Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury
Abstract
1. Introduction
2. Results
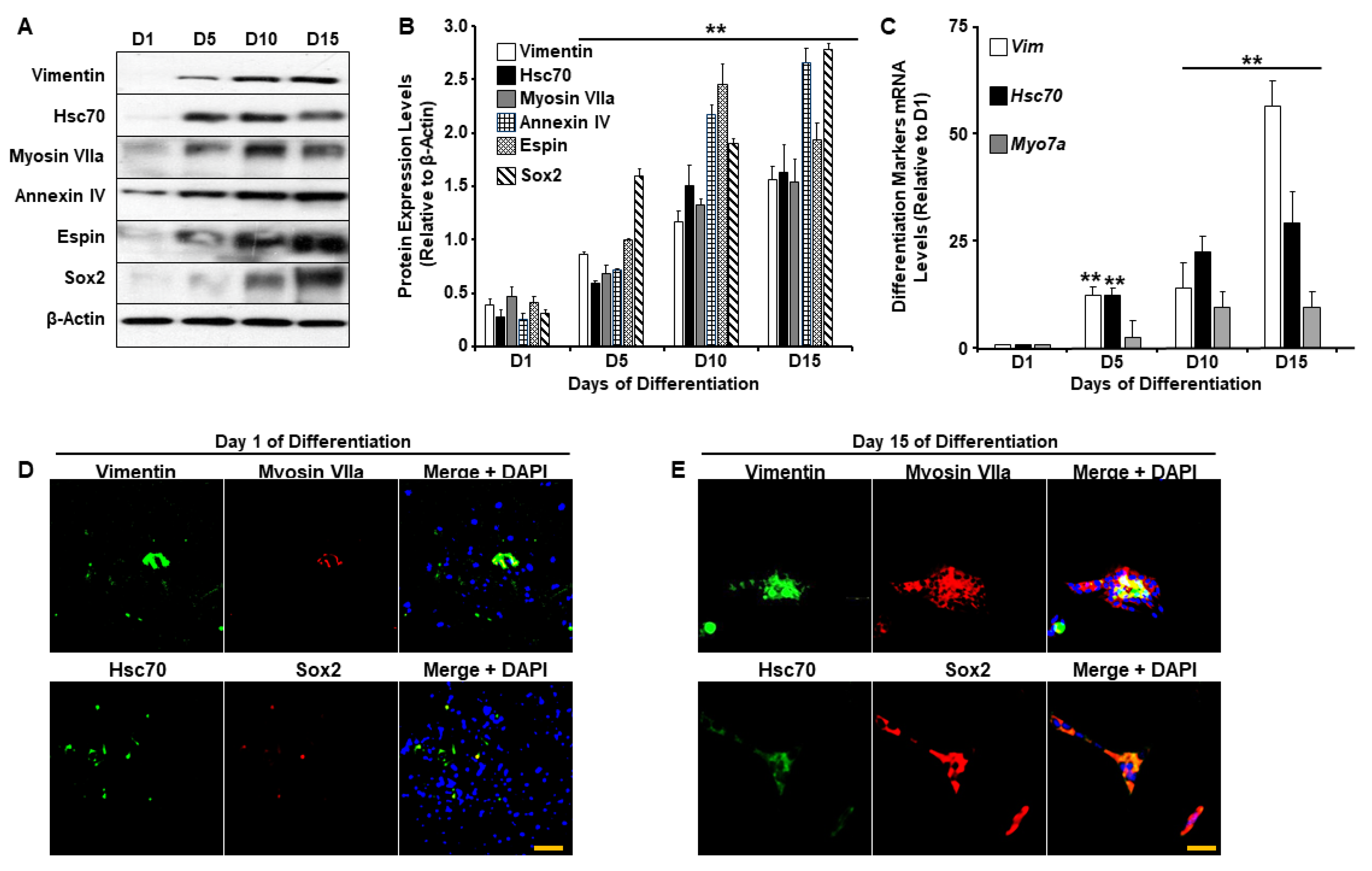
2.1. UB/OC-2 Cells Differentiate into Functional Cochlear Hair Cells
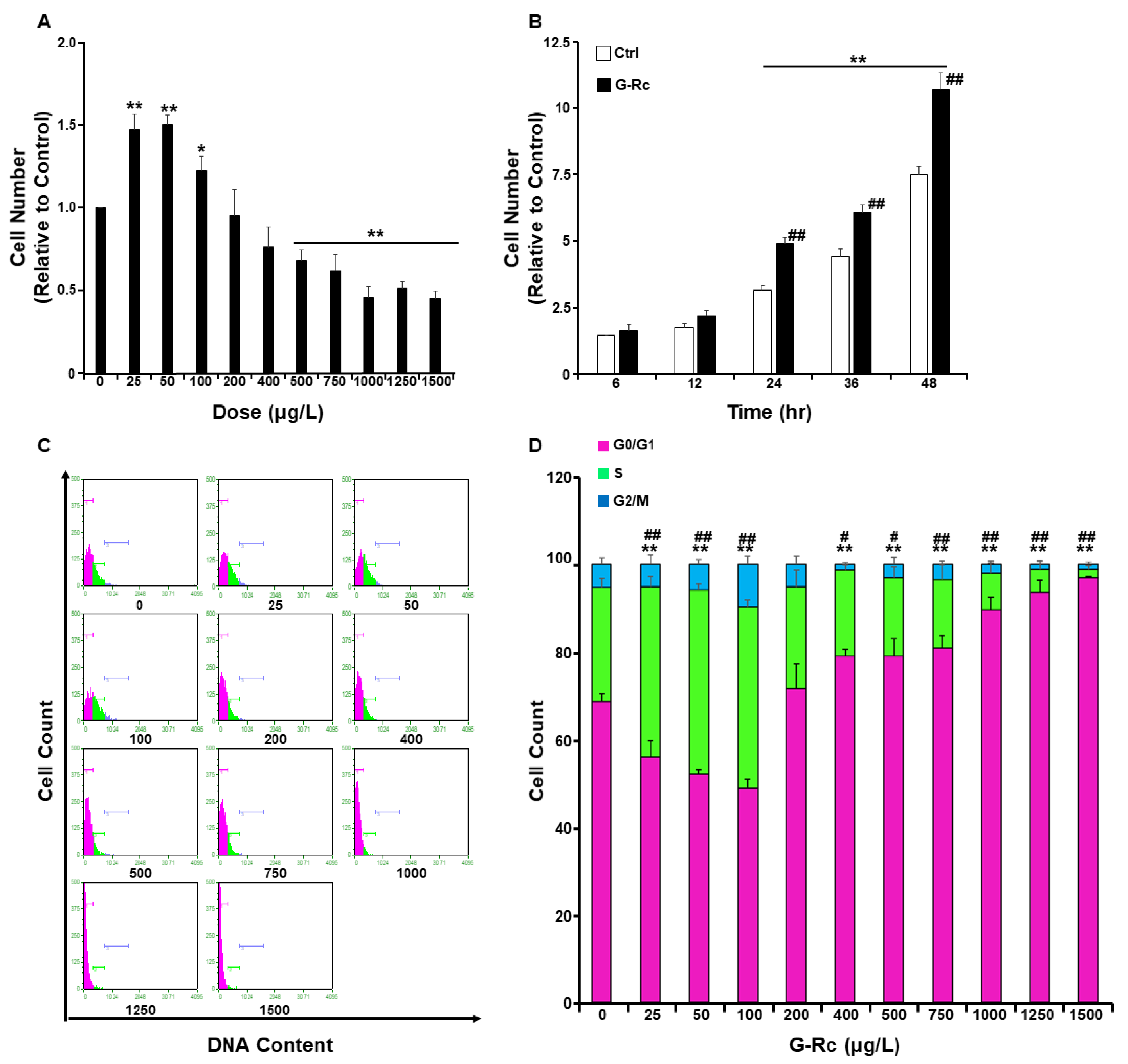
2.2. Effects of G-Rc on Cell Survival/Toxicity, Proliferation, and Cell Cycle
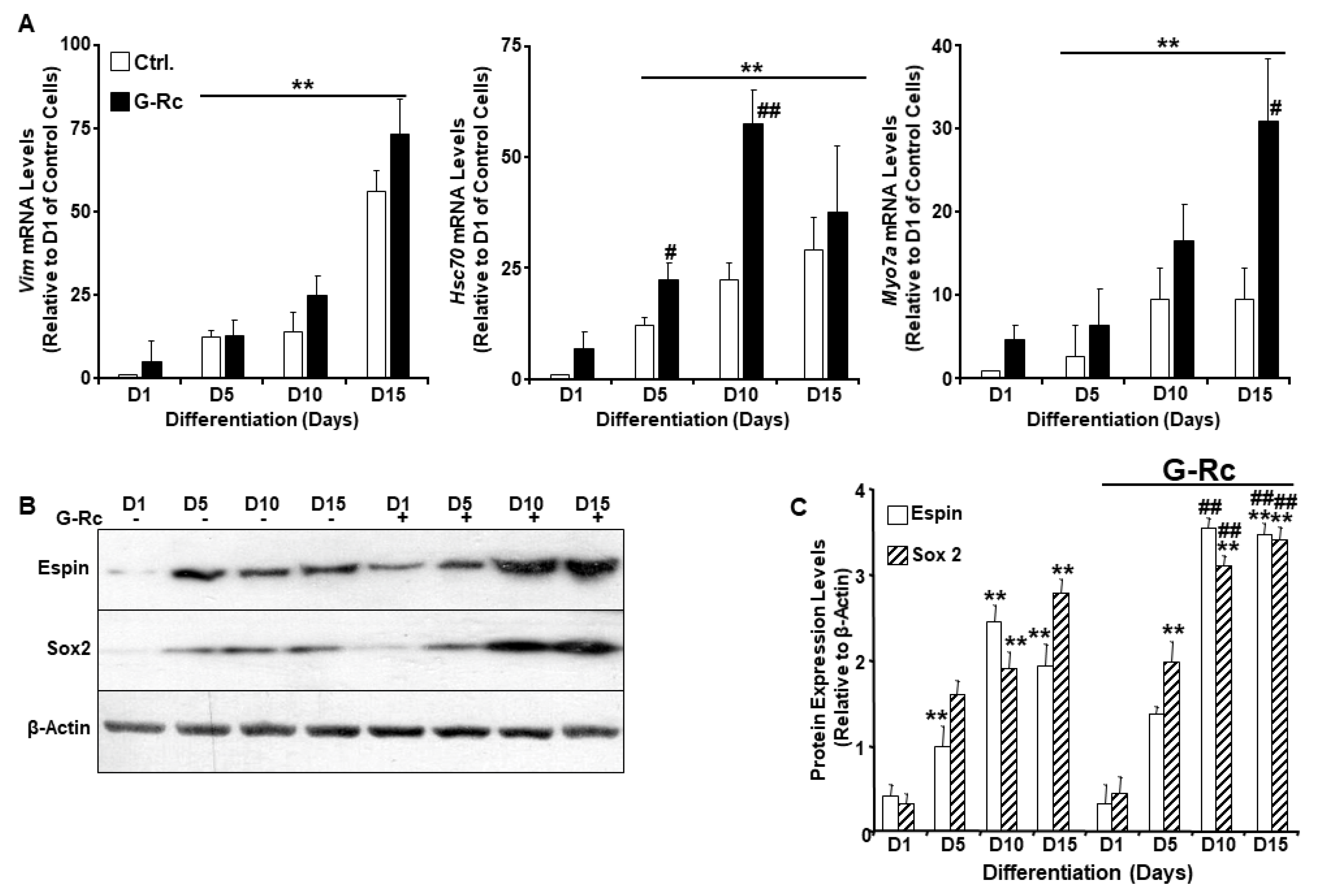
2.3. A Physiologically Relevant Dose of G-Rc Promotes the Differentiation of UB/OC-2 Cells into Cochlear Hair Cells
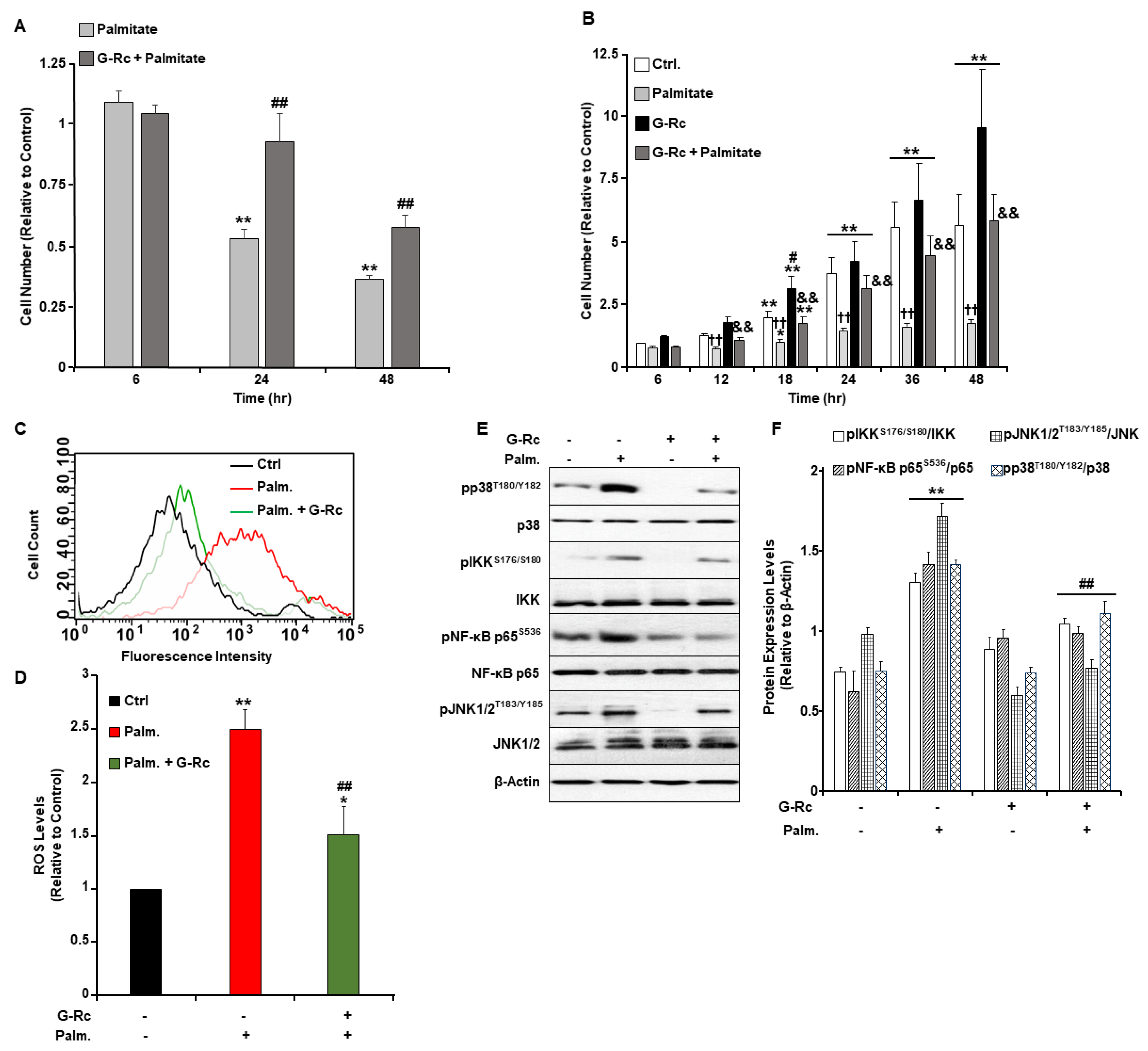
2.4. A Physiologically Relevant Dose of G-Rc Alleviates Palmitate-Induced Alterations to Cell Survival and Proliferation, Oxidative Stress, and Inflammation
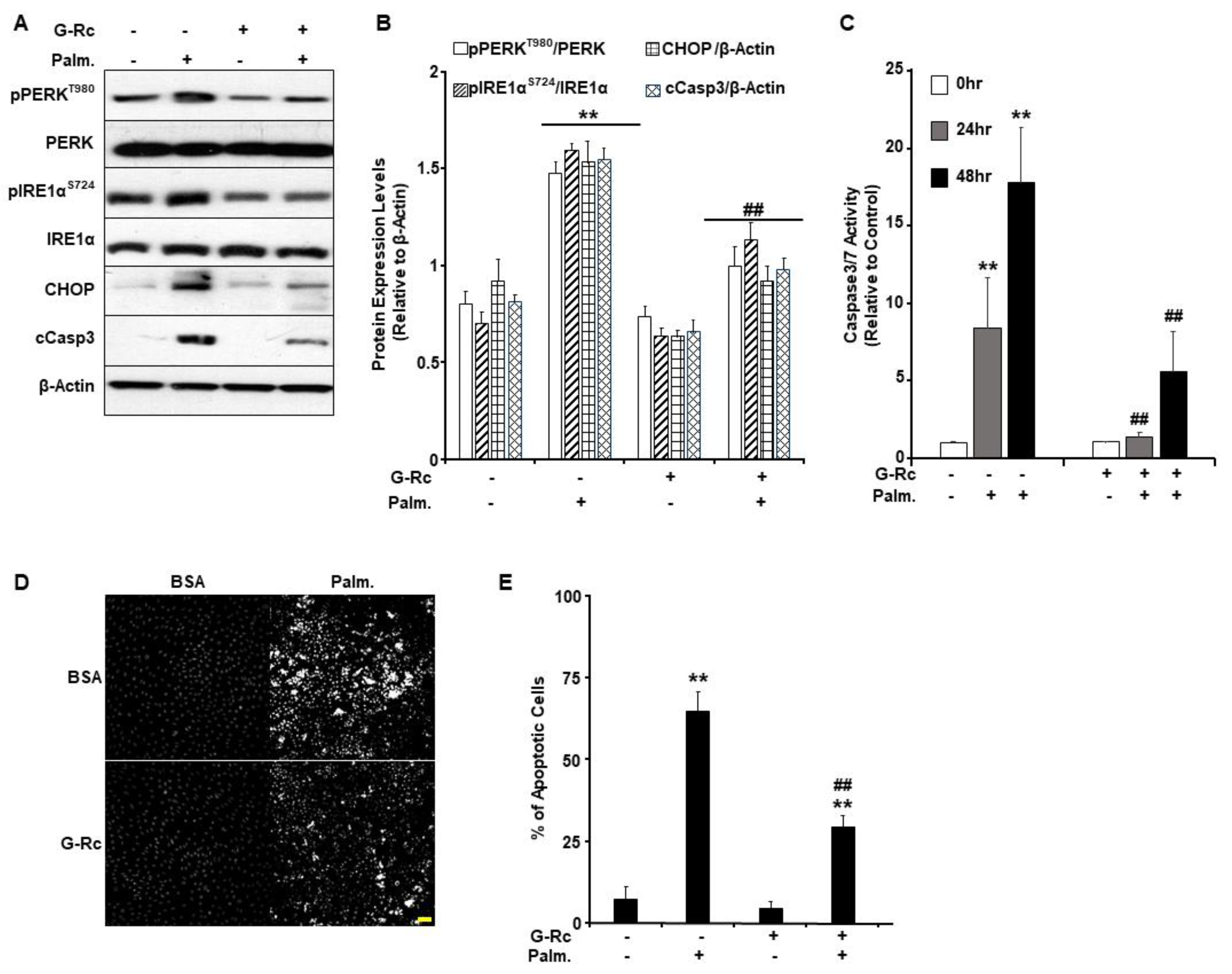
2.5. G-Rc Alleviates Palmitate-Induced ER Stress and Apoptotic Cell Death
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Protein Extraction and Immunoblots
4.4. RNA Isolation and qRT-PCR
4.5. Assessment of Oxidative Stress
4.6. Cell Proliferation Assay
4.7. Cytotoxicity Assay
4.8. Morphological Analysis of Apoptosis
4.9. Cell Cycle Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Michels, T.C.; Duffy, M.T.; Rogers, D.J. Hearing Loss in Adults: Differential Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 98–108. [Google Scholar]
- Nieman, C.L.; Oh, E.S. Hearing Loss. Ann. Intern. Med. 2020, 173, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, R.; Natarajan, S.; Jeong, S.Y.; Hong, B.N.; Kang, T.H. Traditional oriental medicine for sensorineural hearing loss: Can ethnopharmacology contribute to potential drug discovery? J. Ethnopharmacol. 2019, 231, 409–428. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation associated with noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 4020. [Google Scholar] [CrossRef]
- Watson, N.; Ding, B.; Zhu, X.; Frisina, R.D. Chronic inflammation—Inflammaging—In the ageing cochlea: A novel target for future presbycusis therapy. Ageing Res. Rev. 2017, 40, 142–148. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479s–3485s. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517s–520s. [Google Scholar] [CrossRef]
- de Kok, T.M.; van Breda, S.G.; Manson, M.M. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur. J. Nutr. 2008, 47, 51–59. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Shang, H.C.; Gao, X.M.; Zhang, B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother. Res. PTR 2008, 22, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Cuong, T.T.; Yang, C.S.; Yuk, J.M.; Lee, H.M.; Ko, S.R.; Cho, B.G.; Jo, E.K. Glucocorticoid receptor agonist compound K regulates Dectin-1-dependent inflammatory signaling through inhibition of reactive oxygen species. Life Sci. 2009, 85, 625–633. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.H.; Park, D.; Choi, Y.J.; Park, M.H.; Chung, K.W.; Kim, S.R.; Lee, J.S.; Chung, H.Y. Ginsenoside Rc modulates Akt/FoxO1 pathways and suppresses oxidative stress. Arch. Pharm. Res. 2014, 37, 813–820. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Y.; Kwak, Y.S.; Song, G.G.; Kim, M.Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J. Ginseng Res. 2017, 41, 127–133. [Google Scholar] [CrossRef]
- Fujita, K.; Hakuba, N.; Hata, R.; Morizane, I.; Yoshida, T.; Shudou, M.; Sakanaka, M.; Gyo, K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci. Lett. 2007, 415, 113–117. [Google Scholar] [CrossRef]
- Durankaya, S.M.; Olgun, Y.; Aktas, S.; Eskicioglu, H.E.; Gurkan, S.; Altun, Z.; Mutlu, B.; Kolatan, E.; Dogan, E.; Yilmaz, O.; et al. Effect of Korean Red Ginseng on Noise-Induced Hearing Loss. Turk. Arch. Otorhinolaryngol. 2021, 59, 111–117. [Google Scholar] [CrossRef]
- Doosti, A.; Lotfi, Y.; Moossavi, A.; Bakhshi, E.; Talasaz, A.H.; Hoorzad, A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health 2014, 16, 223–227. [Google Scholar] [CrossRef]
- Kim, T.S.; Lee, H.S.; Chung, J.W. The Effect of Korean Red Ginseng on Symptoms and Quality of Life in Chronic Tinnitus: A Randomized, Open-Label Pilot Study. J. Audiol. Otol. 2015, 19, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Morrill, S.; He, D.Z.Z. Apoptosis in inner ear sensory hair cells. J. Otol. 2017, 12, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Lallemend, F.; Lefebvre, P.P.; Hans, G.; Moonen, G.; Malgrange, B. Molecular pathways involved in apoptotic cell death in the injured cochlea: Cues to novel therapeutic strategies. Curr. Pharm. Des. 2005, 11, 2257–2275. [Google Scholar] [CrossRef]
- Li, H.; Roblin, G.; Liu, H.; Heller, S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 13495–13500. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Hashino, E. Generation of inner ear organoids from human pluripotent stem cells. Methods Cell Biol. 2020, 159, 303–321. [Google Scholar] [CrossRef]
- Jagger, D.J.; Griesinger, C.B.; Rivolta, M.N.; Holley, M.C.; Ashmore, J.F. Calcium signalling mediated by the 9 acetylcholine receptor in a cochlear cell line from the Immortomouse. J. Physiol. 2000, 527, 49–54. [Google Scholar] [CrossRef]
- Jagger, D.J.; Holley, M.C.; Ashmore, J.F. Ionic currents expressed in a cell line derived from the organ of Corti of the Immortomouse. Pflug. Arch. 1999, 438, 8–14. [Google Scholar] [CrossRef]
- Choi, M.K.; Jin, S.; Jeon, J.H.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Han, Y.H.; Song, I.S. Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. J. Ginseng Res. 2020, 44, 229–237. [Google Scholar] [CrossRef]
- Gunaratnam, K.; Vidal, C.; Boadle, R.; Thekkedam, C.; Duque, G. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol. Open. 2013, 2, 1382–1389. [Google Scholar] [CrossRef]
- Xu, S.; Nam, S.M.; Kim, J.H.; Das, R.; Choi, S.K.; Nguyen, T.T.; Quan, X.; Choi, S.J.; Chung, C.H.; Lee, E.Y.; et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 2015, 6, e1976. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 2001, 276, 14890–14895. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Schaffer, J.E. Mechanisms of lipoapoptosis: Implications for human heart disease. Trends Cardiovasc. Med. 2002, 12, 134–138. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H(2)S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Pao, H.P.; Liao, W.I.; Tang, S.E.; Wu, S.Y.; Huang, K.L.; Chu, S.J. Suppression of Endoplasmic Reticulum Stress by 4-PBA Protects Against Hyperoxia-Induced Acute Lung Injury via Up-Regulating Claudin-4 Expression. Front. Immunol. 2021, 12, 674316. [Google Scholar] [CrossRef]
- Chauvier, D.; Ankri, S.; Charriaut-Marlangue, C.; Casimir, R.; Jacotot, E. Broad-spectrum caspase inhibitors: From myth to reality? Cell Death Differ. 2007, 14, 387–391. [Google Scholar] [CrossRef]
- Abi-Hachem, R.N.; Zine, A.; Van De Water, T.R. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent. Pat. CNS Drug Discov. 2010, 5, 147–163. [Google Scholar] [CrossRef]
- Li, B.; Zheng, T.; Yan, C.; Wang, W.; Zhang, J.; Zhang, L.; Hu, J.; Zhang, L.; Wan, Y.; Zhang, M.; et al. Chemical chaperone 4-phenylbutyrate prevents hearing loss and cochlear hair cell death in Cdh23erl/erl mutant mice. Neuroreport 2019, 30, 145–150. [Google Scholar] [CrossRef]
- Hu, J.; Li, B.; Apisa, L.; Yu, H.; Entenman, S.; Xu, M.; Stepanyan, R.; Guan, B.J.; Muller, U.; Hatzoglou, M.; et al. ER stress inhibitor attenuates hearing loss and hair cell death in Cdh23(erl/erl) mutant mice. Cell Death Dis. 2016, 7, e2485. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guan, G.; Lei, L.; Lv, Q.; Liu, S.; Zhan, X.; Jiang, Z.; Gu, X. Palmitic acid induces human osteoblast-like Saos-2 cell apoptosis via endoplasmic reticulum stress and autophagy. Cell Stress Chaperones 2018, 23, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Liu, S.; Xi, Y.; Nagata, N.; Matsuo, K.; Matsuo, I.; Chahed, S.; Bakke, J.; Keilhack, H.; Tiganis, T.; et al. Differential regulation of endoplasmic reticulum stress by protein tyrosine phosphatase 1B and T cell protein tyrosine phosphatase. J. Biol. Chem. 2011, 286, 9225–9235. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell. Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Bear, Z.W.; Mikulec, A.A. Intratympanic steroid therapy for treatment of idiopathic sudden sensorineural hearing loss. Mo. Med. 2014, 111, 352–356. [Google Scholar]
- Conlin, A.E.; Parnes, L.S. Treatment of sudden sensorineural hearing loss: II. A Meta-analysis. Arch. Otolaryngol. Head. Neck Surg. 2007, 133, 582–586. [Google Scholar] [CrossRef]
- Burns, J.C.; Corwin, J.T. A historical to present-day account of efforts to answer the question: “what puts the brakes on mammalian hair cell regeneration?”. Hear. Res. 2013, 297, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.L.; Shin, J.B. Mechanisms of Hair Cell Damage and Repair. Trends Neurosci. 2019, 42, 414–424. [Google Scholar] [CrossRef]
- Maharajan, N.; Cho, G.W.; Jang, C.H. Therapeutic Application of Mesenchymal Stem Cells for Cochlear Regeneration. In Vivo 2021, 35, 13–22. [Google Scholar] [CrossRef]
- Mahboubi, H.; Lin, H.W.; Bhattacharyya, N. Prevalence, Characteristics, and Treatment Patterns of Hearing Difficulty in the United States. JAMA Otolaryngol. Head. Neck Surg. 2018, 144, 65–70. [Google Scholar] [CrossRef]
- Kang, T.H.; Hong, B.N.; Jung, S.Y.; Lee, J.H.; So, H.S.; Park, R.; You, Y.O. Curculigo orchioides protects cisplatin-induced cell damage. Am. J. Chin. Med. 2013, 41, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hu, J.; Cheng, Y.; Wang, J.; Zhang, X.; Xu, M. Ginkgolide B protects against cisplatin-induced ototoxicity: Enhancement of Akt-Nrf2-HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother. Pharmacol. 2015, 75, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.; Droy-Lefaix, M.T.; Aurousseau, C.; Cazals, Y. Effects of Ginkgo biloba extract (EGb 761) on cochlear vasculature in the guinea pig: Morphometric measurements and laser Doppler flowmetry. Eur. Arch. Otorhinolaryngol. 1996, 253, 25–30. [Google Scholar] [CrossRef]
- Du, S.; Yao, Q.; Tan, P.; Xie, G.; Ren, C.; Sun, Q.; Zhang, X.; Zheng, R.; Yang, K.; Yuan, Y.; et al. Protective effect of tanshinone IIA against radiation-induced ototoxicity in HEI-OC1 cells. Oncol. Lett. 2013, 6, 901–906. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Im, G.J.; Chang, J.W.; Choi, J.; Chae, S.W.; Ko, E.J.; Jung, H.H. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother. Res. PTR 2010, 24, 614–621. [Google Scholar] [CrossRef]
- Kim, S.J.; Kwak, H.J.; Kim, D.S.; Choi, H.M.; Sim, J.E.; Kim, S.H.; Um, J.Y.; Hong, S.H. Protective mechanism of Korean Red Ginseng in cisplatin-induced ototoxicity through attenuation of nuclear factor-kappaB and caspase-1 activation. Mol. Med. Rep. 2015, 12, 315–322. [Google Scholar] [CrossRef]
- Choung, Y.H.; Kim, S.W.; Tian, C.; Min, J.Y.; Lee, H.K.; Park, S.N.; Lee, J.B.; Park, K. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope 2011, 121, 1294–1302. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, H.Y.; Bae, C.H.; Lee, Y.; Kim, S. Korean Red Ginseng Regulates Intestinal Tight Junction and Inflammation in the Colon of a Parkinson’s Disease Mouse Model. J. Med. Food 2020, 23, 1231–1237. [Google Scholar] [CrossRef]
- Jeon, H.; Bae, C.H.; Lee, Y.; Kim, H.Y.; Kim, S. Korean red ginseng suppresses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced inflammation in the substantia nigra and colon. Brain Behav. Immun. 2021, 94, 410–423. [Google Scholar] [CrossRef]
- Zaafan, M.A.; Abdelhamid, A.M.; Ibrahim, S.M. The Protective Effect of Korean Red Ginseng Against Rotenone-Induced Parkinson’s Disease in Rat Model: Modulation of Nuclear Factor-kappabeta and Caspase-3. Curr. Pharm. Biotechnol. 2019, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Kim, S.K.; Cha, K.M.; Jeong, M.S.; Ghosh, P.; Rhee, D.K. Korean Red Ginseng alleviates neuroinflammation and promotes cell survival in the intermittent heat stress-induced rat brain by suppressing oxidative stress via estrogen receptor beta and brain-derived neurotrophic factor upregulation. J. Ginseng Res. 2020, 44, 593–602. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, M.J.; Jang, M.; Kim, H.J.; Lee, S.; Lee, S.W.; Kim, Y.O.; Cho, I.H. Panax ginseng exerts antidepressant-like effects by suppressing neuroinflammatory response and upregulating nuclear factor erythroid 2 related factor 2 signaling in the amygdala. J. Ginseng Res. 2018, 42, 107–115. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, L. Ginsenoside Rg3 ameliorates lipopolysaccharide-induced acute lung injury in mice through inactivating the nuclear factor-kappaB (NF-kappaB) signaling pathway. Int. Immunopharmacol. 2016, 34, 53–59. [Google Scholar] [CrossRef]
- Seong, M.A.; Woo, J.K.; Kang, J.H.; Jang, Y.S.; Choi, S.; Jang, Y.S.; Lee, T.H.; Jung, K.H.; Kang, D.K.; Hurh, B.S.; et al. Oral administration of fermented wild ginseng ameliorates DSS-induced acute colitis by inhibiting NF-kappaB signaling and protects intestinal epithelial barrier. BMB Rep. 2015, 48, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, S.J.; Lee, A.Y.; Huang, J.; Chung, H.Y.; Im, D.S. Ginsenoside Rg3 promotes inflammation resolution through M2 macrophage polarization. J. Ginseng Res. 2018, 42, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ming, X.F. Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef]
- Na, Y.R.; Yoon, Y.N.; Son, D.I.; Seok, S.H. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef]
- Luo, J.Z.; Luo, L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured beta cells. Evid. Based Complement. Altern. Med. Ecam 2006, 3, 365–372. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, I.H.; Lee, M.J.; Thach Nguyen, C.; Ha, J.A.; Lee, S.C.; Choi, S.; Choi, K.T.; Pyo, S.; Rhee, D.K. Anti-oxidative stress effect of red ginseng in the brain is mediated by peptidyl arginine deiminase type IV (PADI4) repression via estrogen receptor (ER) beta up-regulation. J. Ethnopharmacol. 2013, 148, 474–485. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Magd, M.A.; El-Sayed, R.A. Panax ginseng modulates oxidative stress, DNA damage, apoptosis, and inflammations induced by silicon dioxide nanoparticles in rats. Environ. Toxicol. 2021, 36, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Fan, C.; Shen, Y.; Wang, Q.; Hu, H.; Xiang, M. The Antioxidative Role of Autophagy in Hearing Loss. Front. Neurosci. 2018, 12, 1010. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Guo, L.; Shu, Y.; Fang, Q.; Zhou, H.; Liu, Y.; Liu, D.; Lu, L.; Zhang, X.; Ding, X.; et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy 2017, 13, 1884–1904. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cao, W.; Niu, Y.; He, S.; Chai, R.; Yang, J. Autophagy Regulates the Survival of Hair Cells and Spiral Ganglion Neurons in Cases of Noise, Ototoxic Drug, and Age-Induced Sensorineural Hearing Loss. Front. Cell. Neurosci. 2021, 15, 760422. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Hill, K.; Chen, J.; Lemasters, J.; Yang, S.M.; Sha, S.H. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid. Redox Signal. 2015, 22, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Gul, F.; Muderris, T.; Yalciner, G.; Sevil, E.; Bercin, S.; Ergin, M.; Babademez, M.A.; Kiris, M. A comprehensive study of oxidative stress in sudden hearing loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 1301–1308. [Google Scholar] [CrossRef]
- Capaccio, P.; Pignataro, L.; Gaini, L.M.; Sigismund, P.E.; Novembrino, C.; De Giuseppe, R.; Uva, V.; Tripodi, A.; Bamonti, F. Unbalanced oxidative status in idiopathic sudden sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2012, 269, 449–453. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Chen, S.; Zhou, X.; Wu, X.; Kong, W.; Kong, W. Impaired unfolded protein response in the degeneration of cochlea cells in a mouse model of age-related hearing loss. Exp. Gerontol. 2015, 70, 61–70. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Kitamura, M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am. J. Physiology. Ren. Physiol. 2008, 295, F323–F334. [Google Scholar] [CrossRef]
- Back, S.H.; Kaufman, R.J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 2012, 81, 767–793. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.S.; Lee, D.H.; Lee, S.M.; Kim, M.Y.; Choi, B.Y.; Kim, S.Y. Tauroursodeoxycholic acid attenuates cisplatin-induced hearing loss in rats. Neurosci. Lett. 2020, 722, 134838. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Liu, T.; Wan, F.; Chen, P.; Luo, P.; Xiao, H. Endoplasmic Reticulum Stress Is Involved in Cochlear Cell Apoptosis in a Cisplatin-Induced Ototoxicity Rat Model. Audiol. Neurootol. 2017, 22, 160–168. [Google Scholar] [CrossRef]
- Wen, Y.; Zong, S.; Liu, T.; Du, P.; Li, H.; Xiao, H. Tauroursodeoxycholic acid attenuates cisplatin-induced ototoxicity by inhibiting the accumulation and aggregation of unfolded or misfolded proteins in the endoplasmic reticulum. Toxicology 2021, 453, 152736. [Google Scholar] [CrossRef]
- Jia, Z.; He, Q.; Shan, C.; Li, F. Tauroursodeoxycholic acid attenuates gentamicin-induced cochlear hair cell death in vitro. Toxicol. Lett. 2018, 294, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Herranen, A.; Ikaheimo, K.; Lankinen, T.; Pakarinen, E.; Fritzsch, B.; Saarma, M.; Lindahl, M.; Pirvola, U. Deficiency of the ER-stress-regulator MANF triggers progressive outer hair cell death and hearing loss. Cell Death Dis. 2020, 11, 100. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Luo, Y.; Wang, T.; Li, X.; Li, A.; Li, J.; Liu, K.; Liu, B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J. Ginseng Res. 2016, 40, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Bai, X.; Xiao, L.; Shangguan, J.; Zhang, W.; Zhang, X.; Wang, S.; Liu, G. Inhibition of Autophagy Prevents Panax Notoginseng Saponins (PNS) Protection on Cardiac Myocytes Against Endoplasmic Reticulum (ER) Stress-Induced Mitochondrial Injury, Ca(2+) Homeostasis and Associated Apoptosis. Front. Pharmacol. 2021, 12, 620812. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, H.J.; Kim, J.W.; Hong, S.H.; Kim, J.A.; Lee, Y.B.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Ginsenoside Mc1 improves liver steatosis and insulin resistance by attenuating ER stress. J. Ethnopharmacol. 2020, 259, 112927. [Google Scholar] [CrossRef]
- An, M.Y.; Lee, S.R.; Hwang, H.J.; Yoon, J.G.; Lee, H.J.; Cho, J.A. Antioxidant and Anti-Inflammatory Effects of Korean Black Ginseng Extract through ER Stress Pathway. Antioxidants 2021, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Clough, R.L.; Sud, R.; Davis-Silberman, N.; Hertzano, R.; Avraham, K.B.; Holley, M.; Dawson, S.J. Brn-3c (POU4F3) regulates BDNF and NT-3 promoter activity. Biochem. Biophys. Res. Commun. 2004, 324, 372–381. [Google Scholar] [CrossRef]
- Urso, C.J.; Zhou, H. Palmitic Acid Lipotoxicity in Microglia Cells Is Ameliorated by Unsaturated Fatty Acids. Int. J. Mol. Sci. 2021, 22, 9093. [Google Scholar] [CrossRef]
- Ito, Y.; Hsu, M.F.; Bettaieb, A.; Koike, S.; Mello, A.; Calvo-Rubio, M.; Villalba, J.M.; Haj, F.G. Protein tyrosine phosphatase 1B deficiency in podocytes mitigates hyperglycemia-induced renal injury. Metab. Clin. Exp. 2017, 76, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Chahed, S.; Tabet, G.; Yang, J.; Morisseau, C.; Griffey, S.; Hammock, B.D.; Haj, F.G. Effects of soluble epoxide hydrolase deficiency on acute pancreatitis in mice. PLoS ONE 2014, 9, e113019. [Google Scholar] [CrossRef]
- Yang, C.S.; Lee, H.M.; Lee, J.Y.; Kim, J.A.; Lee, S.J.; Shin, D.M.; Lee, Y.H.; Lee, D.S.; El-Benna, J.; Jo, E.K. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J. Neuroinflammation 2007, 4, 27. [Google Scholar] [CrossRef]
- Souslova, T.; Averill-Bates, D.A. Multidrug-resistant hela cells overexpressing MRP1 exhibit sensitivity to cell killing by hyperthermia: Interactions with etoposide. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1538–1551. [Google Scholar] [CrossRef]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaew, S. Potential of Coffee Fruit Extract and Quinic Acid on Adipogenesis and Lipolysis in 3T3-L1 Adipocytes. Kobe J. Med. Sci. 2018, 64, E84–E92. [Google Scholar]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a mild temperature of 40 degrees C protects cells against heat shock-induced apoptosis. J. Cell. Physiol. 2005, 205, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yu, J.H.; Wu, J.N.; Tashiro, S.; Onodera, S.; Minami, M.; Ikejima, T. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol. Sin. 2007, 28, 1057–1066. [Google Scholar] [CrossRef]
| Antibody | Source | Catalog Number | Observed MW (kDa) | Host | Dilution |
|---|---|---|---|---|---|
| Annexin IV | Santa Cruz Biotechnology | sc-46693 | 35 | Mouse | 1:500 |
| CHOP | Santa Cruz Biotechnology | sc-7351 | 31 | Mouse | 1:5000 |
| Cleaved Caspase-3 | Cell Signaling Technology | 9662 | 17 | Rabbit | 1:5000 |
| Espin | Santa Cruz Biotechnology | sc-393469 | 27 | Mouse | 1:500 |
| Hsc70 | Santa Cruz Biotechnology | sc-7298 | 70 | Mouse | 1:1000 |
| IKKα | Cell Signaling Technology | 2682 | 87 | Rabbit | 1:1000 |
| IRE1α | Cell Signaling Technology | 3294 | 115 | Rabbit | 1:1000 |
| IκBα | Cell Signaling Technology | 4814 | 40 | Mouse | 1:1000 |
| JNK1/2 | Santa Cruz Biotechnology | sc-7345 | 46/54 | Mouse | 1:1000 |
| Myosin VIIa | Santa Cruz Biotechnology | sc-74516 | 200 | Mouse | 1:1000 |
| NF-κBp65 | Cell Signaling Technology | 8242 | 65 | Rabbit | 1:1000 |
| p38 | Santa Cruz Biotechnology | sc-7972 | 42 | Mouse | 1:1000 |
| PERK | Cell Signaling Technology | 3192 | 140 | Rabbit | 1:1000 |
| Phosoho-IκBαS32 | Cell Signaling Technology | 2852 | 40 | Rabbit | 1:1000 |
| Phospho-IRE1αS724 | Abcam | ab 48187 | 115 | Rabbit | 1:10,000 |
| Phospho-JNK1/2T183/Y185 | Santa Cruz Biotechnology | sc-6254 | 46/54 | Mouse | 1:1000 |
| Phospho-P38T180/Y182 | Cell Signaling Technology | 4511 | 43 | Mouse | 1:10,000 |
| Phospho-PERKT980 | Santa Cruz Biotechnology | sc-32577 | 160 | Rabbit | 1:1000 |
| Phospho-PKM2S37 | ThermoFisher | PA5-37684 | 61 | Rabbit | 1:500 |
| Phospho-IKKαS176/S180 | Cell Signaling Technology | 2697 | 87 | Rabbit | 1:1000 |
| Phospho-NF-κBp65S536 | Cell Signaling Technology | 3033 | 65 | Rabbit | 1:1000 |
| Sox2 | Santa Cruz Biotechnology | Sc-365823 | 35 | Mouse | 1:500 |
| Vimentin | Santa Cruz Biotechnology | Sc-6260 | 58 | Mouse | 1:500 |
| β-Actin | Santa Cruz Biotechnology | sc-47778 | 44 | Mouse | 1:20,000 |
| Gene | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|
| Hsc70 | GAAGGTGCTGGACAAGTGC | GCCAGCAGAGGCCTCTAATC |
| Myo7a | CTCAAGCTGCTCAGCAATCTATTT | GGAGCGCAAGTTTGTCATAAGT |
| Tbp | TTGGCTAGGTTTCTGCGGTC | GCCCTGAGCATAAGGTGGAA |
| Vim | CGGCTGCGAGAGAAATTGC | CCACTTTCCGTTCAAGGTCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, N.B.; Dowker-Key, P.D.; Hubbard, K.; Voy, B.H.; Whelan, J.; Hedrick, M.; Bettaieb, A. Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury. Int. J. Mol. Sci. 2023, 24, 7345. https://doi.org/10.3390/ijms24087345
Gill NB, Dowker-Key PD, Hubbard K, Voy BH, Whelan J, Hedrick M, Bettaieb A. Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury. International Journal of Molecular Sciences. 2023; 24(8):7345. https://doi.org/10.3390/ijms24087345
Chicago/Turabian StyleGill, Nicholas B., Presley D. Dowker-Key, Katelin Hubbard, Brynn H. Voy, Jay Whelan, Mark Hedrick, and Ahmed Bettaieb. 2023. "Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury" International Journal of Molecular Sciences 24, no. 8: 7345. https://doi.org/10.3390/ijms24087345
APA StyleGill, N. B., Dowker-Key, P. D., Hubbard, K., Voy, B. H., Whelan, J., Hedrick, M., & Bettaieb, A. (2023). Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury. International Journal of Molecular Sciences, 24(8), 7345. https://doi.org/10.3390/ijms24087345







