1. Introduction
Vibrio cholerae is a group of Gram-negative pathogens causing cholerae and other severe diarrheal infectious diseases in humans; the main pathogenic mechanism of these bacteria is the production of toxins. Although the immune cells producing IL-1β are not restricted to macrophages, these are cells in the first line of infection facing pathogens; therefore, the study of the Vibrio cholerae cytotoxin (VCC) as a PAMP in the earliest phases of infection is a field of investigation.
The innate immune response is the first line of protection against pathogens, and its components get sensed by pattern recognition receptors (PRR). In turn, these receptors recognize pathogen-associated molecular patterns (PAMPs) for a prompt inflammatory response to begin [
1,
2]. Eukaryotic cells rely on the family of toll-like receptors (TLRs), which are the innate immune receptors, best characterized in macrophages, since macrophages perform in the first line of immune response (together with dendritic cells). It is known that TLRs stimulation activates signaling pathways, such as mitogen-activated protein kinases (MAPK) and the nuclear transcription factor NF-κB, to stimulate the synthesis and release of pro-inflammatory cytokines. The MAPK signaling controls a wide range of cellular activities of the innate response, and they are particularly important in the regulation of pro-inflammatory cytokine gene expression and programmed cell death [
3]. The best characterized MAPK families are: (i) ERK 1 and 2, the extracellular regulated signal protein kinases; (ii) JNK 1, 2 and 3, the c-Jun amino terminal kinases and (iii) MAPK p38, in charge of the onset of the innate immune response [
4]. Another important group of PRRs is the Nod-like receptor (NLR) family, which is present primarily in macrophages and dendritic cells and is composed of three domains: the C-terminal region in leucine-rich repeats (LRR), the central nucleotide domain NACHT (NOD) and the N-terminal domain that has either a Caspase recruitment domain (CARD) or a pyrin domain (PYD). The NLRs task is responsible for the constitution of outstanding protein complexes with enzymatic activity denominated inflammasomes, in charge of the launching of the inflammatory process by the further secretion of IL-1β and IL-18. The mechanism used by pore-forming toxins to induce the innate response involves the complementary assembly of the inflammasome, which is a very exciting subject that is still susceptible to further study.
Pore-forming toxins (PFT) such as VCC usually show complex membrane damage mechanisms; they are bacterial soluble monomeric proteins inserting themselves into the lipid bilayer of eukaryotic cells to later assemble five to seven monomers, producing a beta barrel structure responsible for cytoplasmic membrane perforation, resembling and behaving as an active ion permeable channel. Due to its very successful pathogenicity mechanism, PFTs are produced by a large variety of pathogenic bacteria. The toxin pores damaging the cytoplasmic membrane are a cause of profuse diarrhea in patients, also triggering cellular rescue mechanisms that end up acting as subversive processes against the target cell. The
Vibrio cholerae cytolysin (VCC), formerly known as hemolysin, a very efficiently synthesized PFT, turned out to be a conserved protein efficiently exported by these Gram-negative bacteria. The monomeric VCC is released as a 65 kDa water soluble mature protein; depending on the strain, its production may be very efficient, and in some strains, it is an actively secreted toxin. Once released to the bacterial surroundings, the monomeric form of VCC inserts itself into the cytoplasmic membrane of the target cell due to its transmembrane domain. After the insertion in the cytoplasmic membrane, monomers group one after another, forming a molecular pore of approximately 1–2 nm in diameter [
5,
6,
7], a self-assembled heptameric holotoxin in the shape of a β-barrel structure. Numerous heptameric pores get to disrupt the membrane permeability [
8,
9]. Each heptameric pore means an injury to the cell when injuries are multiple; eventually, they trigger endocytosis of the damaged membrane, followed by autophagy as a cell-surviving mechanism, which we see at the microscopic level as a striking vacuolating effect. This process of vacuolization involves numerous events of autophagy, eventually leading to apoptotic cell death [
10,
11].
Although the cytotoxin process of vacuolization has been studied profusely in vitro and in vivo, the innate immune response initiated by the toxin has been usually studied in murine models of infection, using knockout mutants deleting the hlyA gene (encoding the monomeric toxin). In efforts to address, in vitro, the innate response to this cytotoxin, the oligomeric form of the VCC was studied by self-assembly of heptameric pores on liposome membranes and then used to stimulate murine monocytes and macrophages in vitro, showing a pro-inflammatory response [
12]. Although all of the above was a good start, testing the monomeric form of the VCC in human macrophages treated in vitro, in our opinion, would be the most direct means to observe the innate response in such a way that it would very probably occur at the early stages of
Vibrio cholerae infection in patients. Still, the VCC, as a pathogenicity mechanism, is an exciting subject that entertains more possibilities involved in the process of
V. cholerae bacterial pathogenesis, not only by causing vacuolization or cell lysis but also by starting a pro-inflammatory innate immune response in macrophages.
In the present study, the soluble form of VCC released by the bacteria was used, and the working concentration was empirically determined in dose-response experiments until a tiny concentration was reached (picograms), which allowed us to avoid cytoplasmic vacuolization but still observe changes due to the pro-inflammatory response (probably by TLRs) and the MAPK activation in response to treatments with the cytotoxin. These changes were determined by performing kinetics of protein activation using Western blots with specific antibodies recognizing MAPK p38 and ERK. The monomeric form of VCC activates the MAPKs pathway in the first hour of interaction with macrophages in vitro. The toxin induces a dual stimulus of initial survival and is then pro-inflammatory, starting the assembly of the inflammasome.
To better describe the consequences of the MAPK kinases activation, the transcriptional activation of modulators responsible for innate immune response and survival, NF-κB and AP-1, respectively, were determined by quantitative real-time PCR, which revealed the activation of both regulators; this is a striking observation, since AP-1 first conduces to an eventual cell survival program, and the other one, NF-κB, eventually triggers pro-inflammatory cell death (pyroptosis).
3. Discussion
Cell receptors starting MAPK signaling after the interaction with PAMPs, such as the toxin monomers, belong to the TLR family. Thus, this study considers that the signaling response is due to a response originating from either TLRs or perhaps the internal receptors NLRs or both, since the TLR binding the monomer might be internalized by the process of external membrane recycling by the endocytic pathway. To be able to demonstrate this interaction in the system, experimental goals had to be switched, so, since the TLR-4/MAPK pathway is active in the THP-1 cell line, we assume that, perhaps, TLR-4 (previously reported in mice) actually initiates the activation of the MAPKs cascade, yielding the experimentally observed cell response.
Then, since TLRs activate the transcription factors NF-κB and AP-1, through MAPKs signaling cascade pathways, VCC actually stimulates one or more TLRs. In the model proposed here, the VCC monomer is detected by a signaling receptor TLR (based on the literature, TLR4 or TLR2/TLR6) [
12,
24]. In turn, the VCC triggers the activation of the MAPKs p38 and ERK, further triggering the transcriptional activation of Fos/Jun proteins, which are constituents of the heterodimeric transcription factor AP-1. Moreover, VCC also activates the transcription of p50, which, together with p30, bind each other to assemble the heterodimeric transcription factor NF-κB. Both the AP-1 and NF-κB transcription factors are implicated in the inflammatory response; therefore, the monomeric VCC is capable by itself of starting the inflammatory response depending on how long the interaction with membrane receptors is taking place, in response to this particular PAMP.
Colleagues [
12,
24] previously reported that VCC is sensed by TLR4 or TLR2/TLR6. This work did not address a direct TLR response, instead the signaling pathway MAPKs, the activation of MAPK and the later transcriptional activation of NF-κB and AP-1 in human macrophages. Certainly, further studies are needed to identify whether the recognition receptor may be TLR4 [
25], since the signaling is started after intervals shorter than 1 h (as described for LPS responses).
Consistent with this study are the results elegantly obtained by Tetsuya [
26], where KO-p38 baby hamster kidney cells were assayed with Aerolysin, the PFT produced by
Aeromonas, and the cell death rate increased by at least four to five times in comparison to the parental cells [
27]. These authors found that part of the transcriptional response towards PFTs is functionally important for defense; it is specifically responsive to PFTs and not the whole bacteria. To our delight, the results from this study concord with those found using the whole bacteria [
27]. Two of the transcriptionally induced MAPKs were p38 and JNK; alas, the reason why both p38 and JNK are required to protect the cell in front of PFTs is still under debate, so a proposal mechanism will be offered here.
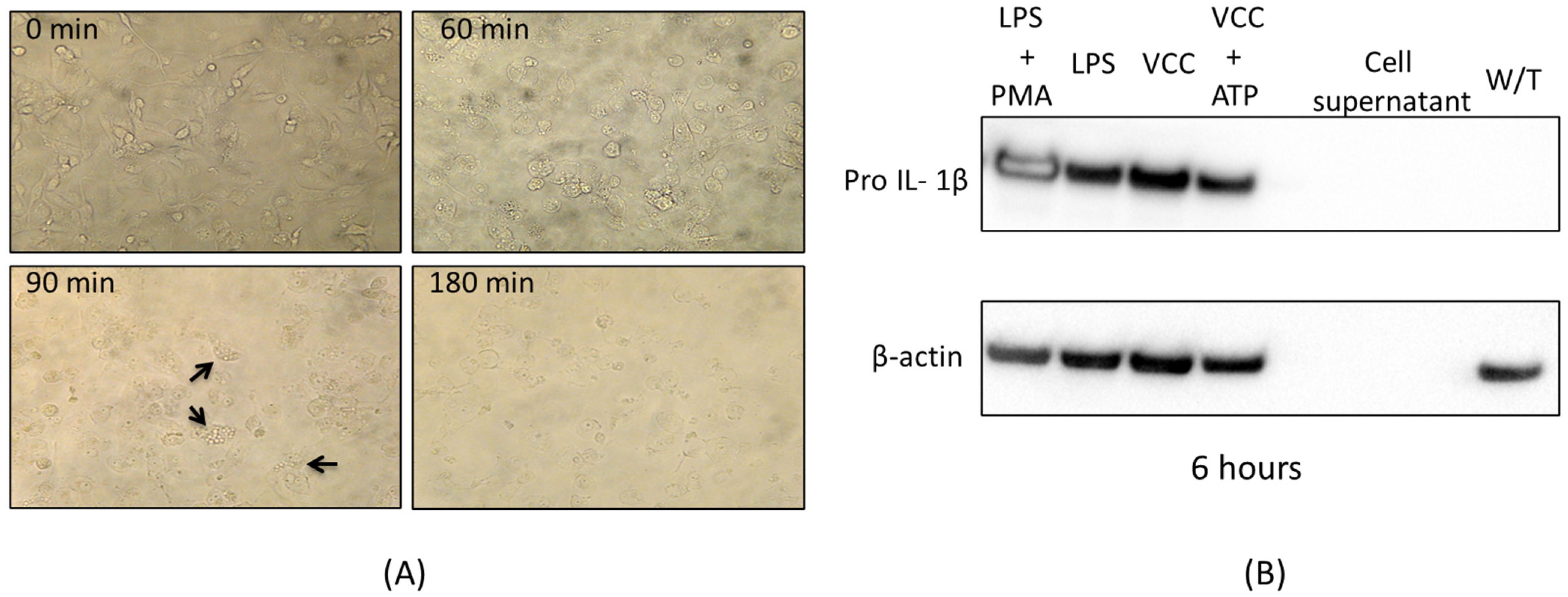
The results from this work support that the VCC monomeric protein, at very low concentrations of the order of picograms, comparable to the onset of an actual infection, is an early alert to the macrophage and perhaps surrounding cells, starting the signaling from TLRs or NLRs (
Figure 8). The treatments with VCC started responses shortly, making it obvious that, at least in macrophages, the most likely response to be stimulated is the one in charge of transcription factor p38, since the innate immune cytokines such as IL-1β production would be the main goal for the first line of protection in charge. As important as the activation of the innate immune response in charge of p38 is, we also wondered whether the cytotoxin also initiates cell death by the pyroptotic pathway. An experimental approach to partially answering the question resulted in the following: cell death led by the toxin was indirectly recognized by the leaking of LDH to the conditioned media, determined after 24 h of treatment (
Figure 7A). Cleaved and thus activated Caspase-1 was observed in western blots from the treated cells (
Figure 7B).
Moreover, the reasoning suggested by our colleagues describing autophagy [
27] as a cell rescue mechanism after a PFT attack now becomes a little undermined. The autophagy and assembly of the inflammasome are perhaps mutually excluding mechanisms regulating cell homeostasis, but the activation of MAPKs is involved in both systems. The concept of the “protection” of the cell towards the injured cell membrane, at least in our study. gets challenged, since results with monomeric VCC show that ERK gets activated perhaps earlier but almost at the same time as p38 (however, within the first hour of incubation,
Figure 5). Interaction with various pathogens stimulates the regulation of these pathways in different ways [
28]. In this case, the monomer of VCC is a PAMP that, at concentrations of picograms, activates p38 (
Figure 2) during the first 15 min of interaction, meaning that the involved mechanisms of the inflammatory response are activated in the first minutes; perhaps, the sensitivity of the macrophages may explain such an efficient response. Additionally, since the kind of mechanism to be activated may depend on the extent of the damage the cell is enduring, it can be the case that the assembly of the inflammasome platform is preferred when the stimulus of the toxin is less severe and the toxin is only added in picograms, rather than the process of autophagy. The process of autophagy may take place only after the cell experiments’ multiple pore formations, such as when concentrations of nanograms are used.
The activation of ERK turns out to be paradoxical, since it is a kinase not directly involved in the innate immune response; nevertheless, it gets activated by treatments with picograms of the monomeric VCC. Activated ERK is indicative of a survival response, since it occurs here under the exposure of a restricted concentration of the monomeric form of a PFT; this fact would only make sense in association with the need for a rather prolonged survival of the cell. Perhaps a prolonged survival response may be needed in a cell destined to undergo the pyroptotic cell death. Before the pyroptotic macrophage becomes necrotic, the cell is actually alive and committed to ensuring an optimal release of pro-inflammatory cytokines such as IL-1β, which are actively produced by the inflammasome and therefore there´s need of an active and ongoing survival response. In other words, the innate immune response program remains in charge of the p38 activated pathway, so the innate cytokines are profusely synthesized by the inflammasome. Once the inflammasome is leading, this response must be kept going as long as possible; therefore, the survival response in charge of ERK (and perhaps JNK) is taking care of ensuring that the inflammasome would continue to be productive until the last minute of the cell viability. In the particular case of pyroptotic macrophages responding to a pathogenic cytotoxin, the ERK pathway relies on keeping the survival signals on before the necrotic cell death strikes (
Figure 8).
Because the macrophage is not dead yet; the inflammasome is actively secreting cytokines of the innate response to effectively initiate activation of the second line of defense of the immune system, such as T cells, but very importantly, the T lymphocytes in charge of generating the specific immune response. In vitro studies like these have shown that the monomeric form of VCC is actually a suitable tool for studying cellular functions [
29,
30,
31], such as membrane remodeling and the inhibition of the pore-forming mechanism. With the above reasoning and the results of the present study, we may speculate the following: treated macrophages are setting up a pro-inflammatory response towards the toxin, where an active production of IL-1β is necessary. In order to maintain the most efficient production of IL-1β, the cell must induce a survival response; yet, at the same time, cell death is initiated going forward, as part of the mechanism of the assembly of the inflammasome. A timeline explaining the occurrence of the events is also proposed (
Figure 9).
The experimental evidence shown here suggests that the monomeric soluble form of the VCC acts as a modulator of the innate immune response in the THP-1 macrophage system, which is consistent with the assembly of the NLRP3 inflammasome actively releasing IL-1β. Cell death events consistent with pyroptosis were monitored in the treated macrophages by revealing the mature form of Caspase-1, which is consistent with the further release of LDH into the conditioned medium, after 24 h exposure treatments. This study, to some extent, may help in understanding the VCC contribution as an early stimulator of the macrophage; although, until now, it has not shed light onto the lack of long-term protection achieved by engineered Vibrio cholerae vaccines.
4. Materials and Methods
4.1. Chemical Agents and Antibodies
Antibodies against the kinase activated by extracellular receptor (ERK) 1/2, phosphorylated ERK, p38, phosphorylated p38, Caspase-1 and interleukin-1 were all obtained from Cell Signaling (Danvers, MA, USA), and β-actin was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The kit of reagents for the determination of the release of LDH was obtained from Biovision (Milpitas, CA, USA).
4.2. Preparation of the VCC Toxin
The strain of
Vibrio cholerae 69750 is a super producer of VCC previously reported in [
10]. Bacteria were grown in 5 mL of the LB medium at 37 °C, with a constant agitation of 270 rpm overnight. A total of 1 mL of the supernatant was taken from the cells and centrifuged at 14,000×
g for 1 min in a micro-centrifuge at 4 °C. Subsequently, the supernatant was collected and sterilized by a 0.22 μm membrane filter; finally, the toxin was concentrated by molecular weight exclusion columns (100 kDa and then 50 kDa of Amicon Ultra 0.5 Ultrafiltration system at 14,000×
g for 10 min); the concentrated toxin was identified by 12% PAGE and then collected and preserved at −4 °C until its use. The biologic effect of the final toxin preparation was evaluated by dose response experiments.
4.3. Cell Culture
The human monocyte cell line THP-1 was obtained from ATCC and donated by the Department of Biochemistry of the National Institute of Nutrition and Medical Sciences; it was generously provided by Dr. Sigifredo Pedraza Sánchez. The cells were cultured as recommended: in RPMI 1640 medium supplemented with 2 mM L-glutamine, 10 nM HEPES, 1 mM sodium pyruvate, 0.05 mM β-mercaptoethanol and 10% fetal bovine serum. The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2. For the tests, six-well plates were used in which 1.5 × 106 cells per well were used and 50 ng/mL of PMA was added in 2 mL of RPMI 1640 supplemented medium. Subsequently the cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 48 h, they were exchanged for fresh medium and re-incubated at 37 °C in a humidified atmosphere with 5% CO2 overnight and the old medium was removed. A new medium was added, this time using supplemented RPMI, without phenol red and without FBS.
4.4. Titration of Biologic Activity (Vacuolating Effect)
A 96-well plate with 1 × 10
6 Vero cells was seeded with supplemented DMEM and incubated at 37 °C in a humidified atmosphere with 5% CO
2 overnight; then, the fresh medium was added to the cells without FBS. One volume of the concentrated toxin was added to the first well of the plate; then, 1:2 serial dilutions were made in the following wells. Then, the plate was incubated at 37 °C in a humidified atmosphere with 5% CO
2 and was observed by inverted optic microscopy every 30 min, keeping aware of the vacuolating effect on the cells, as reported in Figueroa-Arredondo et al., 2001 [
10]. The conditions used to standardize the toxin treatments in THP-1-activated phorbol 12-myristate 13-acetate (PMA) macrophages were taken from the Vero cell line results where no vacuolization was found (40 pg/mL) and later adjusted to their protein concentrations (see below).
The determination of protein concentration of the VCC was performed by the Bradford Method, as described in [
32]. The concentration of VCC to be used is determined by its biologic activity; the shorter concentration showing at least 50% of the cells with heavily vacuolated cytoplasm is considered a vacuolating concentration, as established in Figueroa-Arredondo et al., 2001 [
10]. In this work, the sub-vacuolating cytotoxin concentration is determined by the lowest concentration and does not produce a vacuolating effect 24 h after the addition of the toxin; instead, LDH is released to the cell culture medium.
4.5. Kinetics of the VCC in THP-1 Cells
THP cells that were already differentiated were treated with the sub-vacuolating concentration of VCC standardized as described above, incubating the following periods: 10, 15, 30 and 60 min, respectively. The positive control was 1 µg/mL of LPS; the negative control was cells with no treatment. After incubation, the supernatants were collected and stored at −80 °C, the cells were suspended in 200 μL of RIPA lysis buffer and the plate was scraped with cell lifters. The supernatant was collected and centrifuged at 14,000× g for 10 min at 4 °C. The protein concentration was determined by the method (Pierce BCA protein assay kit).
4.6. Immunoblot
Collected cells from the treatments described above were kept frozen at −80 °C in lysis buffer added with a cocktail of protein cOmplete Lysis, Roche, Basel, Switzerland). From here 10 μg of the protein from each treatment was loaded in a pre-made denaturing electrophoresis gel of 10% acrylamide (Invitrogen Novex, Carlsbad, CA, USA), electrophoresed for 15 min at 80 V and then electrophoresed for 75 min at 150 V. Electrophoresis-separated protein bands were transferred to a polyvinylidene fluoride membrane (Invitrogen Novex PVDF membrane) and then pre-treated with 5% bovine serum albumin in Tris buffer plus 0.1% Tween 20 at room temperature for 1 h. Blotted membranes were incubated overnight in the primary antibody at 4 °C and then subsequently incubated with the secondary antibody for 1 h at room temperature. Luminol was used in the detection of electrophoretic bands and analyzed on a photo-documenter (ChemiDoc XRS+). The relative densities from the bands were estimated with the ImageJ software (NIH, Bethesda, MD, USA). The antibodies used in this study were mentioned above.
4.7. LDH Test
Supernatants from each kinetic assay (25 μL) were taken and added to a 96-well plate, and 25 μL of the working solution was added to each well. The appropriate standard curve of LDH and the working solution were made according to the manufacturer’s instructions (Biovision, Waltham, MA, USA). The optical density was measured at 450 nm (time zero), followed by incubation at 37 °C for 30 min with constant shaking (300 rpm). The optical density (450 nm) was measured after a 30 min period of activity. The LDH activity of every treatment was determined according to the formula provided by the manufacturer. These measurements were performed in duplicate from three different experiments, and the graphics were constructed from the collected data.
4.8. qRT-PCR
The sub-vacuolating concentration of VCC was added to 1 × 10
6 THP-differentiated cells, followed by incubation for 10, 15, 30 and 60 min, respectively. The positive control was 1 mg of LPS, and the negative control was the cells without treatment. Total RNA was extracted using Roche’s High Pure RNA Tissue kit, and then the cDNA from each treatment was synthesized from approximately 500 ng of RNA using Roche’s First Strand cDNA Synthesis Transcription Kit. The generated cDNA was used to perform duplicates of qRT-PCR assays using Roche’s Light Cycler TaqMan Master. The following primers were used: p50_Fwd:5′-caccgaagcaattgaagtga-3′, p50_Rev: 5′-ggcctgagaggtggtcttc-3′, jun_Fwd: 5′-ccaaaggatagtgcgatgttt-3′, jun_Rev: 5′-ctgtccctctccactgcaac-3′, fos_Fwd: 5′-ctaccactcacccgcagact-3′, fos_Rev: 5′-aggtccgtgcagaagtcct-3′, gapdh_Fwd: 5′-agccacatcgctcagacac-3′ and gapdh_Rev: 5′-gcccaatacgaccaaatcc-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping control gene. All reactions were performed in the LightCycler 2.0 (Roche, Basel, Switzerland) real-time equipment, and the relative transcription results were normalized against GAPDH using the ΔΔCt method [
33].
4.9. VCC Super Producer V. cholerae 69750
Purified VCC was obtained from the
V. cholerae 69750 strain [
10] it was cultivated, selected by its natural overexpression of the VCC. Bacterial culture supernatants were taken, filter sterilized (0.22 μm). Since the VCC toxin in its monomeric form has a molecular weight of 65 kDa, molecular weight chromatography was performed by centrifugation, to exclude molecules greater than 100 kDa. Then, another chromatography was performed to exclude molecular weight molecules lower than 50 kDa. In the end the range of molecules kept were between 50 and 100 kDa. The last step was performing PAGE in acrylamide-bis for electroelution of the 65 kDa in 1 mL of PBS and that was the concentrated stock. Aliquots of every step of purification were then electrophoresed as evidence that the protein purification has been carried out successfully. As expected, a band corresponding to 65 kDa was observed in the lane where the concentrate stock was loaded, corresponding to the VCC molecular weight. For corroboration, Purification step samples were electrophoresed then WB, and revealed with an anti-VCC-specific antibody (rabbit polyclonal kindly provided by Professor Takeshi Honda) as it is shown in
Supplementary Information. The 65 kDa protein corresponding to the VCC in the western blot was identified.
4.10. Standardization of the Signalling Responsive Concentration of VCC
In order to define the optimum concentration of the VCC to be used in signaling experiments that specifically avoid the vacuolating effect more related to autophagy [
34], the standardization of a sub-vacuolating concentration was empirically determined. Initially, the use of the Vero cell line was considered to be convenient, since the vacuolating effect was previously characterized in this cell line [
10]. The sub-vacuolating concentration was estimated after serial decimal dilutions of the concentrated stock, considered to be the last dilution where no vacuolating effect was observed after 12 h of incubation. different concentrations were tested, by optical microscopy. Observations were performed after 1 h until 24 h were completed. The typical vacuolating effect was observed at concentrations of 168 pg/mL (
Supplementary Information); however, after serial dilutions concentrations of the order of 40 pg/mL were reached and although not vacuolating effect was observed after 12 h, other cytotoxic changes consistent with lysis were noticed. For example, LDH release was detected from the treated cell supernatants after 24 h; therefore, the concentration of 40 pg/mL was selected, since the goal of this project is to study the toxin concentrations only capable of starting signaling cascades towards the activation of the MAPK pathways.
Previous studies reported the cytotoxin of
V. cholerae to be able to induce an innate immune response through the ensemble and activation of the inflammasome protein complex [
23,
35], leading to pyroptosis, the pro-inflammatory cell death [
36]. To determine if the monomer VCC was able to induce such type of cell death, the presence of LDH released in the treatment supernatants was determined at 10, 15, 30 and 60 min of treatments with the VCC and after 24 h of treatment (
Figure 1B). LDH unequivocally indicates lysis of the treated cells; therefore, pyroptosis is highly probable to occur.
4.11. Standardization of the Signalling Responsive Concentration of VCC
For each experiment, data were obtained in triplicate and were reported as the means ± S.D. Comparisons of the means were analyzed using ANOVA plus the Student–Newman–Keuls method, and significant values are represented at p < 0.05. The p values are indicated as follows: * p < 0.001 and ** p < 0.05.
5. Conclusions
The use of limited non-vacuolating concentrations of VCC, in the order of picograms, was sufficient for the induction of a complex transcriptional response of THP-1 differentiated macrophages. This study presents experimental evidence supporting that the soluble monomeric form of the V. cholerae cytolysin VCC elicits a proinflammatory response in human macrophages, activating the p38 MAPK early after exposure with the toxin and the production of pro-IL-1β, followed by NF-κB activation. As usual, NF-κB activates its own transcription for the further amplification of the started pro-inflammatory response. Exposure to the toxin involves not only the transcriptional activation of pro-IL-1β but also the one of Caspase-1, which eventually will render the mature IL-1β by the assembly and activation of the molecular platform of the inflammasome.
On the other hand, almost synchronically, a cell survival response comes up as early as 10 min after exposure to the toxin, since the survival member of the MAPK pathway, the ERK MAPK, was activated, with further cell survival activity revealed by the activation of the transcription factor AP-1 (Jun and Fos phospholylation). It is necessary to point out that a survival response only makes sense in a cell such as the activated macrophage, destined to undergo the pro-inflammatory lytic cell death known as pyroptosis (suggested strongly by the release of LDH to the culture supernatants). The cell is programmed for the assembly and activation of the inflammasome for actively producing IL-1β to be the innate immune cytokine destinated to recruit the cells that will initiate the secondary, more specific immune response. In this particular case, the activated macrophage, starting and maintaining a program of cell survival, will ensure a maximum release of IL-1β before the cell actually undergoes the classical lysis, characteristic of the proinflammatory cell death named pyroptosis. More studies further supporting these conclusions are definitely needed.