Identification of Signature Genes of Dilated Cardiomyopathy Using Integrated Bioinformatics Analysis
Abstract
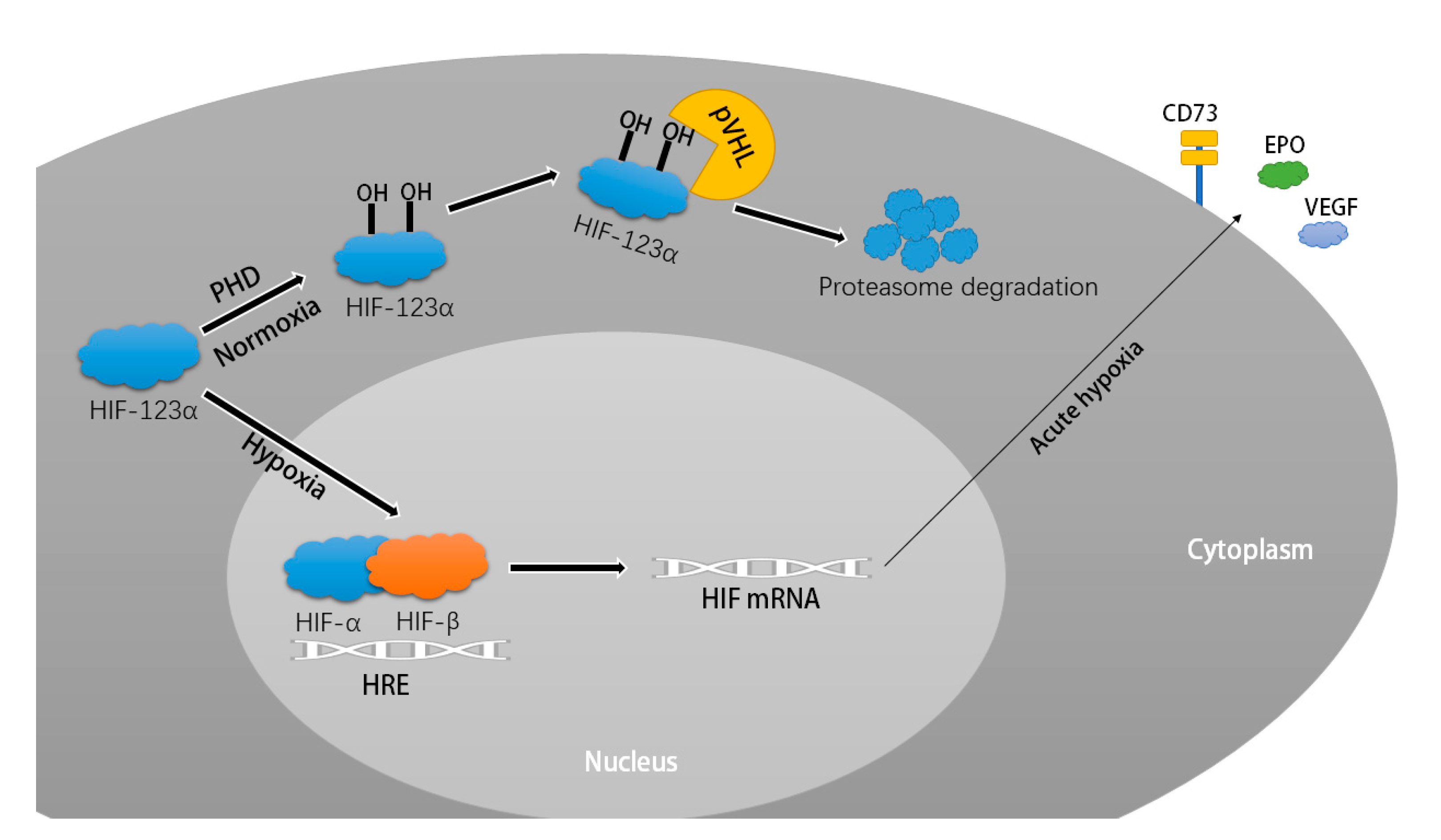
1. Introduction
2. Results
2.1. Microarray Information and DEGs in Six Datasets
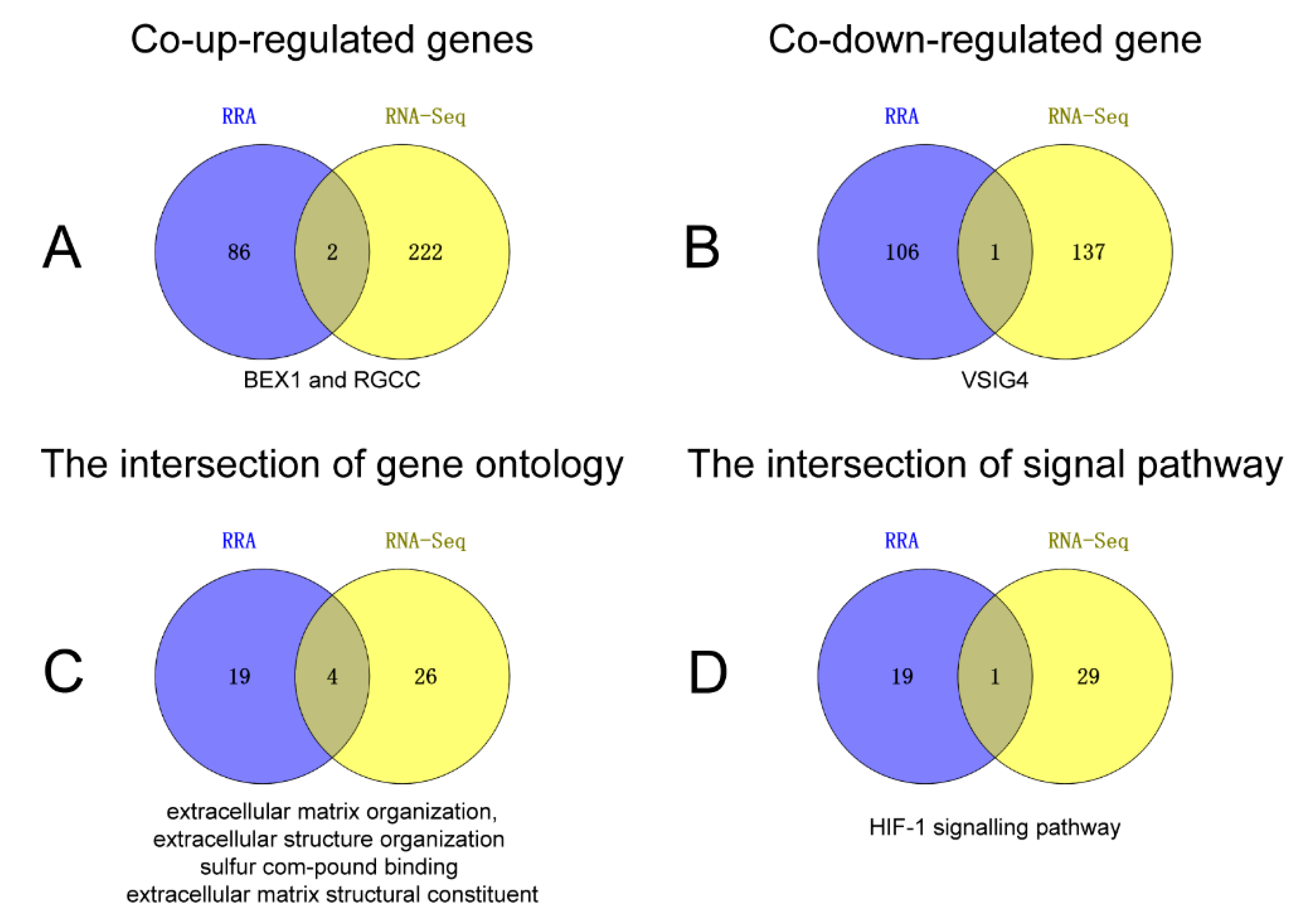
2.2. RRA Integrated Analysis
2.3. Functional Annotation of RRA
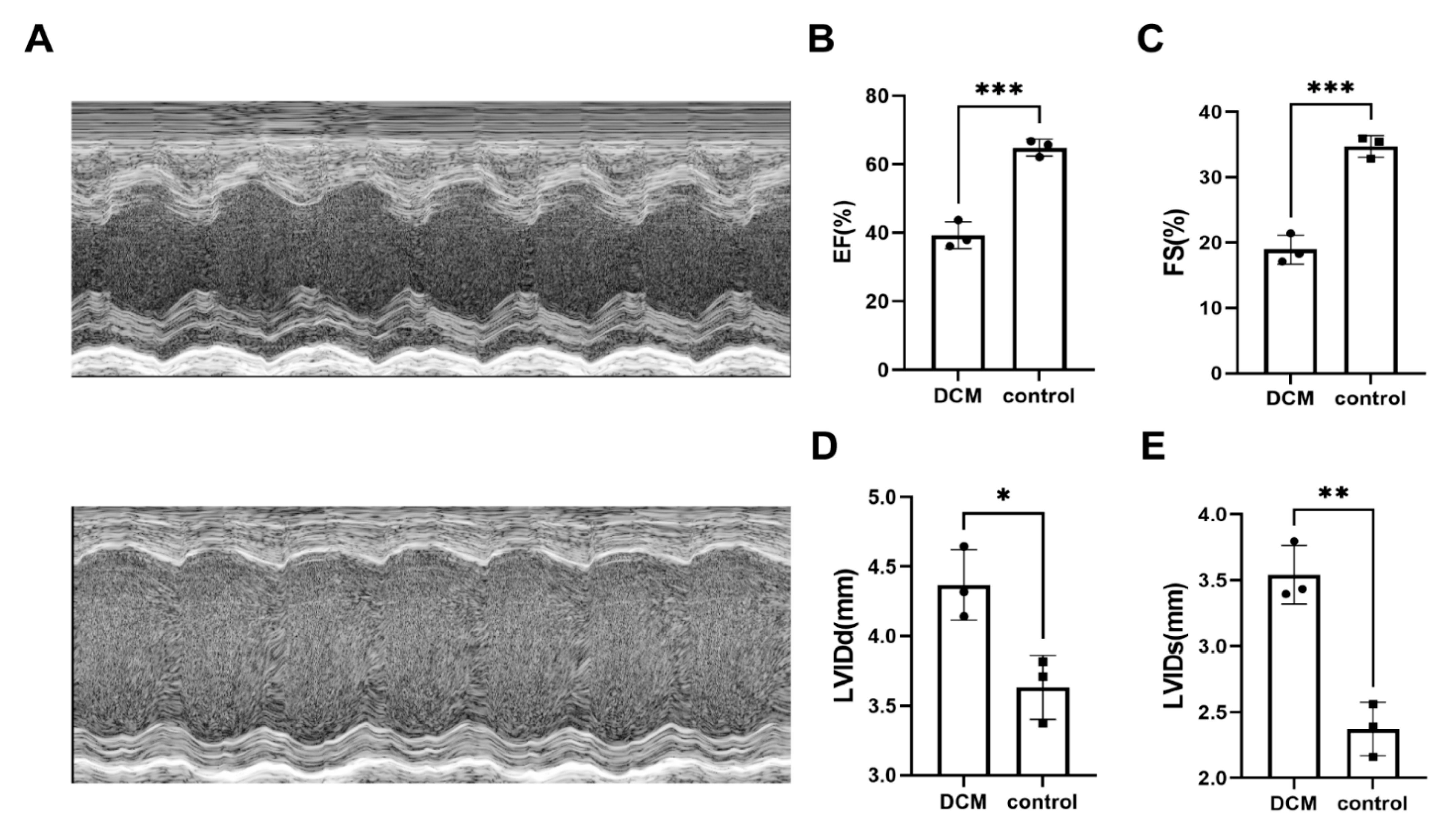
2.4. DEGs in Animal Experiments
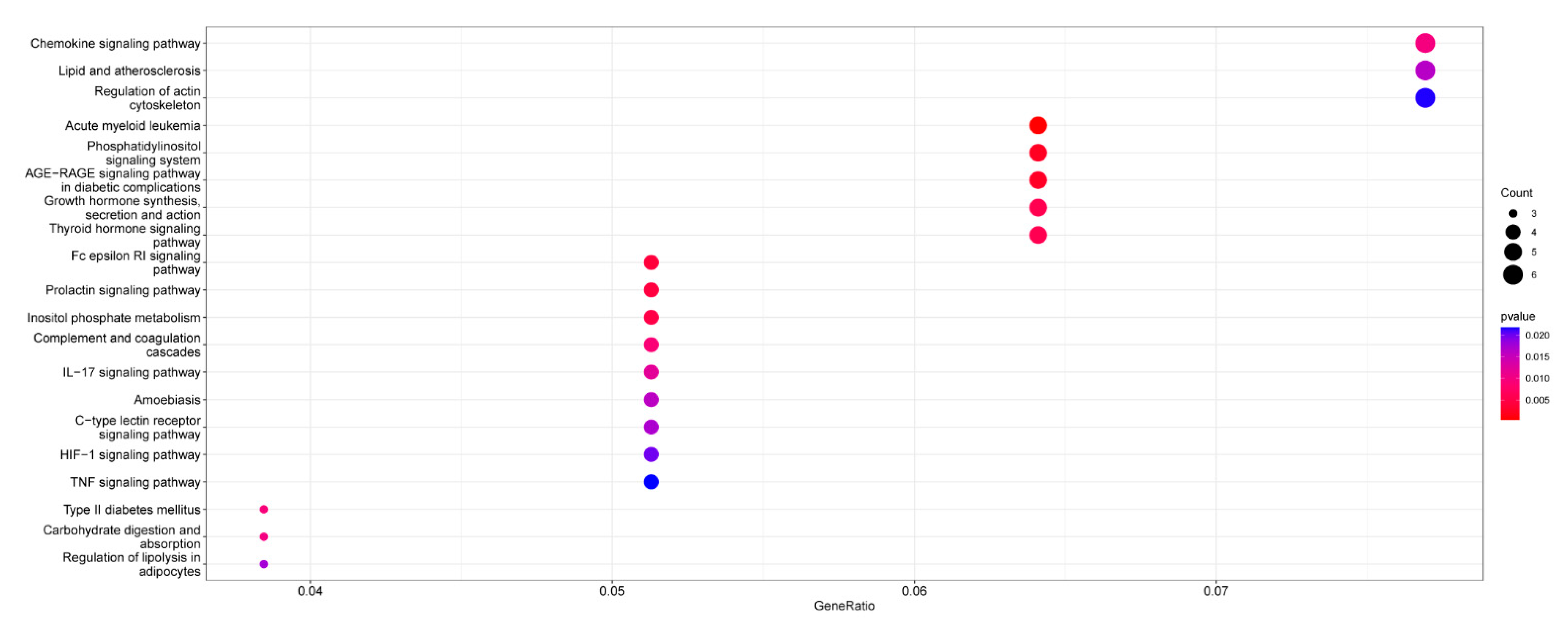
2.5. Enrichment Analysis of RNA-Seq
2.6. Results of Binary Logistic Regression Statistical Analysis
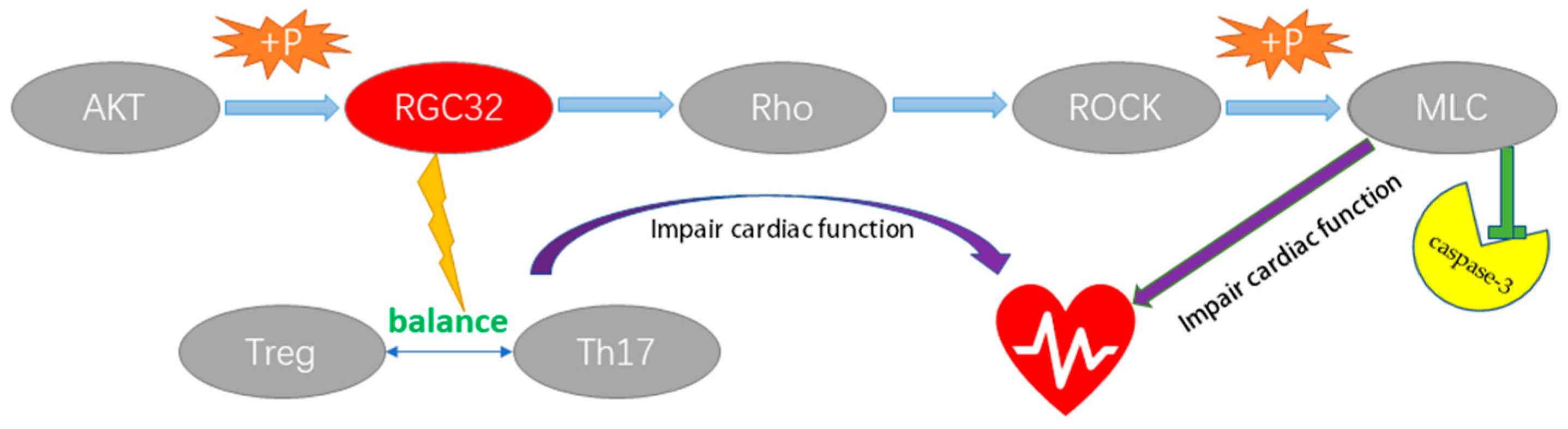
3. Discussion
4. Materials and Methods
4.1. DCM Related Microarray Datasets
4.2. Acquisition and Analysis of Microarray Datasets
4.3. Integration Analysis of RRA Algorithm
4.4. Functional Enrichment Analysis of RRA Results
4.5. DCM Animal Model Establishment
4.6. RNA-Seq and Data Analysis
4.7. Enrichment Analysis of RNA-Seq Results
4.8. Binary Logistic Regression Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merlo, M.; Cannata, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving concepts in dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Reichart, D.; Magnussen, C.; Zeller, T.; Blankenberg, S. Dilated cardiomyopathy: From epidemiologic to genetic phenotypes: A translational review of current literature. J. Intern. Med. 2019, 286, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.; Ahn, E.; Soor, G.S.; Butany, J. Dilated cardiomyopathy: A review. J. Clin. Pathol. 2009, 62, 219–225. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Agre, K.E.; Pereira, N.L. Genetics of dilated cardiomyopathy: Practical implications for heart failure management. Nat. Rev. Cardiol. 2020, 17, 286–297. [Google Scholar] [CrossRef]
- Kolde, R.; Laur, S.; Adler, P.; Vilo, J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics 2012, 28, 573–580. [Google Scholar] [CrossRef]
- Barth, A.S.; Kuner, R.; Buness, A.; Ruschhaupt, M.; Merk, S.; Zwermann, L.; Kääb, S.; Kreuzer, E.; Steinbeck, G.; Mansmann, U.; et al. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J. Am. Coll. Cardiol. 2006, 48, 1610–1617. [Google Scholar] [CrossRef]
- Gaertner, A.; Schwientek, P.; Ellinghaus, P.; Summer, H.; Golz, S.; Kassner, A.; Schulz, U.; Gummert, J.; Milting, H. Myocardial transcriptome analysis of human arrhythmogenic right ventricular cardiomyopathy. Physiol. Genom. 2012, 44, 99–109. [Google Scholar] [CrossRef]
- Molina-Navarro, M.M.; Roselló-Lletí, E.; Ortega, A.; Tarazón, E.; Otero, M.; Martínez-Dolz, L.; Lago, F.; González-Juanatey, J.R.; España, F.; García-Pavía, P.; et al. Differential gene expression of cardiac ion channels in human dilated cardiomyopathy. PLoS ONE 2013, 8, e79792. [Google Scholar] [CrossRef]
- Koczor, C.A.; Torres, R.A.; Fields, E.J.; Boyd, A.; He, S.; Patel, N.; Lee, E.K.; Samarel, A.M.; Lewis, W. Thymidine kinase and mtDNA depletion in human cardiomyopathy: Epigenetic and translational evidence for energy starvation. Physiol. Genom. 2013, 45, 590–596. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Al Khiami, B.; Efimov, I.R.; Evans, S.; Vader, J.; Jain, A.; Brownstein, B.H.; Hotchkiss, R.S.; Mann, D.L. Widespread Down-Regulation of Cardiac Mitochondrial and Sarcomeric Genes in Patients With Sepsis. Crit. Care Med. 2017, 45, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Laugier, L.; Frade, A.F.; Ferreira, F.M.; Baron, M.A.; Teixeira, P.C.; Cabantous, S.; Ferreira, L.R.P.; Louis, L.; Rigaud, V.O.C.; Gaiotto, F.A.; et al. Whole-Genome Cardiac DNA Methylation Fingerprint and Gene Expression Analysis Provide New Insights in the Pathogenesis of Chronic Chagas Disease Cardiomyopathy. Clin. Infect. Dis. 2017, 65, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, J.C. Logistic regression: A brief primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Hibino, E.; Ichiyama, Y.; Tsukamura, A.; Senju, Y.; Morimune, T.; Ohji, M.; Maruo, Y.; Nishimura, M.; Mori, M. Bex1 is essential for ciliogenesis and harbours biomolecular condensate-forming capacity. BMC Biol. 2022, 20, 42. [Google Scholar] [CrossRef]
- Aronow, B.J.; Toyokawa, T.; Canning, A.; Haghighi, K.; Delling, U.; Kranias, E.; Molkentin, J.D.; Dorn, G.W., 2nd. Divergent transcriptional responses to independent genetic causes of cardiac hypertrophy. Physiol. Genom. 2001, 6, 19–28. [Google Scholar] [CrossRef]
- Accornero, F.; Schips, T.G.; Petrosino, J.M.; Gu, S.Q.; Kanisicak, O.; van Berlo, J.H.; Molkentin, J.D. BEX1 is an RNA-dependent mediator of cardiomyopathy. Nat. Commun. 2017, 8, 1875. [Google Scholar] [CrossRef]
- Badea, T.; Niculescu, F.; Soane, L.; Fosbrink, M.; Sorana, H.; Rus, V.; Shin, M.L.; Rus, H. RGC-32 increases p34CDC2 kinase activity and entry of aortic smooth muscle cells into S-phase. J. Biol. Chem. 2002, 277, 502–508. [Google Scholar] [CrossRef]
- Fosbrink, M.; Cudrici, C.; Tegla, C.A.; Soloviova, K.; Ito, T.; Vlaicu, S.; Rus, V.; Niculescu, F.; Rus, H. Response gene to complement 32 is required for C5b-9 induced cell cycle activation in endothelial cells. Exp. Mol. Pathol. 2009, 86, 87–94. [Google Scholar] [CrossRef]
- Vlaicu, S.I.; Tatomir, A.; Boodhoo, D.; Ito, T.; Fosbrink, M.; Cudrici, C.; Mekala, A.P.; Ciriello, J.; Crişan, D.; Boţan, E.; et al. RGC-32 is expressed in the human atherosclerotic arterial wall: Role in C5b-9-induced cell proliferation and migration. Exp. Mol. Pathol. 2016, 101, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Stone, N.L.; Pittman, R.N. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J. Cell Biol. 1999, 146, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Sebbagh, M.; Renvoizé, C.; Hamelin, J.; Riché, N.; Bertoglio, J.; Bréard, J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001, 3, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xie, M.; Shah, V.R.; Schneider, M.D.; Entman, M.L.; Wei, L.; Schwartz, R.J. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc. Natl. Acad. Sci. USA 2006, 103, 14495–14500. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, W.; Tang, X.; Wang, W.; Pan, J.; Tan, M. Response Gene to Complement-32 Promotes the Imbalance of Treg/Th17 in Patients with Dilated Cardiomyopathy. Cell. Physiol. Biochem. 2017, 43, 1515–1525. [Google Scholar] [CrossRef]
- Baldeviano, G.C.; Barin, J.G.; Talor, M.V.; Srinivasan, S.; Bedja, D.; Zheng, D.; Gabrielson, K.; Iwakura, Y.; Rose, N.R.; Cihakova, D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010, 106, 1646–1655. [Google Scholar] [CrossRef]
- Chen, P.; Baldeviano, G.C.; Ligons, D.L.; Talor, M.V.; Barin, J.G.; Rose, N.R.; Cihakova, D. Susceptibility to autoimmune myocarditis is associated with intrinsic differences in CD4+ T cells. Clin. Exp. Immunol. 2012, 169, 79–88. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, Y.; Jeon, C.; Lee, S.-J.; Lee, B.-H.; Choi, K.D.; Bae, Y.-S. Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis. Blood 2002, 100, 1742–1754. [Google Scholar] [CrossRef]
- Munawara, U.; Perveen, K.; Small, A.G.; Putty, T.; Quach, A.; Gorgani, N.N.; Hii, C.S.; Abbott, C.A.; Ferrante, A. Human Dendritic Cells Express the Complement Receptor Immunoglobulin Which Regulates T Cell Responses. Front. Immunol. 2019, 10, 2892. [Google Scholar] [CrossRef]
- Jung, K.; Kang, M.; Park, C.; Hyun Choi, Y.; Jeon, Y.; Park, S.H.; Seo, S.K.; Jin, D.; Choi, I. Protective role of V-set and immunoglobulin domain-containing 4 expressed on kupffer cells during immune-mediated liver injury by inducing tolerance of liver T- and natural killer T-cells. Hepatology 2012, 56, 1838–1848. [Google Scholar] [CrossRef]
- Mora-Ruíz, M.D.; Blanco-Favela, F.; Chávez Rueda, A.K.; Legorreta-Haquet, M.V.; Chávez-Sánchez, L. Role of interleukin-17 in acute myocardial infarction. Mol. Immunol. 2019, 107, 71–78. [Google Scholar] [CrossRef]
- Rahman, A.; Jafry, S.; Jeejeebhoy, K.; Nagpal, A.D.; Pisani, B.; Agarwala, R. Malnutrition and Cachexia in Heart Failure. JPEN. J. Parenter. Enter. Nutr. 2016, 40, 475–486. [Google Scholar] [CrossRef]
- Levick, S.P.; Goldspink, P.H. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail. Rev. 2014, 19, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Rurik, J.G.; Aghajanian, H.; Epstein, J.A. Immune Cells and Immunotherapy for Cardiac Injury and Repair. Circ. Res. 2021, 128, 1766–1779. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl. Res. J. Lab. Clin. Med. 2018, 191, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Diao, B.; Guo, S.; Huang, X.; Yang, C.; Feng, Z.; Yan, W.; Ning, Q.; Zheng, L.; Chen, Y.; et al. VSIG4 inhibits proinflammatory macrophage activation by reprogramming mitochondrial pyruvate metabolism. Nat. Commun. 2017, 8, 1322. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Schmitz, N.; Kurrer, M.O.; Bauer, M.; Hinton, H.I.; Behnke, S.; Gatto, D.; Sebbel, P.; Beerli, R.R.; Sonderegger, I.; et al. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J. Clin. Investig. 2006, 116, 2817–2826. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Russo, I.; Cavalera, M.; Huang, S.; Su, Y.; Hanna, A.; Chen, B.; Shinde, A.V.; Conway, S.J.; Graff, J.; Frangogiannis, N.G. Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program. Circ. Res. 2019, 124, 1214–1227. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T. Cardiac interstitium in health and disease: The fibrillar collagen network. J. Am. Coll. Cardiol. 1989, 13, 1637–1652. [Google Scholar] [CrossRef]
- Dixon, I.M.; Ju, H.; Reid, N.L.; Scammell-La Fleur, T.; Werner, J.P.; Jasmin, G. Cardiac collagen remodeling in the cardiomyopathic Syrian hamster and the effect of losartan. J. Mol. Cell. Cardiol. 1997, 29, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J. Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochem. Soc. Trans. 2004, 32, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Qabar, A.N.; Lin, Z.; Wolf, F.W.; O’Shea, K.S.; Lawler, J.; Dixit, V.M. Thrombospondin 3 is a developmentally regulated heparin binding protein. J. Biol. Chem. 1994, 269, 1262–1269. [Google Scholar] [CrossRef]
- Collins, A.R.; Schnee, J.; Wang, W.; Kim, S.; Fishbein, M.C.; Bruemmer, D.; Law, R.E.; Nicholas, S.; Ross, R.S.; Hsueh, W.A. Osteopontin modulates angiotensin II- induced fibrosis in the intact murine heart. J. Am. Coll. Cardiol. 2004, 43, 1698–1705. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Hiroe, M.; Nishikawa, T.; Ishiyama, S.; Shimojo, T.; Ohta, Y.; Sakakura, T.; Yoshida, T. Tenascin-C Modulates Adhesion of Cardiomyocytes to Extracellular Matrix during Tissue Remodeling after Myocardial Infarction. Lab. Investig. 2001, 81, 1015–1024. [Google Scholar] [CrossRef]
- Li, G.; Jin, R.; Norris, R.A.; Zhang, L.; Yu, S.; Wu, F.; Markwald, R.R.; Nanda, A.; Conway, S.J.; Smyth, S.S.; et al. Periostin mediates vascular smooth muscle cell migration through the integrins ανβ3 and ανβ5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 2010, 208, 358–365. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Huang, L.E.; Gu, J.; Schau, M.; Bunn, H.F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 7987–7992. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Hu, C.J.; Wang, L.Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Investig. 2002, 110, 993–1002. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, S.; Glover, L.; Miller, S.M.; Shannon, J.M.; Guo, X.; Franklin, W.A.; Bridges, J.P.; Schaack, J.B.; Colgan, S.P.; et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 10684–10689. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Wiesener, M.S.; Jürgensen, J.S.; Rosenberger, C.; Scholze, C.K.; Hörstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003, 17, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef]
- Hölscher, M.; Schäfer, K.; Krull, S.; Farhat, K.; Hesse, A.; Silter, M.; Lin, Y.; Pichler, B.J.; Thistlethwaite, P.; El-Armouche, A.; et al. Unfavourable consequences of chronic cardiac HIF-1α stabilization. Cardiovasc. Res. 2012, 94, 77–86. [Google Scholar] [CrossRef]
- Lei, L.; Mason, S.; Liu, D.; Huang, Y.; Marks, C.; Hickey, R.; Jovin, I.S.; Pypaert, M.; Johnson, R.S.; Giordano, F.J. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol. Cell. Biol. 2008, 28, 3790–3803. [Google Scholar] [CrossRef]
- Moslehi, J.; Minamishima, Y.A.; Shi, J.; Neuberg, D.; Charytan, D.M.; Padera, R.F.; Signoretti, S.; Liao, R.; Kaelin, W.G., Jr. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation 2010, 122, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.-G.; Lu, M.-J.; Chang, H.; Sun, H.-Y.; Wang, B.-W.; Kuan, P. Carvedilol Modulates the Expression of Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor in a Rat Model of Volume-Overload Heart Failure. J. Card. Fail. 2005, 11, 152–159. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Gautier, L.; Cope, L.; Bolstad, B.M.; Irizarry, R.A. Affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3. [Google Scholar] [CrossRef]



| GSE ID | Participants | Platform | Year | Number of DEGs | Reference | |
|---|---|---|---|---|---|---|
| Up | Down | |||||
| GSE3585 | 7 cases and 5 controls | GPL96 | 2005 | 147 | 123 | Barth et al. [7] |
| GSE29819 | 7 cases and 6 controls | GPL570 | 2011 | 1132 | 1103 | Gaertner et al. [8] |
| GSE42955 | 12 cases and 5 controls | GPL6244 | 2012 | 79 | 102 | Molina-Navarro et al. [9] |
| GSE43435 | 10 cases and 10 controls | GPL15338 | 2013 | 365 | 518 | Koczor et al. [10] |
| GSE79962 | 9 cases and 11 controls | GPL6244 | 2016 | 349 | 252 | Matkovich et al. [11] |
| GSE84796 | 10 cases and 7 controls | GPL14550 | 2016 | 3223 | 4022 | Laugier et al. [12] |
| Term | Correlation Coefficient | SE | Wald | p | OR | 95% Confidence Interval of OR | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| BEX1 | 1.513 | 0.528 | 8.197 | 0.004 *** | 4.538 | 1.611 | 12.782 |
| RGCC | 1.005 | 0.51 | 3.882 | 0.049 ** | 2.731 | 1.005 | 7.42 |
| VSIG4 | 1.102 | 0.509 | 4.692 | 0.030 ** | 3.009 | 1.11 | 8.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Wang, X.; Liang, H.; Liu, F.; Li, Y.; Zhang, H.; Wang, C.; Wang, Q. Identification of Signature Genes of Dilated Cardiomyopathy Using Integrated Bioinformatics Analysis. Int. J. Mol. Sci. 2023, 24, 7339. https://doi.org/10.3390/ijms24087339
Wu Z, Wang X, Liang H, Liu F, Li Y, Zhang H, Wang C, Wang Q. Identification of Signature Genes of Dilated Cardiomyopathy Using Integrated Bioinformatics Analysis. International Journal of Molecular Sciences. 2023; 24(8):7339. https://doi.org/10.3390/ijms24087339
Chicago/Turabian StyleWu, Zhimin, Xu Wang, Hao Liang, Fangfang Liu, Yingxuan Li, Huaxing Zhang, Chunying Wang, and Qiao Wang. 2023. "Identification of Signature Genes of Dilated Cardiomyopathy Using Integrated Bioinformatics Analysis" International Journal of Molecular Sciences 24, no. 8: 7339. https://doi.org/10.3390/ijms24087339
APA StyleWu, Z., Wang, X., Liang, H., Liu, F., Li, Y., Zhang, H., Wang, C., & Wang, Q. (2023). Identification of Signature Genes of Dilated Cardiomyopathy Using Integrated Bioinformatics Analysis. International Journal of Molecular Sciences, 24(8), 7339. https://doi.org/10.3390/ijms24087339





