Simultaneous Inhibition of Thrombosis and Inflammation Is Beneficial in Treating Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
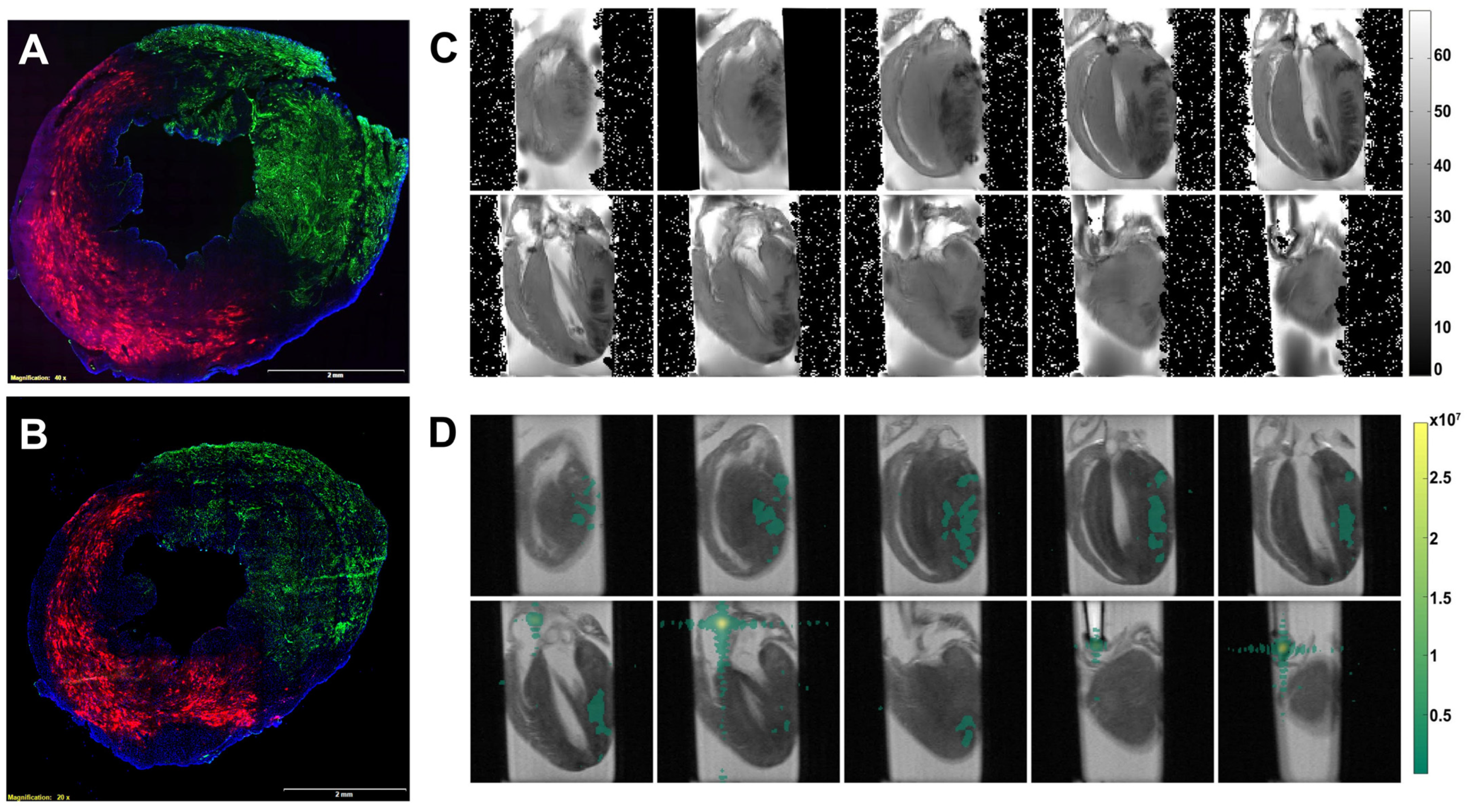
2.1. Delivery of Perfluorocarbon Nanoparticles to the Heart with Acute Myocardial Infarction
2.2. PPACK PFC NP Treatment Preserved Ventricular Structure and Function
2.3. PPACK Perfluorocarbon Nanoparticles Treatment Reduced Thrombin Deposition in the Border Zone Areas
2.4. PPACK Perfluorocarbon Nanoparticles Treatment Preserved Border Zone Vasculature
2.5. PPACK PFC NP Treatment Reduced Border Zone Endothelial Activation
2.6. PPACK PFC NP Treatment Reduces Border Zone Inflammasome Signaling
2.7. PPACK PFC NP Treatment Reduced Border Zone Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Formulation
4.2. Formulation of PPACK PFC Nanoparticles
4.3. Nanoparticle Distribution and Zeta Potential Measurement
4.4. Acute Myocardial Infarction Animal Model
4.4.1. Mouse Acute Myocardial Infarction Model
4.4.2. Rat Acute Myocardial Infarction Model
4.5. Tissue Collection and Preservation
4.6. Echocardiogram
4.7. 19F Magnetic Resonance Spectroscopy and Magnetic Resonance Imaging
4.8. Histology
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kloner, R.A. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ. Res. 2013, 113, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Przyklenk, K.; Whittaker, P. The Future of Remote Ischemic Conditioning. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 295–296. [Google Scholar] [CrossRef]
- Garratt, K.N.; Whittaker, P.; Przyklenk, K. Remote Ischemic Conditioning and the Long Road to Clinical Translation: Lessons Learned From ERICCA and RIPHeart. Circ. Res. 2016, 118, 1052–1054. [Google Scholar] [CrossRef]
- Ong, S.B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Targeting Myocardial Reperfusion Injury—The Search Continues. N. Engl. J. Med. 2015, 373, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Schwartz Longacre, L.; Kloner, R.A.; Arai, A.E.; Baines, C.P.; Bolli, R.; Braunwald, E.; Downey, J.; Gibbons, R.J.; Gottlieb, R.A.; Heusch, G.; et al. New horizons in cardioprotection: Recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 2011, 124, 1172–1179. [Google Scholar] [CrossRef]
- Ibanez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef] [PubMed]
- Hood, W.B., Jr. Modification of infarct size. Cardiovasc. Clin. 1975, 7, 259–278. [Google Scholar] [PubMed]
- Dixon, J.A.; Spinale, F.G. Pathophysiology of myocardial injury and remodeling: Implications for molecular imaging. J. Nucl. Med. 2010, 51 (Suppl. S1), 102S–106S. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Barrabes, J.A.; Botker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic. Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Botker, H.E.; Heusch, G.; Ibanez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Resendiz, S.; Munoz-Vega, M.; Contreras, W.E.; Crespo-Avilan, G.E.; Rodriguez-Montesinos, J.; Arias-Carrion, O.; Perez-Mendez, O.; Boisvert, W.A.; Preissner, K.T.; Cabrera-Fuentes, H.A. Responses of Endothelial Cells Towards Ischemic Conditioning Following Acute Myocardial Infarction. Cond. Med. 2018, 1, 247–258. [Google Scholar] [PubMed]
- Damani, S.; Bacconi, A.; Libiger, O.; Chourasia, A.H.; Serry, R.; Gollapudi, R.; Goldberg, R.; Rapeport, K.; Haaser, S.; Topol, S.; et al. Characterization of circulating endothelial cells in acute myocardial infarction. Sci. Transl. Med. 2012, 4, 126ra133. [Google Scholar] [CrossRef] [PubMed]
- Mutin, M.; Canavy, I.; Blann, A.; Bory, M.; Sampol, J.; Dignat-George, F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood 1999, 93, 2951–2958. [Google Scholar] [CrossRef]
- Bhagat, K. Endothelial function and myocardial infarction. Cardiovasc. Res. 1998, 39, 312–317. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; van Geuns, R.M.; Berry, C.; Riksen, N.P.; Escaned, J.; van Royen, N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Chilian, W.; Crea, F.; Davidson, S.M.; Ferdinandy, P.; Garcia-Dorado, D.; van Royen, N.; Schulz, R.; Heusch, G. The coronary circulation in acute myocardial ischaemia/reperfusion injury: A target for cardioprotection. Cardiovasc. Res. 2019, 115, 1143–1155. [Google Scholar] [CrossRef]
- Tantry, U.S.; Navarese, E.P.; Myat, A.; Chaudhary, R.; Gurbel, P.A. Combination oral antithrombotic therapy for the treatment of myocardial infarction: Recent developments. Expert. Opin. Pharmacother. 2018, 19, 653–665. [Google Scholar] [CrossRef]
- Hale, S.L.; Kloner, R.A. Dabigatran treatment: Effects on infarct size and the no-reflow phenomenon in a model of acute myocardial ischemia/reperfusion. J. Thromb. Thrombolysis 2015, 39, 50–54. [Google Scholar] [CrossRef]
- Ito, B.R.; Schmid-Schonbein, G.; Engler, R.L. Effects of leukocyte activation on myocardial vascular resistance. Blood Cells 1990, 16, 145–163, discussion 146–163. [Google Scholar]
- Myerson, J.; He, L.; Lanza, G.; Tollefsen, D.; Wickline, S. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. J. Thromb. Haemost. 2011, 9, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vemuri, C.; Palekar, R.U.; Gaut, J.P.; Goette, M.; Hu, L.; Cui, G.; Zhang, H.; Wickline, S.A. Antithrombin nanoparticles improve kidney reperfusion and protect kidney function after ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2015, 308, F765–F773. [Google Scholar] [CrossRef] [PubMed]
- Vargas, I.; Stephenson, D.J.; Baldwin, M.; Gaut, J.P.; Chalfant, C.E.; Pan, H.; Wickline, S.A. Sustained local inhibition of thrombin preserves renal microarchitecture and function after onset of acute kidney injury. Nanomedicine 2021, 38, 102449. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pan, H.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy. Adv. Chronic Kidney Dis. 2013, 20, 466–478. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Caruthers, S.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann. Biomed. Eng. 2009, 37, 1922–1933. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Lanza, G.M.; Pan, H.; Wickline, S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine 2015, 10, 1817–1832. [Google Scholar] [CrossRef]
- Morawski, A.M.; Lanza, G.A.; Wickline, S.A. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr. Opin. Biotechnol. 2005, 16, 89–92. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Wickline, S.A. Fluorine ((19)F) MRI to Measure Renal Oxygen Tension and Blood Volume: Experimental Protocol. Methods Mol. Biol. 2021, 2216, 509–518. [Google Scholar] [CrossRef]
- Partlow, K.C.; Chen, J.; Brant, J.A.; Neubauer, A.M.; Meyerrose, T.E.; Creer, M.H.; Nolta, J.A.; Caruthers, S.D.; Lanza, G.M.; Wickline, S.A. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007, 21, 1647–1654. [Google Scholar] [CrossRef]
- Weinheimer, C.J.; Lai, L.; Kelly, D.P.; Kovacs, A. Novel mouse model of left ventricular pressure overload and infarction causing predictable ventricular remodelling and progression to heart failure. Clin. Exp. Pharmacol. Physiol. 2015, 42, 33–40. [Google Scholar] [CrossRef]
- Lau, J.M.; Jin, X.; Ren, J.; Avery, J.; DeBosch, B.J.; Treskov, I.; Lupu, T.S.; Kovacs, A.; Weinheimer, C.; Muslin, A.J. The 14-3-3tau phosphoserine-binding protein is required for cardiomyocyte survival. Mol. Cell. Biol. 2007, 27, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Takagawa, J.; Sievers, R.E.; Khan, M.F.; Viswanathan, M.N.; Springer, M.L.; Foster, E.; Yeghiazarians, Y. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1187–H1192. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Myerson, J.; Bibee, K.; Scott, M.; Allen, J.; Sicard, G.; Lanza, G.; Wickline, S. Quantifying the evolution of vascular barrier disruption in advanced atherosclerosis with semipermeant nanoparticle contrast agents. PLoS ONE 2011, 6, e26385. [Google Scholar] [CrossRef] [PubMed]
- Palekar, R.U.; Jallouk, A.P.; Goette, M.J.; Chen, J.; Myerson, J.W.; Allen, J.S.; Akk, A.; Yang, L.; Tu, Y.; Miller, M.J.; et al. Quantifying progression and regression of thrombotic risk in experimental atherosclerosis. FASEB J. 2015, 29, 3100–3109. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Myerson, J.W.; Pan, H.; Wickline, S.A. Inhibition of Thrombin with PPACK-Nanoparticles Restores Disrupted Endothelial Barriers and Attenuates Thrombotic Risk in Experimental Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 inflammasome in endothelial dysfunction. Cell. Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Matikainen, S.; Nyman, T.A.; Cypryk, W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J. Immunol. 2020, 204, 3063–3069. [Google Scholar] [CrossRef]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell. Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef]
- Cohn, C.S.; Cushing, M.M. Oxygen therapeutics: Perfluorocarbons and blood substitute safety. Crit. Care Clin. 2009, 25, 399–414. [Google Scholar] [CrossRef]
- Spahn, D.R.; van Brempt, R.; Theilmeier, G.; Reibold, J.P.; Welte, M.; Heinzerling, H.; Birck, K.M.; Keipert, P.E.; Messmer, K.; Heinzerling, H.; et al. Perflubron emulsion delays blood transfusions in orthopedic surgery. European Perflubron Emulsion Study Group. Anesthesiology 1999, 91, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Kerins, D.M. Role of the perfluorocarbon Fluosol-DA in coronary angioplasty. Am. J. Med. Sci. 1994, 307, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Flaim, S.F.; Hazard, D.R.; Hogan, J.; Peters, R.M. Characterization and mechanism of side-effects of Oxygent HT (highly concentrated fluorocarbon emulsion) in swine. Artif. Cells Blood Substit. Immobil. Biotechnol. 1994, 22, 1511–1515. [Google Scholar] [CrossRef]
- Lanza, G.M.; Wallace, K.D.; Scott, M.J.; Cacheris, W.P.; Abendschein, D.R.; Christy, D.H.; Sharkey, A.M.; Miller, J.G.; Gaffney, P.J.; Wickline, S.A. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation 1996, 94, 3334–3340. [Google Scholar] [CrossRef]
- Lanza, G.M.; Wickline, S.A. Targeted ultrasonic contrast agents for molecular imaging and therapy. Prog. Cardiovasc. Dis. 2001, 44, 13–31. [Google Scholar] [CrossRef]
- Neubauer, A.M.; Myerson, J.; Caruthers, S.D.; Hockett, F.D.; Winter, P.M.; Chen, J.; Gaffney, P.J.; Robertson, J.D.; Lanza, G.M.; Wickline, S.A. Gadolinium-modulated 19F signals from perfluorocarbon nanoparticles as a new strategy for molecular imaging. Magn. Reson. Med. 2008, 60, 1066–1072. [Google Scholar] [CrossRef]
- Wickline, S.A.; Neubauer, A.M.; Winter, P.; Caruthers, S.; Lanza, G. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Caruthers, S.D.; Lanza, G.M.; Wickline, S.A. Quantitative cardiovascular magnetic resonance for molecular imaging. J. Cardiovasc. Magn. Reson. 2010, 12, 62. [Google Scholar] [CrossRef]
- Pan, H.; Ivashyna, O.; Sinha, B.; Lanza, G.M.; Ratner, L.; Schlesinger, P.H.; Wickline, S.A. Post-formulation peptide drug loading of nanostructures for metered control of NF-kappaB signaling. Biomaterials 2011, 32, 231–238. [Google Scholar] [CrossRef]
- Pan, H.; Myerson, J.W.; Hu, L.; Marsh, J.N.; Hou, K.; Scott, M.J.; Allen, J.S.; Hu, G.; San Roman, S.; Lanza, G.M.; et al. Programmable nanoparticle functionalization for in vivo targeting. FASEB J. 2013, 27, 255–264. [Google Scholar] [CrossRef]
- Myerson, J.W.; He, L.; Allen, J.S.; Williams, T.; Lanza, G.; Tollefsen, D.; Caruthers, S.; Wickline, S. Thrombin-inhibiting nanoparticles rapidly constitute versatile and detectable anticlotting surfaces. Nanotechnology 2014, 25, 395101. [Google Scholar] [CrossRef] [PubMed]
- Palekar, R.U.; Vemuri, C.; Marsh, J.N.; Arif, B.; Wickline, S.A. Antithrombin nanoparticles inhibit stent thrombosis in ex vivo static and flow models. J. Vasc. Surg. 2016, 64, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Babes, E.E.; Zaha, D.C.; Tit, D.M.; Nechifor, A.C.; Bungau, S.; Andronie-Cioara, F.L.; Behl, T.; Stoicescu, M.; Munteanu, M.A.; Rus, M.; et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Erlich, J.H.; Boyle, E.M.; Labriola, J.; Kovacich, J.C.; Santucci, R.A.; Fearns, C.; Morgan, E.N.; Yun, W.; Luther, T.; Kojikawa, O.; et al. Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia-reperfusion injury by reducing inflammation. Am. J. Pathol. 2000, 157, 1849–1862. [Google Scholar] [CrossRef]
- Ambrosio, G.; Weisman, H.F.; Mannisi, J.A.; Becker, L.C. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 1989, 80, 1846–1861. [Google Scholar] [CrossRef]
- Mittal, M.; Urao, N.; Hecquet, C.M.; Zhang, M.; Sudhahar, V.; Gao, X.P.; Komarova, Y.; Ushio-Fukai, M.; Malik, A.B. Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 877–887. [Google Scholar] [CrossRef]
- Kondo, N.; Ogawa, M.; Wada, H.; Nishikawa, S. Thrombin induces rapid disassembly of claudin-5 from the tight junction of endothelial cells. Exp. Cell. Res. 2009, 315, 2879–2887. [Google Scholar] [CrossRef]
- Palekar, R.U.; Myerson, J.W.; Schlesinger, P.H.; Sadler, J.E.; Pan, H.; Wickline, S.A. Thrombin-targeted liposomes establish a sustained localized anticlotting barrier against acute thrombosis. Mol. Pharm. 2013, 10, 4168–4175. [Google Scholar] [CrossRef]
- Minami, T.; Aird, W.C. Thrombin stimulation of the vascular cell adhesion molecule-1 promoter in endothelial cells is mediated by tandem nuclear factor-kappa B and GATA motifs. J. Biol. Chem. 2001, 276, 47632–47641. [Google Scholar] [CrossRef]
- Kaplanski, G.; Marin, V.; Fabrigoule, M.; Boulay, V.; Benoliel, A.M.; Bongrand, P.; Kaplanski, S.; Farnarier, C. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106). Blood 1998, 92, 1259–1267. [Google Scholar] [CrossRef]
- Minami, T.; Sugiyama, A.; Wu, S.Q.; Abid, R.; Kodama, T.; Aird, W.C. Thrombin and phenotypic modulation of the endothelium. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Fender, A.C.; Kleeschulte, S.; Stolte, S.; Leineweber, K.; Kamler, M.; Bode, J.; Li, N.; Dobrev, D. Thrombin receptor PAR4 drives canonical NLRP3 inflammasome signaling in the heart. Basic. Res. Cardiol. 2020, 115, 10. [Google Scholar] [CrossRef]
- Toldo, S.; Marchetti, C.; Mauro, A.G.; Chojnacki, J.; Mezzaroma, E.; Carbone, S.; Zhang, S.; Van Tassell, B.; Salloum, F.N.; Abbate, A. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int. J. Cardiol. 2016, 209, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Sandanger, O.; Ranheim, T.; Vinge, L.E.; Bliksoen, M.; Alfsnes, K.; Finsen, A.V.; Dahl, C.P.; Askevold, E.T.; Florholmen, G.; Christensen, G.; et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2013, 99, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Gao, Y.; Dong, Z.; Yang, J.; Gao, R.; Li, X.; Zhang, S.; Ma, L.; Sun, X.; Wang, Z.; et al. GSDMD-Mediated Cardiomyocyte Pyroptosis Promotes Myocardial I/R Injury. Circ. Res. 2021, 129, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Mezzaroma, E.; Van Tassell, B.W.; Marchetti, C.; Carbone, S.; Abbate, A.; Toldo, S. Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. Mol. Med. 2014, 20, 221–229. [Google Scholar] [CrossRef]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Considerations in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef]
- Winter, P.M.; Morawski, A.M.; Caruthers, S.D.; Fuhrhop, R.W.; Zhang, H.; Williams, T.A.; Allen, J.S.; Lacy, E.K.; Robertson, J.D.; Lanza, G.M.; et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation 2003, 108, 2270–2274. [Google Scholar] [CrossRef]
- Nossuli, T.O.; Lakshminarayanan, V.; Baumgarten, G.; Taffet, G.E.; Ballantyne, C.M.; Michael, L.H.; Entman, M.L. A chronic mouse model of myocardial ischemia-reperfusion: Essential in cytokine studies. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1049–H1055. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, P.; Kloner, R.A.; Przyklenk, K. Intramyocardial injections and protection against myocardial ischemia. An attempt to examine the cardioprotective actions of adenosine. Circulation 1996, 93, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Lerner, D.L.; Schuessler, R.B.; Betsuyaku, T.; Yamada, K.A.; Saffitz, J.E.; Kovacs, A. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J. Am. Soc. Echocardiogr. 2002, 15, 601–609. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, I.; Grabau, R.P.; Chen, J.; Weinheimer, C.; Kovacs, A.; Dominguez-Viqueira, W.; Mitchell, A.; Wickline, S.A.; Pan, H. Simultaneous Inhibition of Thrombosis and Inflammation Is Beneficial in Treating Acute Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 7333. https://doi.org/10.3390/ijms24087333
Vargas I, Grabau RP, Chen J, Weinheimer C, Kovacs A, Dominguez-Viqueira W, Mitchell A, Wickline SA, Pan H. Simultaneous Inhibition of Thrombosis and Inflammation Is Beneficial in Treating Acute Myocardial Infarction. International Journal of Molecular Sciences. 2023; 24(8):7333. https://doi.org/10.3390/ijms24087333
Chicago/Turabian StyleVargas, Ian, Ryan P. Grabau, Junjie Chen, Carla Weinheimer, Attila Kovacs, William Dominguez-Viqueira, Adam Mitchell, Samuel A. Wickline, and Hua Pan. 2023. "Simultaneous Inhibition of Thrombosis and Inflammation Is Beneficial in Treating Acute Myocardial Infarction" International Journal of Molecular Sciences 24, no. 8: 7333. https://doi.org/10.3390/ijms24087333
APA StyleVargas, I., Grabau, R. P., Chen, J., Weinheimer, C., Kovacs, A., Dominguez-Viqueira, W., Mitchell, A., Wickline, S. A., & Pan, H. (2023). Simultaneous Inhibition of Thrombosis and Inflammation Is Beneficial in Treating Acute Myocardial Infarction. International Journal of Molecular Sciences, 24(8), 7333. https://doi.org/10.3390/ijms24087333





