ban, Z.; Chen, C.; Zhu, Y. Partial compression increases acidity, but decreases phenolics in jujube fruit: Evidence from targeted metabolomics. Food Res. Int. 2023, 164, 112388. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Vidyarthi, S.K.; Zhang, R. Active components and antioxidant activity of thirty-seven varieties of Chinese jujube fruits (Ziziphus jujuba Mill.). Int. J. Food Prop. 2021, 24, 1479–1494. [Google Scholar] [CrossRef]
- Lu, Y.; Bao, T.; Mo, J.; Ni, J.; Chen, W. Research advances in bioactive components and health benefits of jujube (Ziziphus jujuba Mill.) fruit. J. Zhejiang Univ. Sci. B 2021, 22, 431–449. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid. Based Complement. Altern. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.T.; Yuan, Q.; Guo, H.; Fu, Y.; Li, F.; Wang, S.P.; Gan, R.Y. Dynamic changes of structural characteristics of snow chrysanthemum polysaccharides during in vitro digestion and fecal fermentation and related impacts on gut microbiota. Food Res. Int. 2021, 141, 109888. [Google Scholar] [CrossRef]
- Chi, A.; Kang, C.; Zhang, Y.; Tang, L.; Guo, H.; Li, H.; Zhang, K. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus Jujube on Chronic Fatigue Syndrome rats. Carbohydr. Polym. 2015, 122, 189–196. [Google Scholar] [CrossRef]
- Liu, X.-X.; Liu, H.-M.; Yan, Y.-Y.; Fan, L.-Y.; Yang, J.-N.; Wang, X.-D.; Qin, G.-Y. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. LWT 2020, 117, 108645. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiao, J.; Jiang, D.; Liu, Y.; Gou, Z.; Li, J.; Shi, M.; Wang, X.; Guo, Y.; Ma, L.; et al. Inhibitory effects of a water-soluble jujube polysaccharide against biofilm-forming oral pathogenic bacteria. Int. J. Biol. Macromol. 2022, 208, 1046–1062. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, X.; Yang, S.; Yu, L.; Gao, Q.; Yang, X.; Zhao, Y. Characterization of the antioxidative polysaccharides from Ziziphus jujube cv. Goutouzao and its tumor-inhibitory effects on human colorectal carcinoma LoVo cells via immunocyte activation. J. Food Biochem. 2020, 44, e13462. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, Y.; Ren, W.C.; Su, Y.; Li, J.; Du, Y.Q.; Wang, Q.H.; Kuang, H.X. Rapid determination and origin identification of total polysaccharides contents in Schisandra chinensis by near-infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120327. [Google Scholar] [CrossRef]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Wang, H.; Yu, W.; Li, Q. Structural diversity and physicochemical properties of polysaccharides isolated from pumpkin (Cucurbita moschata) by different methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, C.; Zhang, R. A novel acidic polysaccharide from blackened jujube: Structural features and antitumor activity in vitro. Front. Nutr. 2022, 9, 1001334. [Google Scholar] [CrossRef]
- Cheong, K.L.; Wu, D.T.; Deng, Y.; Leong, F.; Zhao, J.; Zhang, W.J.; Li, S.P. Qualitation and quantification of specific polysaccharides from Panax species using GC-MS, saccharide mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016, 153, 47–54. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, W.; Zhao, J.; Chen, B.; Liu, F.; Li, S. Characterization and Comparison of Bioactive Polysaccharides from Grifola frondosa by HPSEC-MALLS-RID and Saccharide Mapping Based on HPAEC-PAD. Polymers 2022, 15, 208. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Huangfu, L.-T.; Dong, L.-L.; Liu, S.-L. Functional groups and antioxidant activities of polysaccharides from five categories of tea. Ind. Crops Prod. 2014, 58, 31–35. [Google Scholar] [CrossRef]
- Li, L.F.; Wong, T.L.; Zhang, J.X.; Zhou, L.S.; Bai, S.P.; Fung, H.Y.; Cheng, H.Y.; Zhang, Q.W.; Zheng, H.M.; Bao, W.R.; et al. An oligosaccharide-marker approach to quantify specific polysaccharides in herbal formula by LC-qTOF-MS: Danggui Buxue Tang, a case study. J. Pharm. Biomed. Anal. 2020, 185, 113235. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Zhang, Z.; Zhang, X.; Yang, H.; Lu, H.; Liu, M.; Shao, Y.; Zhao, X.; Zhang, H. Oligosaccharide mapping analysis by HILIC-ESI-HCD-MS/MS for structural elucidation of fucoidan from sea cucumber Holothuria floridana. Carbohydr. Polym. 2022, 275, 118694. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.L.; Ma, H.M.; Ke, Q.H.; Wang, S.Y.; Zhou, H.F.; Zheng, M. The determination of monosaccharide in different years Qingzhuan Dark Tea polysaccharide by liquid chromatography-mass spectrometry. Phytochem. Anal. 2022, 33, 577–589. [Google Scholar] [CrossRef]
- Wen, M.; Cui, Y.; Dong, C.X.; Zhang, L. Quantitative changes in monosaccharides of Keemun black tea and qualitative analysis of theaflavins-glucose adducts during processing. Food Res. Int. 2021, 148, 110588. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, R.; Li, H.; Wang, Y.; Luo, Y.; Zou, J.; Zhao, B.; Chen, S. New insight into the joint significance of dietary jujube polysaccharides and 6-gingerol in antioxidant and antitumor activities. RSC Adv. 2021, 11, 33219–33234. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, Y.S.; Park, H.Y. Pectic polysaccharides: Targeting gut microbiota in obesity and intestinal health. Carbohydr. Polym. 2022, 287, 119363. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Q.; Gao, Z.; Lin, X.; Zhou, K.; Cheng, X.; Chitrakar, B.; Chen, H.; Zhao, W. In vitro digestion and fecal fermentation behaviors of polysaccharides from Ziziphus Jujuba cv. Pozao and its interaction with human gut microbiota. Food Res. Int. 2022, 162, 112022. [Google Scholar] [CrossRef]
- Jamshidi, M.; Vij, J. Influence of bias voltage on the orientation of core carbonyl and phenyl groups of a ferroelectric liquid crystal mixture studied by polarised FT-IR spectroscopy. Mol. Cryst. Liq. Cryst. 2000, 12, 153–167. [Google Scholar]
- Moreno, F.J.; Montilla, A.; Villamiel, M.; Corzo, N.; Olano, A. Analysis, structural characterization, and bioactivity of oligosaccharides derived from lactose. Electrophoresis 2014, 35, 1519–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xu, J.; Wu, Q.; Fang, H.; Shao, X.; Ouyang, X.; He, Z.; Deng, Y.; Chen, C. Gut Microbiota Mediates the Susceptibility of Mice to Sepsis-Associated Encephalopathy by Butyric Acid. J. Inflamm. Res. 2022, 15, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Meng, J.; Xu, L.; Chang, M.; Feng, C.; Geng, X.; Cheng, Y.; Guo, D.; Liu, R.; Wang, Z.; et al. In-depth investigation of the hypoglycemic mechanism of Morchella importuna polysaccharide via metabonomics combined with 16S rRNA sequencing. Int. J. Biol. Macromol. 2022, 220, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Wang, C.; Lai, R.; Peng, X.; Luo, J. Lycium barbarum polysaccharide modulates gut microbiota to alleviate rheumatoid arthritis in a rat model. NPJ Sci. Food 2022, 6, 34. [Google Scholar] [CrossRef]
- Jezewska-Frackowiak, J.; Seroczynska, K.; Banaszczyk, J.; Jedrzejczak, G.; Zylicz-Stachula, A.; Skowron, P.M. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Huang, S.; Jiang, X.; Chen, Q.; Hu, Z.; Wang, F.; Zhao, Y.; Xin, G.-Z.; Chen, J. Jujube polysaccharides mitigated anemia in rats with chronic kidney disease: Regulation of short chain fatty acids release and erythropoietin production. J. Funct. Foods 2021, 86, 104673. [Google Scholar] [CrossRef]
- Chang, M.; Shi, S.; Liu, H.; Tu, J.; Yan, Z.; Ding, S. Extraction, characterization, and in vivo antitumor activity of a novel polysaccharide from Coriandrum sativum L. J. Food Biochem. 2022, 46, e14323. [Google Scholar] [CrossRef]
- Wong, T.L.; Li, L.F.; Zhang, J.X.; Bai, S.P.; Zhou, L.S.; Fung, H.Y.; Zhang, Q.W.; Ma, D.L.; Leung, C.H.; Zhao, Z.Z.; et al. Oligosaccharide-marker approach for qualitative and quantitative analysis of specific polysaccharide in herb formula by ultra-high-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry: Dendrobium officinale, a case study. J. Chromatogr. A 2019, 1607, 460388. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lu, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Yu, C.; Ahmadi, S.; Shen, S.; Wu, D.; Xiao, H.; Ding, T.; Liu, D.; Ye, X.; Chen, S. Structure and fermentation characteristics of five polysaccharides sequentially extracted from sugar beet pulp by different methods. Food Hydrocoll. 2022, 126, 107462. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zhang, J.; Yan, M.; Li, D.; Zhou, S.; Feng, J.; Liu, Y. Research on Extraction, Structure Characterization and Immunostimulatory Activity of Cell Wall Polysaccharides from Sparassis latifolia. Polymers 2022, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Cai, Y.; Qian, K.; Li, X.; Ren, J.; Wang, P.; Fu, T.; Zhao, T.; Cheng, L.; Shi, L.; et al. gutMDisorder v2.0: A comprehensive database for dysbiosis of gut microbiota in phenotypes and interventions. Nucleic Acids Res. 2023, 51, D717–D722. [Google Scholar] [CrossRef] [PubMed]
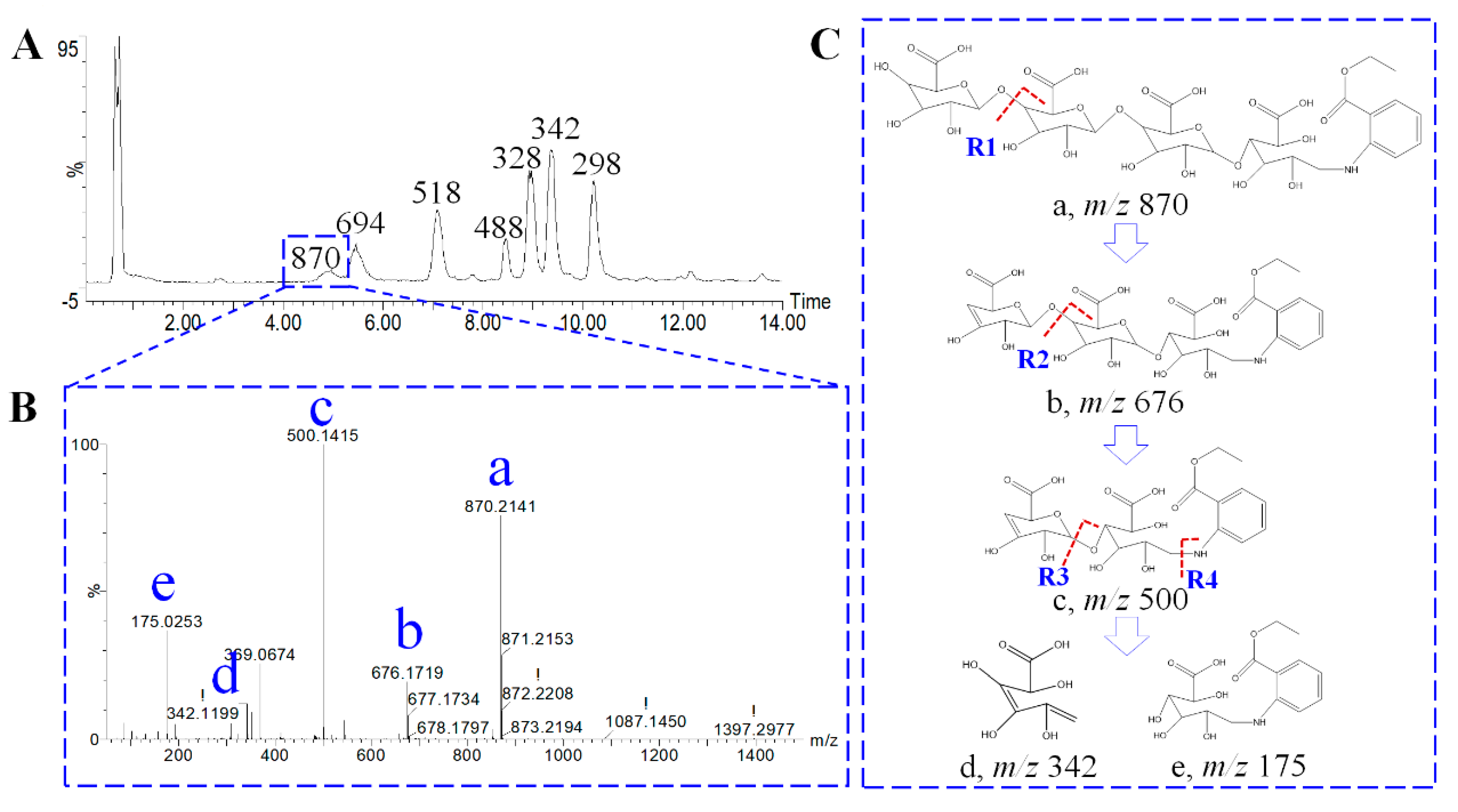


| RT | m/z | Formula | Addition | Composition | DP |
|---|---|---|---|---|---|
| 10.21 | 298.13 | C14H20NO6 | M-H | Arabinose | 1 |
| 9.36 | 342.12 | C15H20NO8 | M-H | Galacturonic acid | 1 |
| 8.92 | 328.14 | C15H22NO7 | M-H | Glucose | 1 |
| 8.46 | 488.17 | C21H30NO12 | M-H | Two glucoses | 2 |
| 7.08 | 518.15 | C21H28NO14 | M-H | Two galacturonic acids | 2 |
| 5.46 | 694.18 | C27H36NO20 | M-H | Three galacturonic acids | 3 |
| 4.97 | 870.21 | C33H44NO26 | M-H | Four galacturonic acids | 4 |
| Group | Chao | Shannon | Shannoneven | pd |
|---|---|---|---|---|
| Blank | 177.7 ± 24.5 | 3.707 ± 0.372 | 0.717 ± 0.062 | 16.99 ± 1.63 |
| Gansu | 169.2 ± 14.0 | 3.782 ± 0.220 | 0.739 ± 0.045 | 16.51 ± 1.03 |
| Hebei | 169.8 ± 25.0 | 3.599 ± 0.192 | 0.704 ± 0.031 | 16.87 ± 1.98 |
| Henan | 186.4 ± 27.1 | 3.812 ± 0.414 | 0.732 ± 0.077 | 16.96 ± 1.56 |
| Ningxia | 178.3 ± 19.7 | 4.129 ± 0.278 | 0.797 ± 0.040 | 16.79 ± 1.02 |
| Shaanxi | 131.4 ± 25.3 * | 2.665 ± 0.640 * | 0.548 ± 0.108 * | 13.92 ± 1.85 * |
| Shandong | 163.6 ± 22.5 | 3.23 ± 0.428 | 0.635 ± 0.074 | 16.11 ± 1.54 |
| Shanxi | 162.8 ± 15.4 | 3.479 ± 0.275 | 0.686 ± 0.049 | 16.77 ± 0.91 |
| Xinjiang | 127 ± 14.1 * | 2.843 ± 0.797 | 0.586 ± 0.155 | 13.71 ± 1.06 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wu, M.; Wei, W.; An, Y.; Li, Y.; Wen, Q.; Zhang, D.; Zhang, J.; Yao, C.; Bi, Q.; et al. Fingerprinting Evaluation and Gut Microbiota Regulation of Polysaccharides from Jujube (Ziziphus jujuba Mill.) Fruit. Int. J. Mol. Sci. 2023, 24, 7239. https://doi.org/10.3390/ijms24087239
Li Z, Wu M, Wei W, An Y, Li Y, Wen Q, Zhang D, Zhang J, Yao C, Bi Q, et al. Fingerprinting Evaluation and Gut Microbiota Regulation of Polysaccharides from Jujube (Ziziphus jujuba Mill.) Fruit. International Journal of Molecular Sciences. 2023; 24(8):7239. https://doi.org/10.3390/ijms24087239
Chicago/Turabian StyleLi, Zhenwei, Menglei Wu, Wenlong Wei, Yaling An, Yun Li, Qiuyi Wen, Daidi Zhang, Jianqing Zhang, Changliang Yao, Qirui Bi, and et al. 2023. "Fingerprinting Evaluation and Gut Microbiota Regulation of Polysaccharides from Jujube (Ziziphus jujuba Mill.) Fruit" International Journal of Molecular Sciences 24, no. 8: 7239. https://doi.org/10.3390/ijms24087239






