Influence of the Structure on Magnetic Properties of Calcium-Phosphate Systems Doped with Iron and Vanadium Ions
Abstract
1. Introduction
2. Results and Discussion
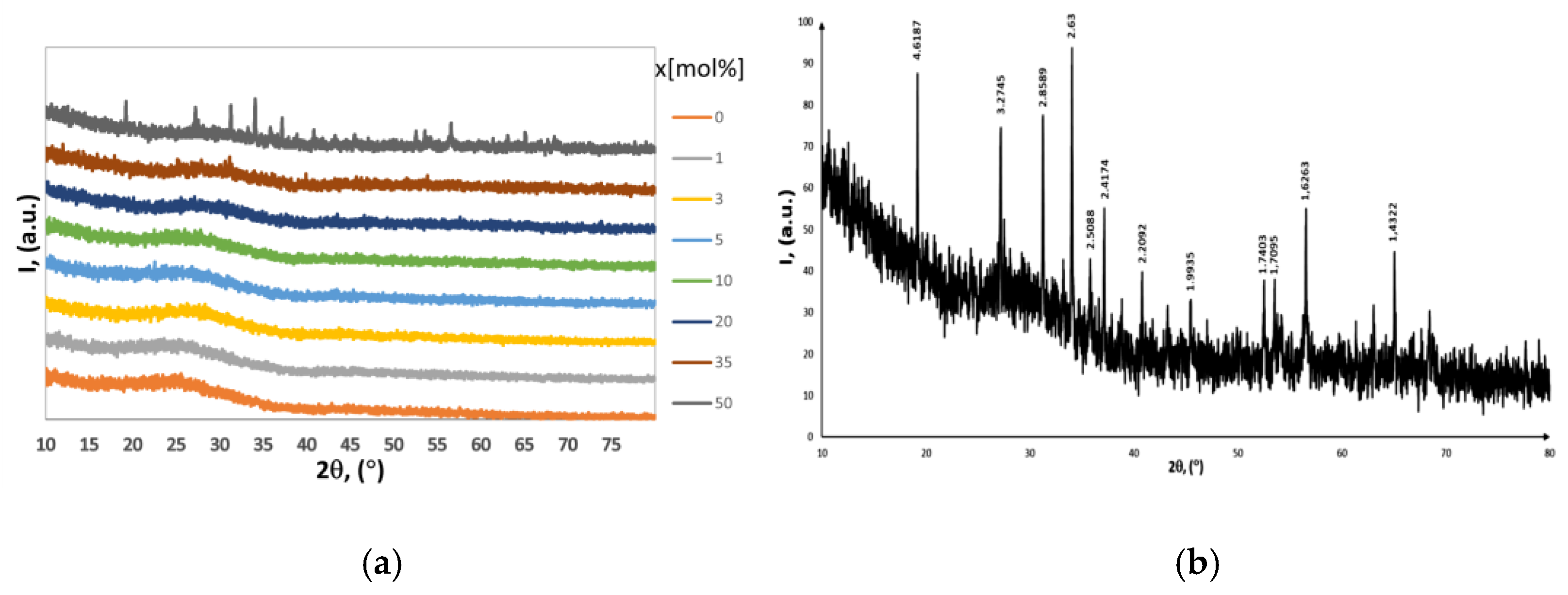
2.1. XRD Data
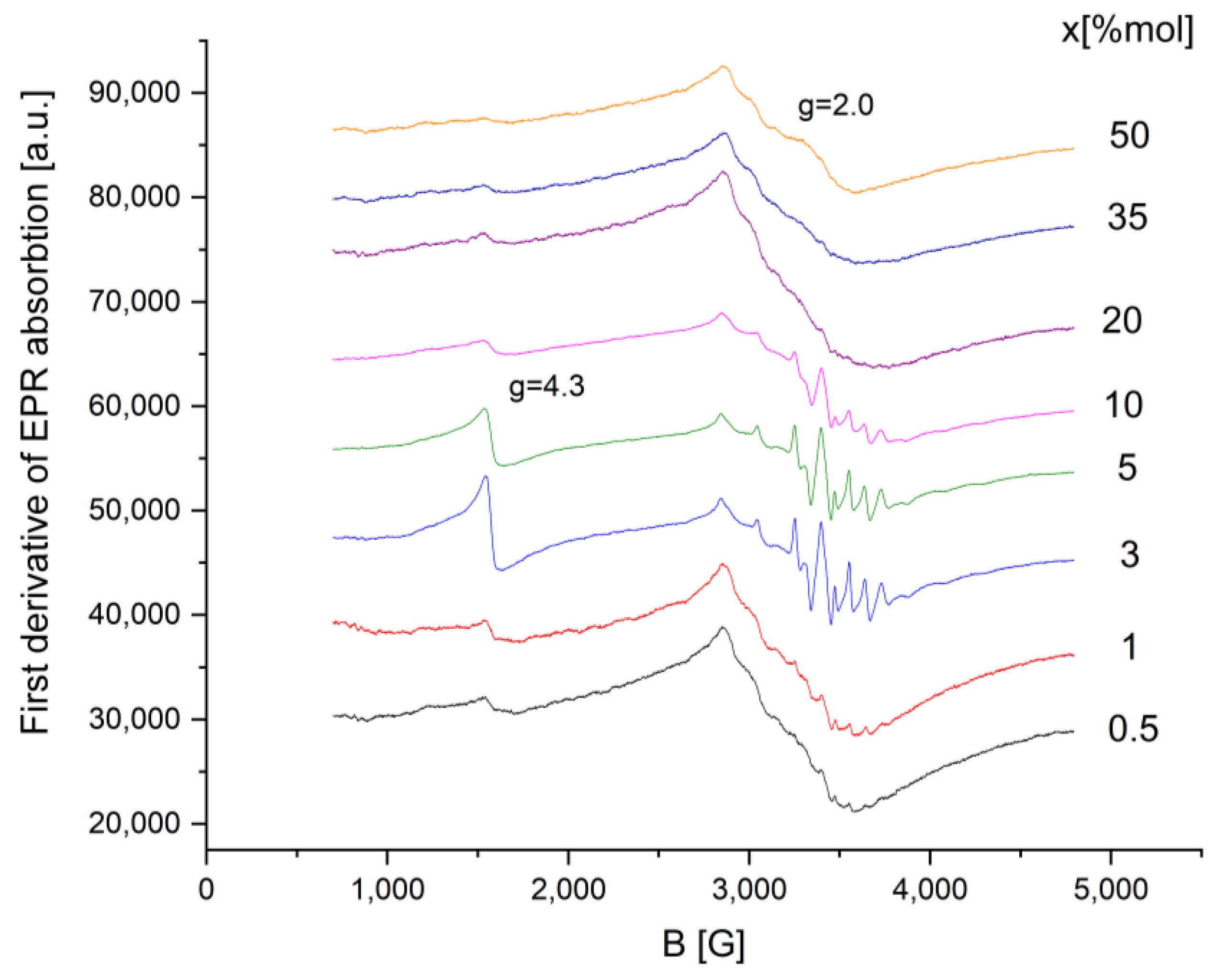
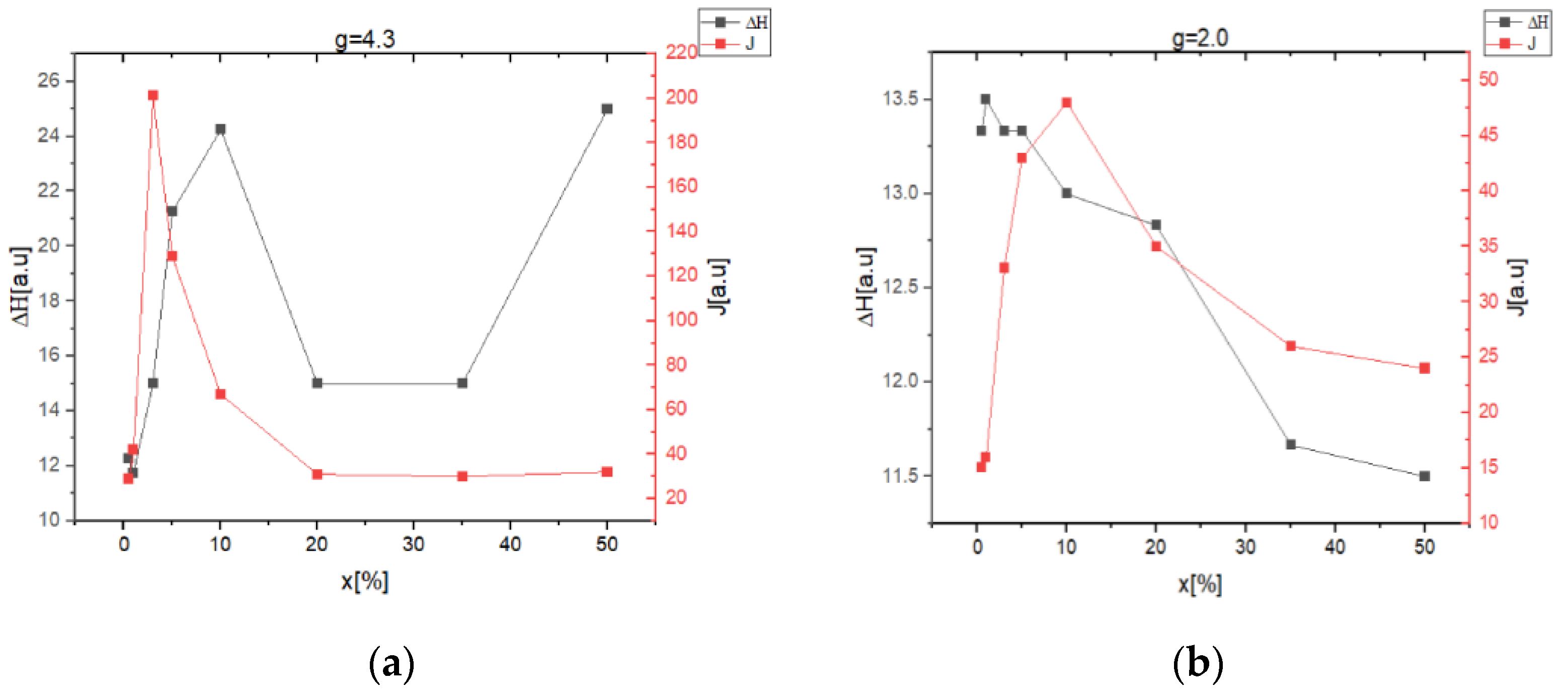
2.2. EPR Data
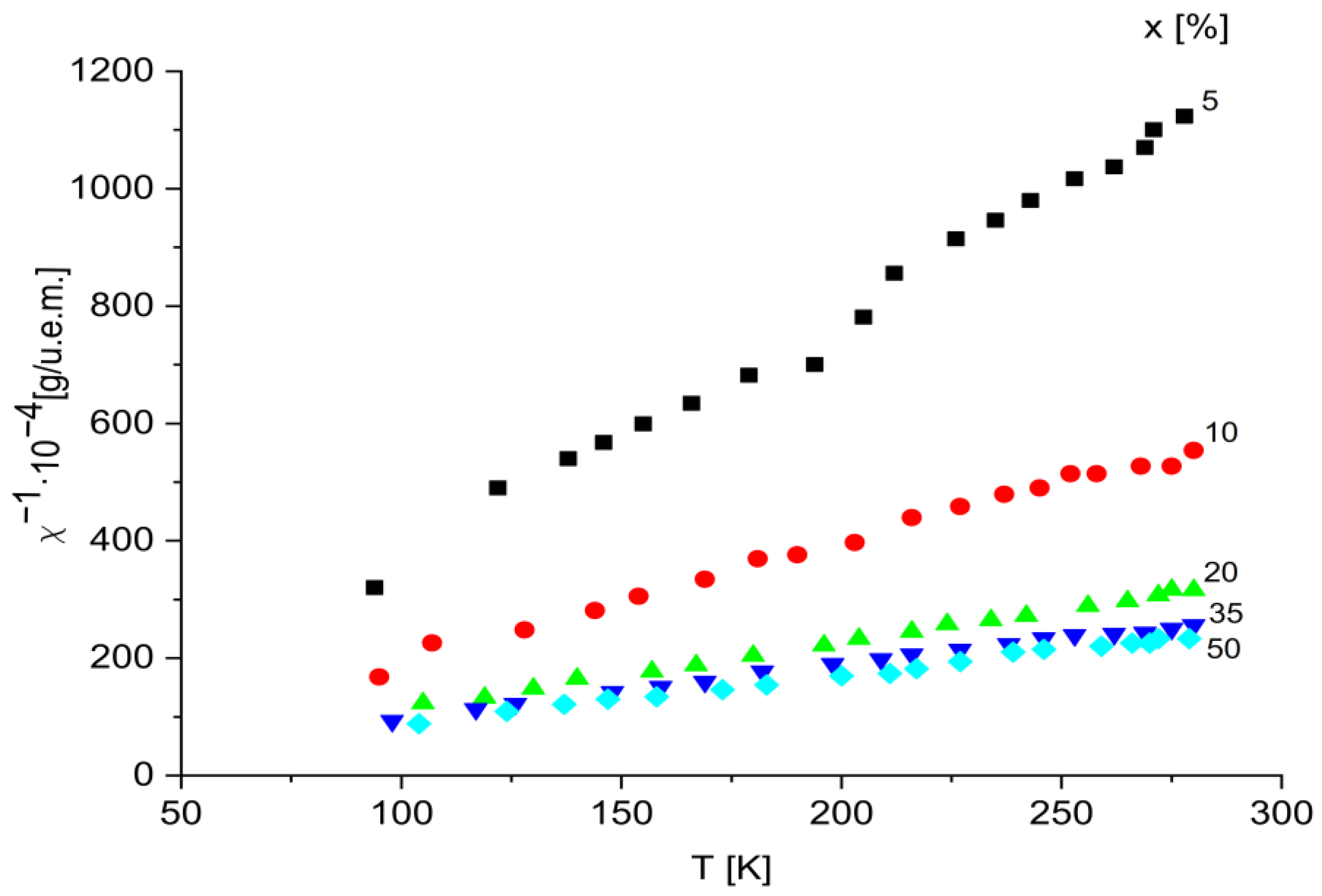
2.3. Magnetic Susceptibility Data
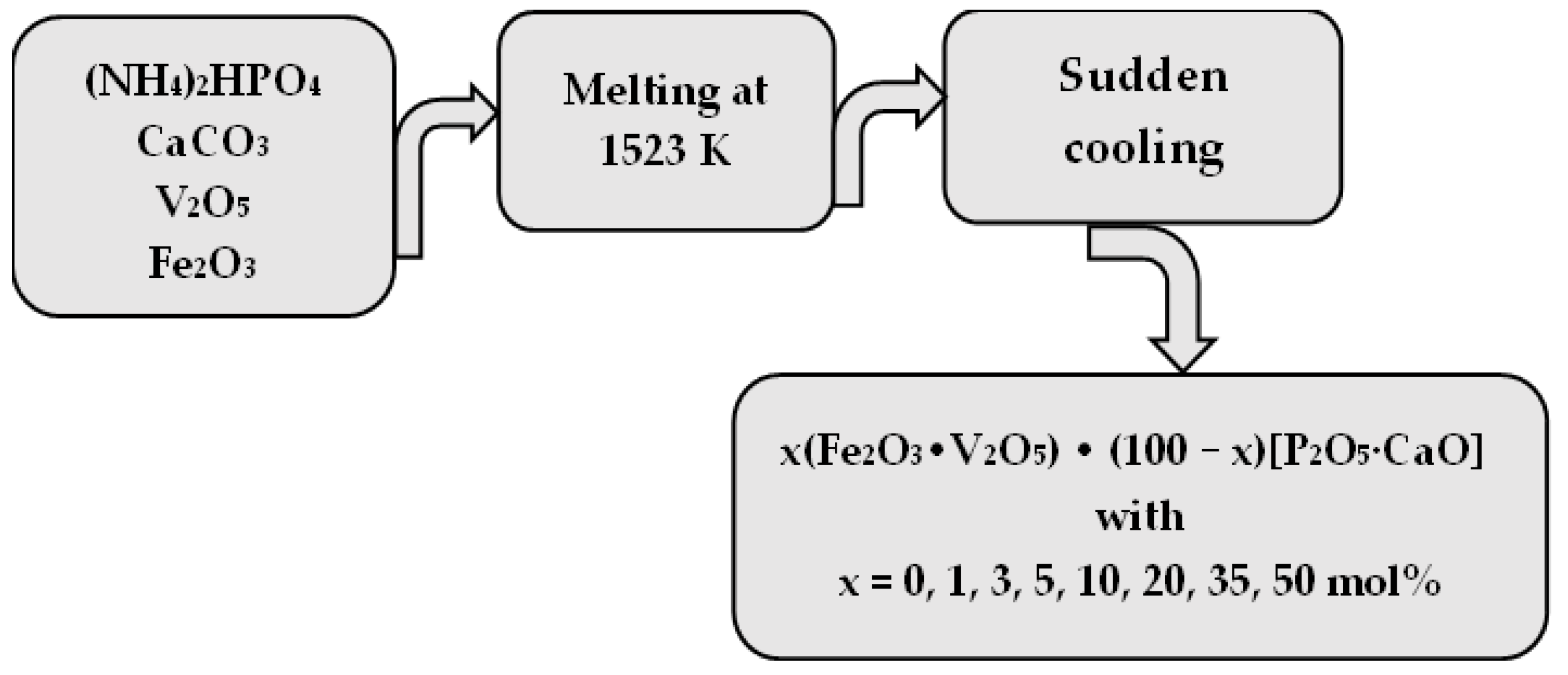
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorzkowska, I.; Jozwiak, P.; Garbarczyk, J.E.; Wasiucionek, M.; Julien, C.M. Studies on glass transition of lithium-iron phosphate glasses. J. Therm. Anal. Calorim. 2008, 93, 759–762. [Google Scholar] [CrossRef]
- Aqdim, S.; Ouchetto, M. Elaboration and Structural Investigation of Iron (III) Phosphate Glasses. Adv. Mater. Phys. Chem. 2013, 3, 332. [Google Scholar] [CrossRef]
- Venkateswara, R.G.; Shashikala, H.D. Optical and mechanical properties of calcium phosphate glasses. Glass. Phys. Chem. 2014, 40, 303–309. [Google Scholar] [CrossRef]
- Singh, S.P.; Chakradhar, R.P.S.; Rao, J.L.; Karmakar, B. EPR optical absorption and photoluminescence properties of MnO2 doped 23B2O3–5ZnO–72Bi2O3 glasses. Phys. B 2010, 405, 2157. [Google Scholar] [CrossRef]
- Chung, W.J.; Choi, J.; Choi, Y.G. Compositional effect on structural and spectroscopic properties of P2O5–SnO–MnO ternary glass system. J. Alloys Compd. 2010, 505, 661. [Google Scholar] [CrossRef]
- Takahashi, H.; Karasawa, T.; Sakuma, T.; Garbarczyk, J.E. Electrical conduction in the vitreous and crystallized Li2O–V2O5–P2O5 system. Solid State Ion. 2010, 181, 27–32. [Google Scholar] [CrossRef]
- Bingham, P.A.; Hand, R.J.; Forder, S.D.; La Vaysierre, A. Vitrified metal finishing wastes: II. Thermal and structural characterization. J. Hazard. Mater. 2005, 122, 129. [Google Scholar] [CrossRef] [PubMed]
- Brow, R.K. Review: The Structure of Simple Phosphate Glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Ahmed, I.; Ren, H.; Booth, J. Developing Unique Geometries of Phosphate-Based Glasses and their Prospective Biomedical Applications. Johns. Matthey Technol. Rev. 2019, 63, 34. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Patel, U.; Kennedy, A.R.; Macri-Pellizzeri, L.; Sottile, V.; Grant, D.M.; Scammell, B.E.; Ahmed, I. Porous calcium phosphate glass microspheres for orthobiologic applications. Acta Biomater. 2018, 72, 396–406. [Google Scholar] [CrossRef]
- Sheng, L.; Hongting, L.; Fengnian, W.; Ziyuan, C.; Yunlong, Y. Effects of alkaline-earth metal oxides on structure and properties of iron phosphate glasses. J. Non-Cryst. Solids 2016, 434, 108–114. [Google Scholar]
- Bingham, P.A.; Hand, R.; Hannant, O.M.; Forder, S.; Kilcoyne, S. Effects of modifier additions on the thermal properties, chemical durability, oxidation state and structure of iron phosphate glasses. J. Non-Cryst. Solids 2009, 355, 1526–1538. [Google Scholar] [CrossRef]
- Ahmed, I.; Collins, C.A.; Lewis, M.P.; Olsen, I.; Knowles, J.C. Processing, characterization and biocompatibility of iron-phosphate glass fibres for tissue engineering. Biomaterials 2004, 25, 3223. [Google Scholar] [CrossRef] [PubMed]
- Vedeanu, N.S.; Lujerdean, C.; Zăhan, M.; Dezmirean, D.S.; Barbu-Tudoran, L.; Damian, G.; Stefan, R. Synthesis and Structural Characterization of CaO-P2O5-CaF:CuO Glasses with Antitumoral Effect on Skin Cancer Cells. Materials 2022, 15, 1526. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.P.; Roy, M.; Mishra, R.K.; Meena, S.S.; Yadav, A.; Kaushik, C.P.; Tyagi, A.K. Structural investigations on Mo, Cs and Ba ions-loaded iron phosphate glass for nuclear waste storage application. J. Alloys Compd. 2021, 850, 156715. [Google Scholar] [CrossRef]
- Hoppe, A.; Nusret, S.G.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Omidian, S.; Nazarpak, M.H.; Bagher, Z.; Moztardeh, F. The effect of vanadium ferrite doping on the bioactivity of mesoporous bioactive glass-ceramics. RSC Adv. 2022, 12, 25639–25653. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioactive Glass: Chronology, Characterization, and Genetic Control of Tissue Regeneration. In Advances in Calcium Phosphate Biomaterials; Springer Series in Biomaterials Science and Engineering; Ben-Nissan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 51–70. [Google Scholar]
- Ardelean, I.; Rusu, D.; Andronache, C.; Ciobotă, V. Raman study of xMeO·(100 − x)[P2O5·Li2O] (MeO⇒Fe2O3 or V2O5) glass systems. Mater. Lett. 2007, 61, 3301–3304. [Google Scholar] [CrossRef]
- Andronache, C. The local structure and magnetic interactions between Fe3+ and V4+ ions in lithium–phosphate glasses. Mater. Chem. Phys. 2012, 136, 281–285. [Google Scholar] [CrossRef]
- Andronache, C.; Balasoiu, M.; Orelovich, A.; Roghacev, V.; Mihaly-Cozmuta, L.; Balasoiu-Gaina, A.M.; Racolta, D. On the structure of Lithium-Phosphate glasses doped with iron and vanadium ions. J. Optoelectron. Adv. Mater. 2019, 21, 726–732. [Google Scholar]
- Ardelean, I.; Andronache, C.; Cimpean, C.; Pascuta, P. EPR and magnetic investigation of calcium—Phosphate glasses containing iron ions. J. Optoelectron. Adv. Mater. 2006, 8, 1372. [Google Scholar]
- Vedeanu, N.S.; Magdas, D.A. The influence of some transition metal ions in lead-and calcium-phosphate glasses. J. Alloys Compd. 2012, 534, 93–96. [Google Scholar] [CrossRef]
- Andronache, C.; Balasoiu, M.; Racolta, D. Magnetic Interaction between Iron Particles in Lithium Phosphate Systems. Russ. J. Phys. Chem. 2017, 91, 2686–2689. [Google Scholar] [CrossRef]
- Stoch, P.; Stoch, A.; Ciecinska, M.; Krakowiak, I.; Sitarz, M. Structure of phosphate and iron-phosphate glasses by DFT calculations and FTIR/Raman spectroscopy. J. Non-Cryst. Solids 2016, 450, 48–60. [Google Scholar] [CrossRef]
- Shapaan, M.; Shabaan, E.R.; Mostafa, A.G. Study of the hyperfine structure, thermal stability and electric–dielectric properties of vanadium iron phosphate glasses. Phys. B Condens. Matter 2009, 404, 2058–2064. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1970. [Google Scholar]
- Chavan, V.K.; Rupa, B.; Rupa, V.R.; Sreenivasulu, M. Transition metal doped phosphate glass. Int. J. Innov. Eng. Res. Manag. 2018, 5, 1–7. [Google Scholar]
- Andronache, C.; Balasoiu, M.; Racolta, D. Structural properties of different phosphate glasses by EPR analysis. AIP Conf. Proc. 2019, 2071, 030004. [Google Scholar]
- Agarwal, A.; Khasa, S.; Seth, V.P.; Arora, M. Study of EPR, optical properties and dc conductivity of VO2+ ion dopedTiO2R2 OB2 O3 (R = Li and K) glasses. J. Alloys Compd. 2013, 568, 112–117. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Ivantsov, R.D.; Edelman, I.S.; Petrakovskaja, E.A.; Velikanov, D.A.; Zubavichus, Y.V.; Zaikovskii, V.I.; Stepanov, S.A. Identification of ε-Fe2O3 nano-phase in borate glasses doped with Fe and Gd. J. Magn. Magn. Mater. 2016, 401, 880–889. [Google Scholar] [CrossRef]
- Akamatsu, H.; Oku, S.; Fujita, K.; Murai, S.; Tanaka, K. Magnetic properties of mixed-valence iron phosphate glasses. Phys. Rev. B 2009, 80, 134408. [Google Scholar] [CrossRef]
- Akamatsu, H.; Oku, S.; Fujita, K.; Murai, S.; Tanaka, K. Magnetic properties and photoluminescence of thulium-doped calcium aluminosilicate glasses. Opt. Mater. Express 2019, 9, 4348–4359. [Google Scholar]
- Khattak, G.D.; Mekki, A.; Wenger, L.E. X-ray photoelectron spectroscopy (XPS) and magnetic susceptibility studies of vanadium phosphate glasses. J. Non-Cryst. Solids 2000, 262, 66–79. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate-based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- Andronache, C.; Racolta, D.; Ardelean, G. Magnetic properties of x(Fe2O3)·(100 − x)[P2O5·Li2O] and x(Fe2O3)·(100 − x)[P2O5·CaO] glass systems. AIP Conf. Proc. 2017, 1916, 030004. [Google Scholar]
- Pascuta, P.; Borodi, G.; Popa, A.; Dan, V.; Culea, E. Influence of iron ions on the structural and magnetic properties of some zinc-phosphate glasses. Mater. Chem. Phys. 2010, 123, 767–771. [Google Scholar] [CrossRef]
- Winterstein, A.; Akamatsu, H.; Möncke, D.; Tanaka, K.; Schmidt, M.A.; Wondraczek, L. Magnetic and magneto-optical quenching in (Mn2+, Sr2+) metaphosphate glasses. Opt. Mater. Express 2013, 3, 184–193. [Google Scholar] [CrossRef]
| x [%] | CM [u.e.m./mol] | μeff [μB] |
|---|---|---|
| 5 | 0.73 | 5.40 |
| 10 20 35 | 1.41 2.54 3.10 | 5.3 5.03 4.20 |
| 50 | 3.38 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racolta, D.; Andronache, C.; Balasoiu, M.; Mihaly-Cozmuta, L.; Sikolenko, V.; Orelovich, O.; Rogachev, A.; Borodi, G.; Iepure, G. Influence of the Structure on Magnetic Properties of Calcium-Phosphate Systems Doped with Iron and Vanadium Ions. Int. J. Mol. Sci. 2023, 24, 7366. https://doi.org/10.3390/ijms24087366
Racolta D, Andronache C, Balasoiu M, Mihaly-Cozmuta L, Sikolenko V, Orelovich O, Rogachev A, Borodi G, Iepure G. Influence of the Structure on Magnetic Properties of Calcium-Phosphate Systems Doped with Iron and Vanadium Ions. International Journal of Molecular Sciences. 2023; 24(8):7366. https://doi.org/10.3390/ijms24087366
Chicago/Turabian StyleRacolta, Dania, Constantin Andronache, Maria Balasoiu, Leonard Mihaly-Cozmuta, Vadim Sikolenko, Oleg Orelovich, Andrey Rogachev, Gheorghe Borodi, and Gheorghe Iepure. 2023. "Influence of the Structure on Magnetic Properties of Calcium-Phosphate Systems Doped with Iron and Vanadium Ions" International Journal of Molecular Sciences 24, no. 8: 7366. https://doi.org/10.3390/ijms24087366
APA StyleRacolta, D., Andronache, C., Balasoiu, M., Mihaly-Cozmuta, L., Sikolenko, V., Orelovich, O., Rogachev, A., Borodi, G., & Iepure, G. (2023). Influence of the Structure on Magnetic Properties of Calcium-Phosphate Systems Doped with Iron and Vanadium Ions. International Journal of Molecular Sciences, 24(8), 7366. https://doi.org/10.3390/ijms24087366






