Derrone Targeting the TGF Type 1 Receptor Kinase Improves Bleomycin-Mediated Pulmonary Fibrosis through Inhibition of Smad Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Low Concentrations of Derrone Exhibit No Cytotoxicity in MRC-5 Cells
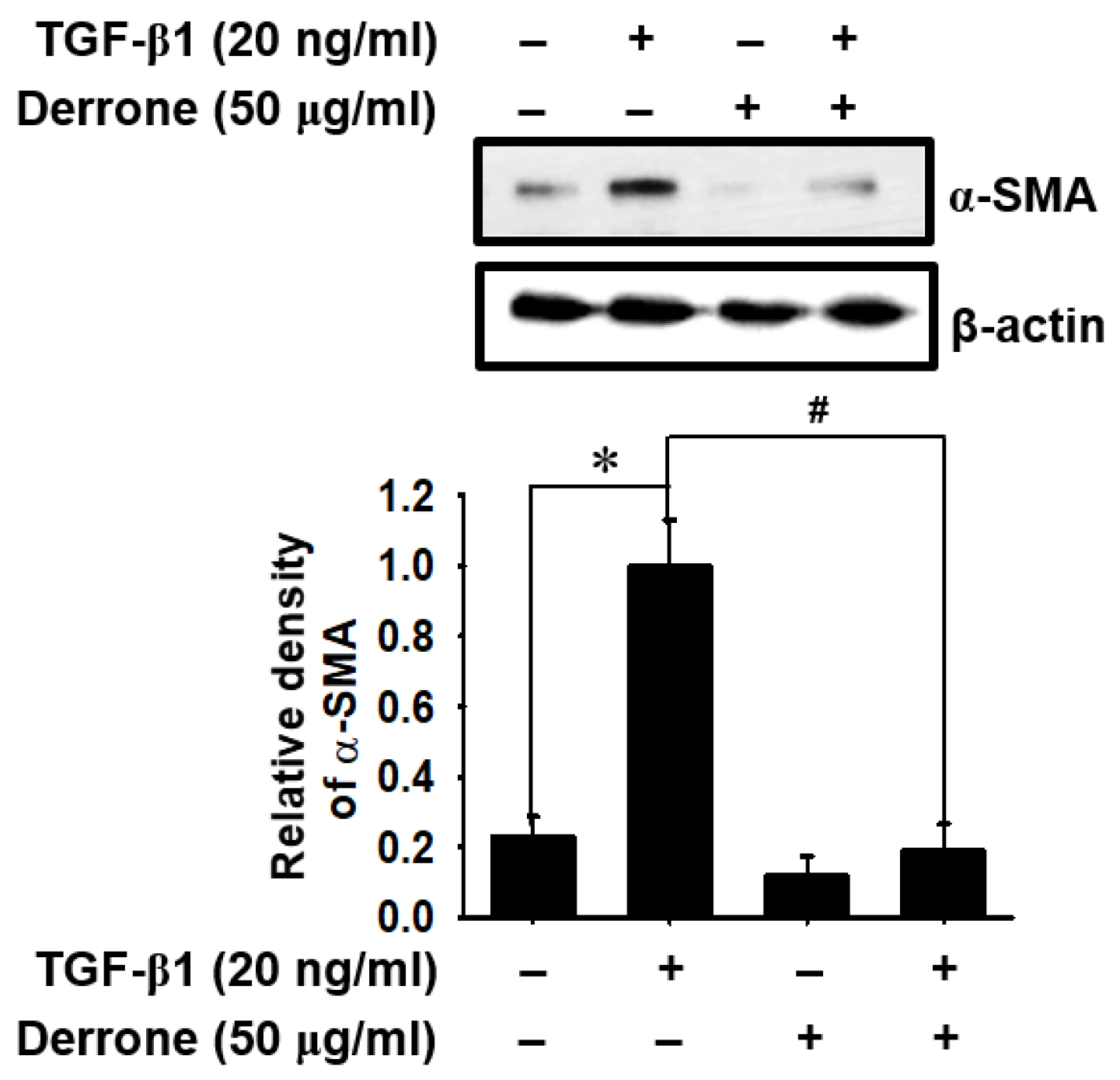
2.2. Derrone Downregulates TGF-β1-Induced Myofibroblast Marker Expression
2.3. Derrone Attenuates Pulmonary Fibrosis in Lungs of Bleomycin-Challenged Mice
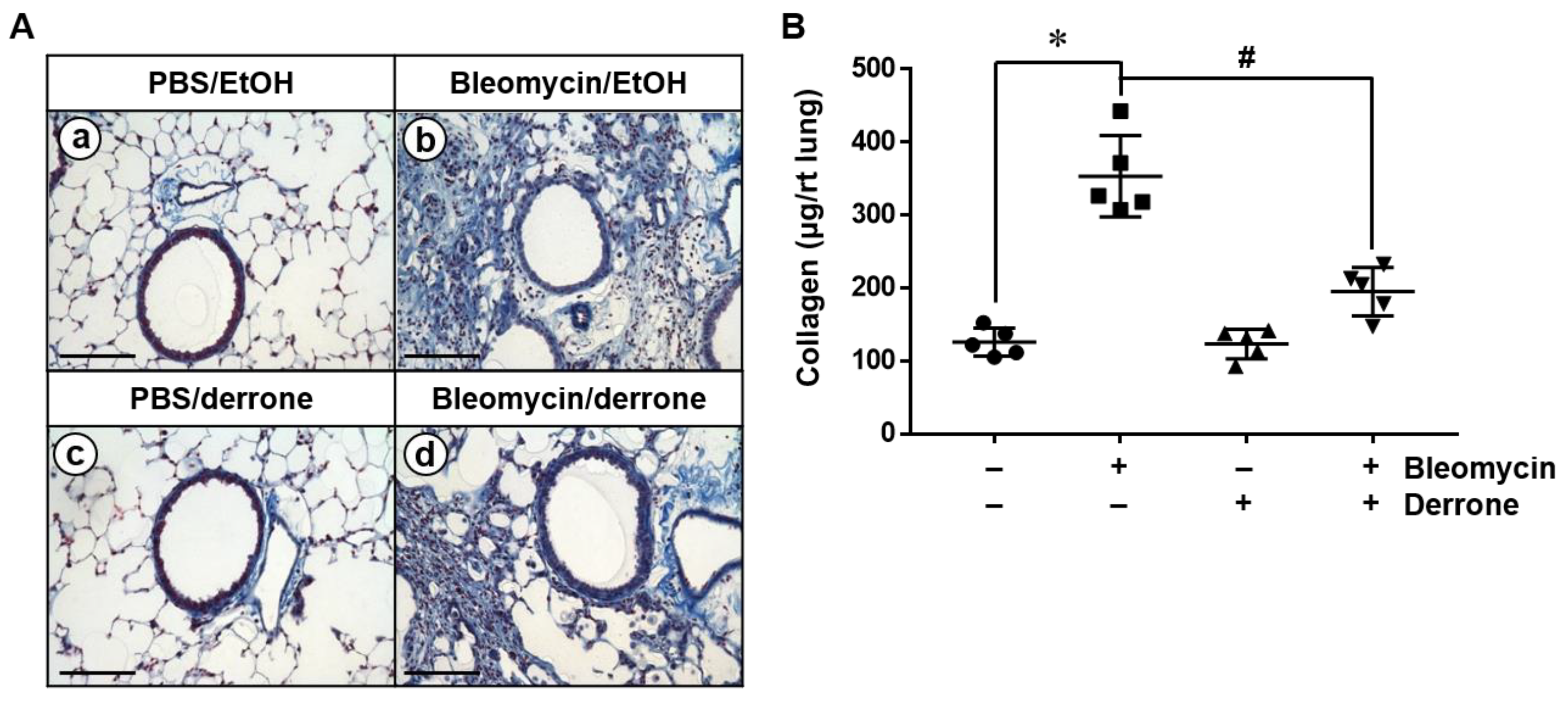
2.4. Derrone Decreases Collagen Deposition in the Lungs of Bleomycin-Challenged Mice
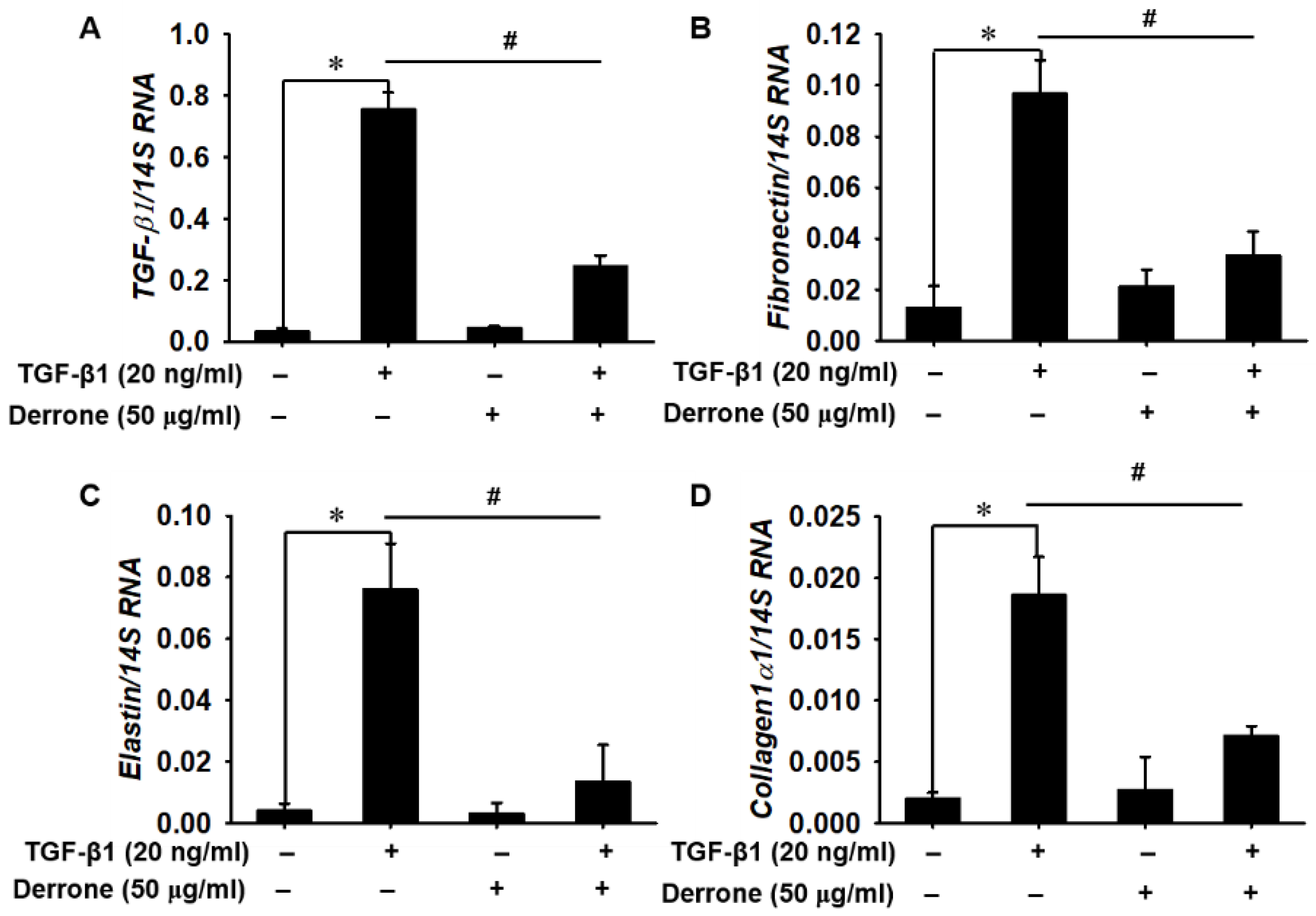
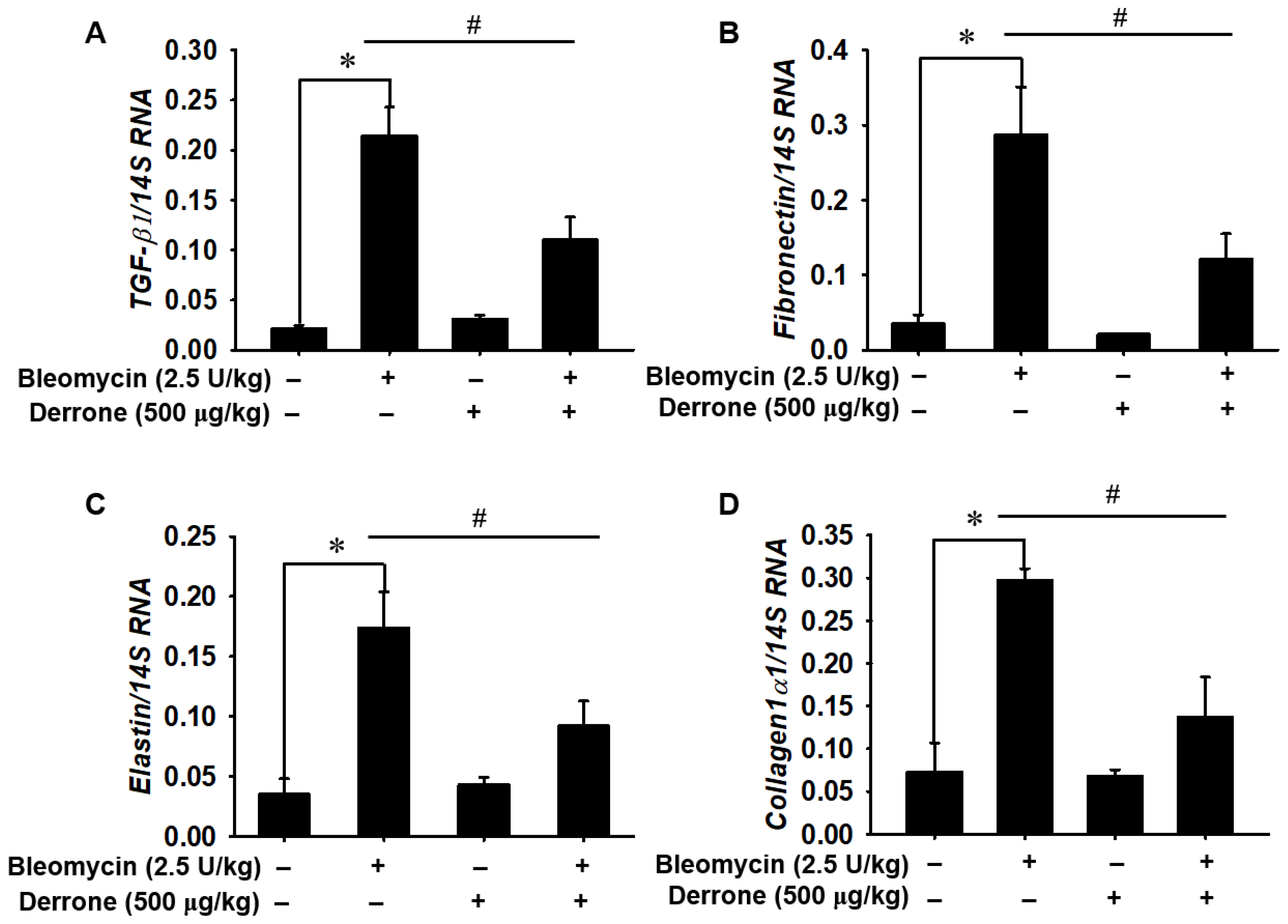
2.5. Derrone Attenuates Expression of Fibrotic Markers in Bleomycin-Challenged Mice
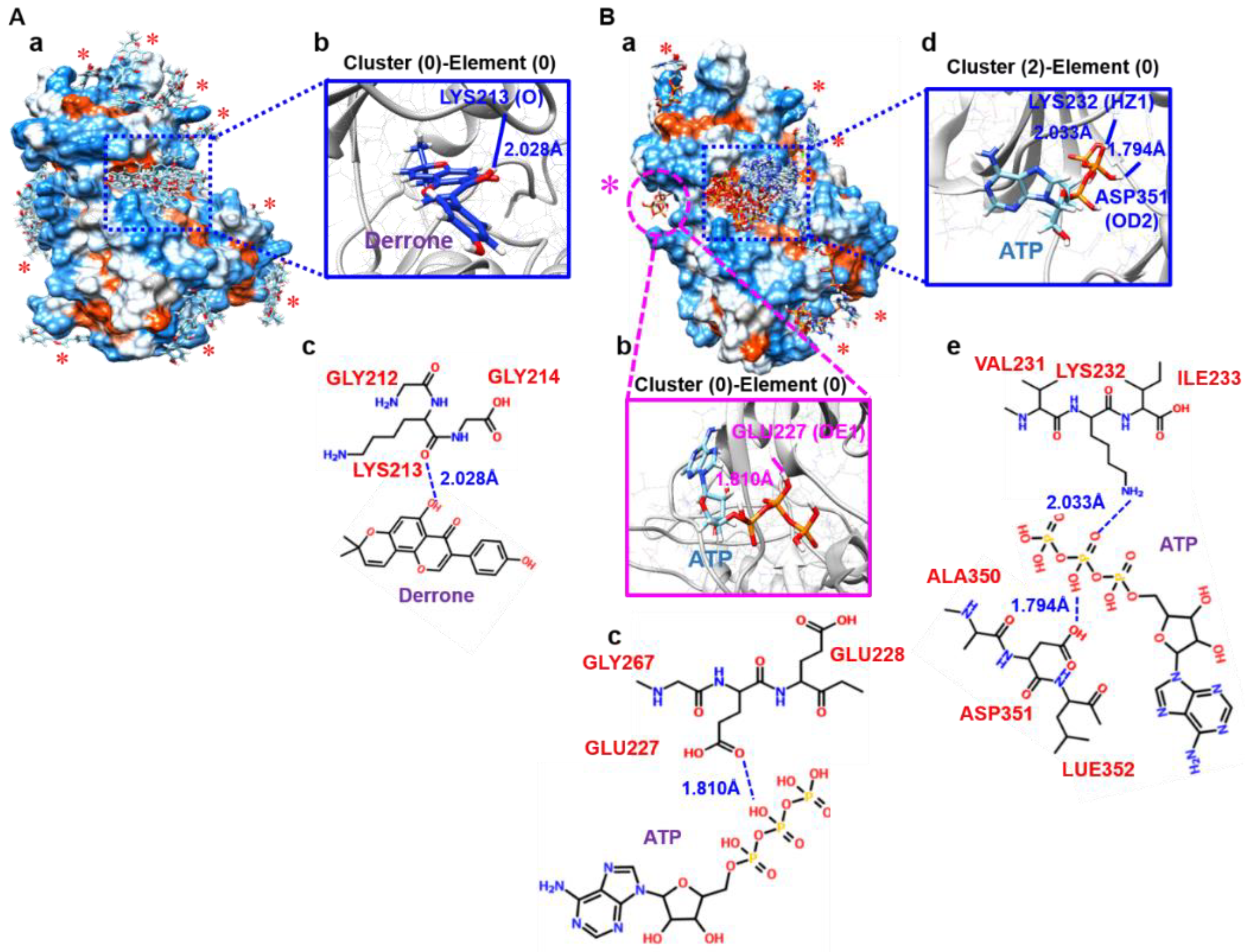
2.6. Molecular Docking Based on SwissDock
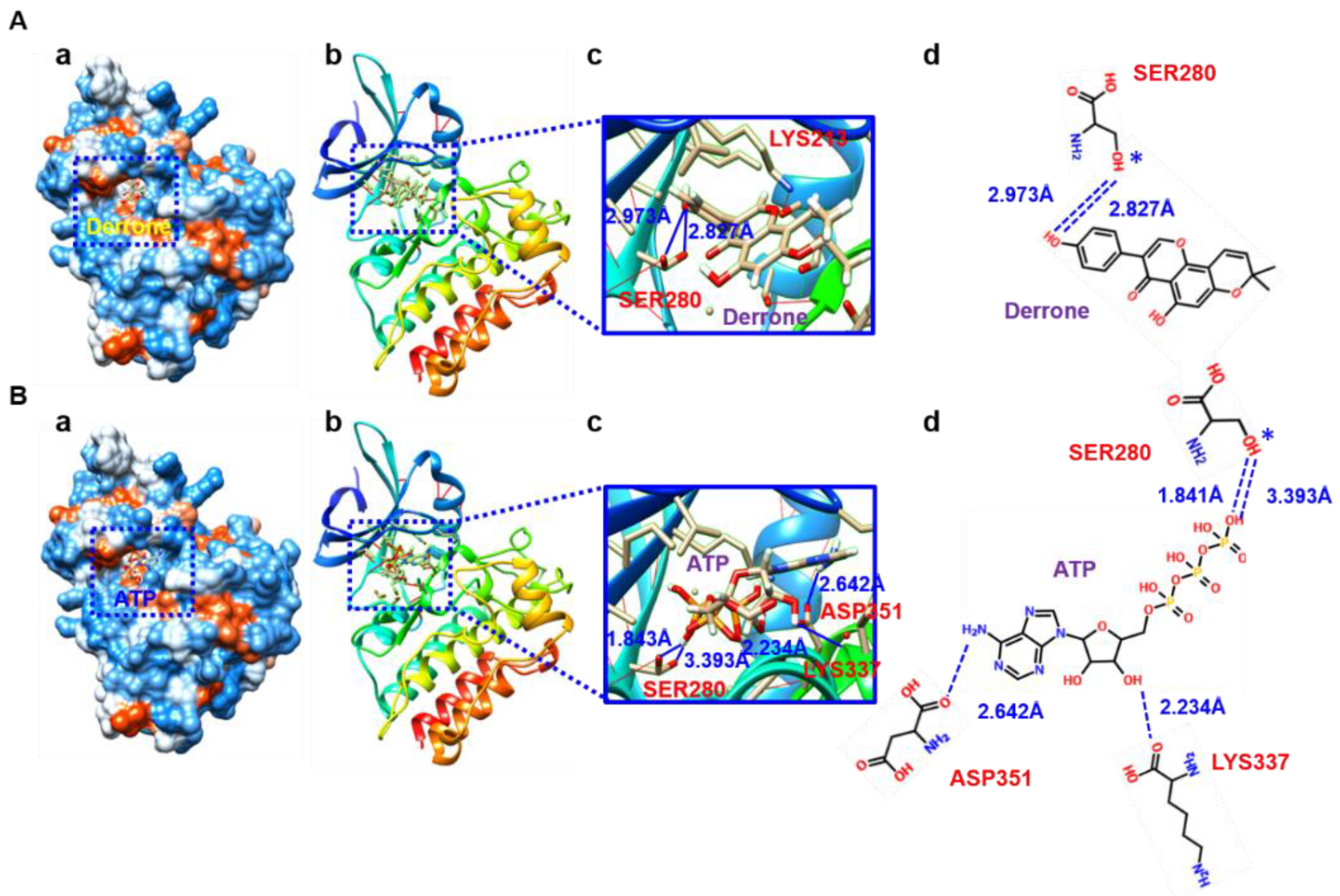
2.7. Molecular Cocking Based on AutoDock Vina
2.8. Derrone Inhibits Phosphorylation and Nuclear Translocation of Smad2/3
3. Discussion
4. Materials and Methods
4.1. Isolation of Derrone
4.2. Reagents and Antibodies
4.3. Cell Culture and Cell Viability Assay
4.4. RNA Isolation and Quantitative Real-Time RT-PCR
4.5. Western Blotting
4.6. Murine Model of Bleomycin-Induced Pulmonary Fibrosis
4.7. Histopathological Analysis
4.8. Immunofluorescence Staining
4.9. Sircol Collagen Assay
4.10. Molecular Docking
4.11. Smad-Binding Element (SBE)-Luciferase Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glassberg, M.K. Overview of idiopathic pulmonary fibrosis, evidence-based guidelines, and recent developments in the treatment landscape. Am. J. Manag. Care 2019, 25, S195–S203. [Google Scholar] [PubMed]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [Green Version]
- Wuyts, W.A.; Antoniou, K.M.; Borensztajn, K.; Costabel, U.; Cottin, V.; Crestani, B.; Grutters, J.C.; Maher, T.M.; Poletti, V.; Richeldi, L.; et al. Combination therapy: The future of management for idiopathic pulmonary fibrosis? Lancet Respir. Med. 2014, 2, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Moua, T.; Daniels, C.E.; Hartman, T.E.; Yi, E.S.; Utz, J.P.; Limper, A.H. Idiopathic pulmonary fibrosis: Evolving concepts. Mayo Clin. Proc. 2014, 89, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.N.; Lee, J.; Yang, H.S.; Cho, J.W.; Kwon, S.; Kim, Y.B.; Her, J.D.; Cho, K.H.; Song, C.W.; Lee, K. Dose-response effects of bleomycin on inflammation and pulmonary fibrosis in mice. Toxicol. Res. 2010, 26, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolahian, S.; Fernandez, I.E.; Eickelberg, O.; Hartl, D. Immune mechanisms in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Shan, B.; Lasky, J.A. TGF-b: Titan of lung fibrogenesis. Curr. Enzym. Inhib. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Aschner, Y.; Downey, G.P. Transforming growth factor-b: Master regulator of the respiratory system in health and disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-b on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-b1 signaling and tissue fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.; Yang, N.; Mahato, R.I. TGF-b1 gene silencing for treating liver fibrosis. Mol. Pharm. 2009, 6, 772–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantal, A.F.; Chen, H.; Shi, T.T.; Lu, C.H.; Fang, A.B.; Buckley, S.; Kolb, M.; Gauldie, J.; Warburton, D.; Shi, W. Overexpression of transforming growth factor-b1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur. Respir. J. 2010, 36, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Tatler, A.L.; Jenkins, G. TGF-b activation and lung fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 130–136. [Google Scholar] [CrossRef]
- Bagnato, G.; Harari, S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur. Respir. Rev. 2015, 24, 102–114. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.M.; Lee, D.S.; Jeong, G.S. Cudraticusxanthone A isolated from the roots of Cudrania tricuspidata inhibits metastasis and induces apoptosis in breast cancer cells. J. Ethnopharmacol. 2016, 194, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Hiep, N.T.; Kim, D.W.; Hwang, B.Y.; Lee, H.J.; Mar, W.; Lee, D. Neuroprotective xanthones from the root bark of Cudrania tricuspidata. J. Nat. Prod. 2014, 77, 1893–1901. [Google Scholar] [CrossRef]
- Nile, S.H.; Kim, D.H. HPLC analysis, antioxidant, anti-inflammatory and xanthine oxidase inhibitory activity of Cudrania tricuspidata. Nat. Prod. Commun. 2015, 10, 1839–1842. [Google Scholar] [CrossRef] [Green Version]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, antifungal and cytotoxic activities of two flavonoids from Retama raetam flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef] [Green Version]
- Hoang, N.T.; Phuong, T.T.; Nguyen, T.T.; Tran, Y.T.; Nguyen, A.T.; Nguyen, T.L.; Bui, K.T. In vitro characterization of derrone as an aurora kinase inhibitor. Biol. Pharm. Bull. 2016, 39, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.J.; Kim, S.Y.; Kwon, E.B.; Jo, Y.H.; Lee, M.K.; Lee, H.S.; Moon, D.O.; Kim, M.O. Derrone induces autophagic cell death through induction of ROS and ERK in A549 cells. PLoS ONE 2019, 14, e0218659. [Google Scholar] [CrossRef] [PubMed]
- Botello-Smith, W.M.; Alsamarah, A.; Chatterjee, P.; Xie, C.; Lacroix, J.J.; Hao, J.; Luo, Y. Polymodal allosteric regulation of type 1 serine/threonine kinase receptors via a conserved electrostatic lock. PLoS Comput. Biol. 2017, 13, e1005711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Ha, H.; Lee, J.K.; Seo, C.S.; Lee, N.H.; Jung, D.Y.; Park, S.J.; Shin, H.K. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother. Res. 2012, 26, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kim, S.B.; Liu, Q.; Do, S.G.; Hwang, B.Y.; Lee, M.K. Comparison of pancreatic lipase inhibitory isoflavonoids from unripe and ripe fruits of Cudrania tricuspidata. PLoS ONE 2017, 12, e0172069. [Google Scholar] [CrossRef] [Green Version]
- Sakai, N.; Tager, A.M. Fibrosis of two: Epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 170062. [Google Scholar] [CrossRef] [Green Version]
- Walters, D.M.; Kleeberger, S.R. Mouse models of bleomycin-induced pulmonary fibrosis. Curr. Protoc. Pharmacol. 2008, 40, 5.46.1–5.46.17. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2015, 97, 4–27. [Google Scholar] [CrossRef]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The extracellular matrix—The under-recognized element in lung disease? J. Pathol. 2016, 240, 397–409. [Google Scholar] [CrossRef]
- Roberts, A.B.; Heine, U.I.; Flanders, K.C.; Sporn, M.B. Transforming growth factor-b. Major role in regulation of extracellular matrix. Ann. N. Y. Acad. Sci. 1990, 580, 225–232. [Google Scholar] [CrossRef]
- D’Alessandro-Gabazza, C.N.; Kobayashi, T.; Boveda-Ruiz, D.; Takagi, T.; Toda, M.; Gil-Bernabe, P.; Miyake, Y.; Yasukawa, A.; Matsuda, Y.; Suzuki, N.; et al. Development and preclinical efficacy of novel transforming growth factor-b1 short interfering RNAs for pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 46, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Meng, Q.; Liu, J.; Wang, C. Thalidomide accelerates the degradation of extracellular matrix in rat hepatic cirrhosis via down-regulation of transforming growth factor-b1. Yonsei Med. J. 2015, 56, 1572–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.M.; Park, J.H.; Park, I.H.; Lee, H.M. Pirfenidone inhibits transforming growth factor b1-induced extracellular matrix production in nasal polyp-derived fibroblasts. Am. J. Rhinol. Allergy 2015, 29, 408–413. [Google Scholar] [CrossRef]
- Hayashi, H.; Sakai, T. Biological significance of local TGF-β activation in liver diseases. Front. Physiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-b Mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-b/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.Y.; Chan, M.K.; Li, J.S.; Chan, A.S.; Tang, P.C.; Leung, K.T.; To, K.F.; Lan, H.Y.; Tang, P.M. TGF-b signaling: From tissue fibrosis to tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 7575. [Google Scholar] [CrossRef]
- Syed, V. TGF-b signaling in cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Oyama, S.D.O.; Souza, L.A.D.; Baldoqui, D.C.; Sarragiotto, M.H.; Silva, A.A. Prenylated flavonoids from Maclura tinctoria fruits. Quim Nova 2013, 36, 800–802. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Species | Gene | Forward Primers (5′→3′) | Reverse Primers (5′→3′) |
|---|---|---|---|
| Mouse | TGF-β1 * | ttgcttcagctccacagaga | tggttgtagagggcaaggac |
| Fibronectin | aatggaaaaggggaatggac | ctcggttgtccttcttgctc | |
| Elastin | gctgatcctcttgctcaacc | tccaaacgttcccagaagtc | |
| Collagen I α1 | gagcggagagtactggatcg | gcttcttttccttggggttc | |
| Human | TGF-β1 | cacgtggagctgtaccagaa | gaacccgttgatgtccactt |
| Fibronectin | cagtgggagacctcgagaag | cactgtgacagcaggagcat | |
| Elastin | ggtggcttaggagtgtctgc | ccagcaaaagctccacctac | |
| Collagen I α1 | gtgctaaaggtgccaatggt | ctcctcgctttccttcctct |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molagoda, I.M.N.; Sanjaya, S.S.; Lee, K.T.; Choi, Y.H.; Lee, J.H.; Lee, M.-H.; Kang, C.-H.; Lee, C.-M.; Kim, G.-Y. Derrone Targeting the TGF Type 1 Receptor Kinase Improves Bleomycin-Mediated Pulmonary Fibrosis through Inhibition of Smad Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 7265. https://doi.org/10.3390/ijms24087265
Molagoda IMN, Sanjaya SS, Lee KT, Choi YH, Lee JH, Lee M-H, Kang C-H, Lee C-M, Kim G-Y. Derrone Targeting the TGF Type 1 Receptor Kinase Improves Bleomycin-Mediated Pulmonary Fibrosis through Inhibition of Smad Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(8):7265. https://doi.org/10.3390/ijms24087265
Chicago/Turabian StyleMolagoda, Ilandarage Menu Neelaka, Sobarathne Senel Sanjaya, Kyoung Tae Lee, Yung Hyun Choi, Joyce H. Lee, Mi-Hwa Lee, Chang-Hee Kang, Chang-Min Lee, and Gi-Young Kim. 2023. "Derrone Targeting the TGF Type 1 Receptor Kinase Improves Bleomycin-Mediated Pulmonary Fibrosis through Inhibition of Smad Signaling Pathway" International Journal of Molecular Sciences 24, no. 8: 7265. https://doi.org/10.3390/ijms24087265
APA StyleMolagoda, I. M. N., Sanjaya, S. S., Lee, K. T., Choi, Y. H., Lee, J. H., Lee, M.-H., Kang, C.-H., Lee, C.-M., & Kim, G.-Y. (2023). Derrone Targeting the TGF Type 1 Receptor Kinase Improves Bleomycin-Mediated Pulmonary Fibrosis through Inhibition of Smad Signaling Pathway. International Journal of Molecular Sciences, 24(8), 7265. https://doi.org/10.3390/ijms24087265









