Characterization and In Vivo Assay of Allantoin-Enriched Pectin Hydrogel for the Treatment of Skin Wounds
Abstract
1. Introduction
2. Results
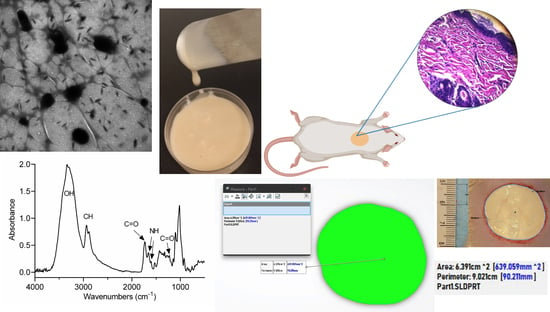
2.1. Pectin Hydrogel Enriched with Allantoin
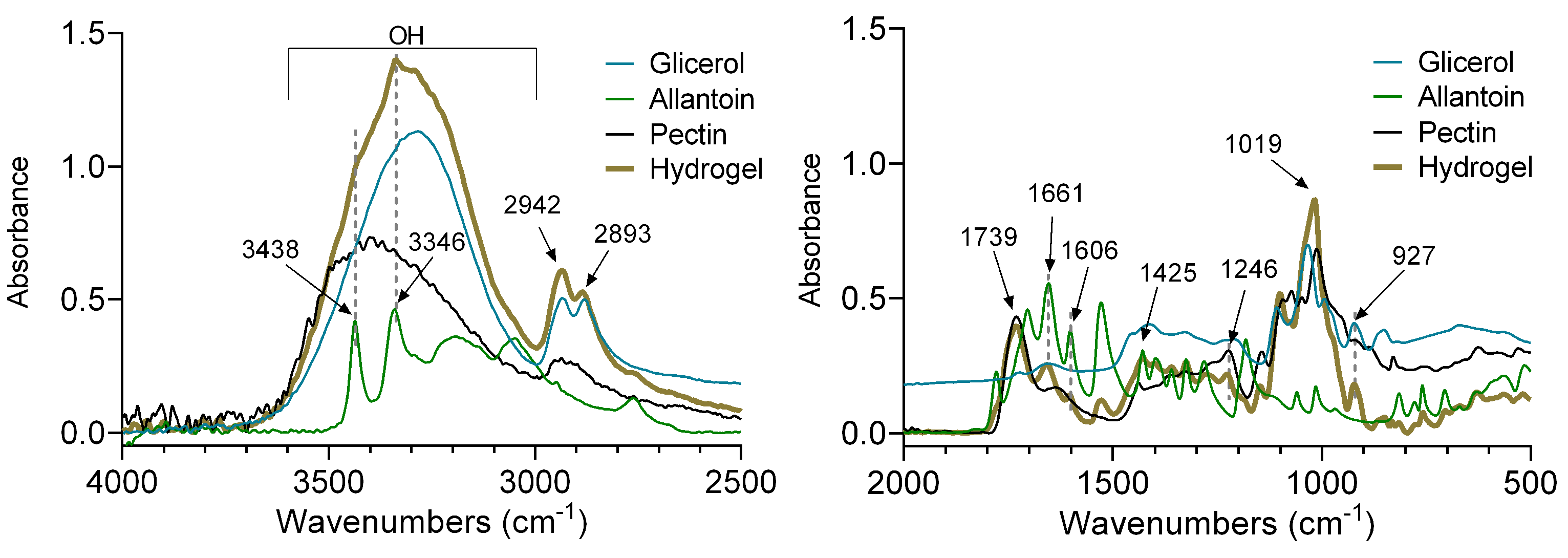
2.2. FT-IR Analysis
2.3. Contact Angle
2.4. Surface Analysis
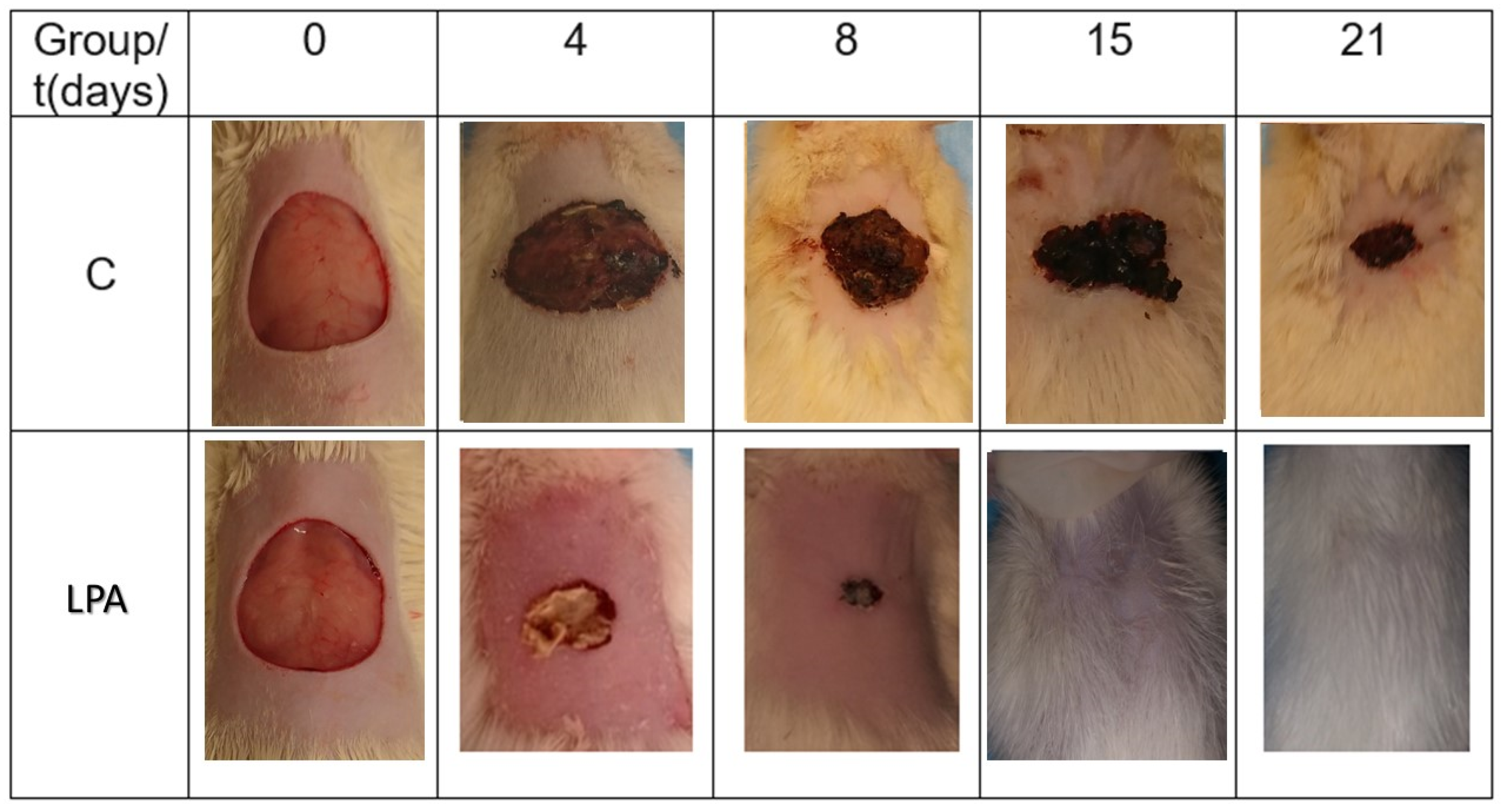
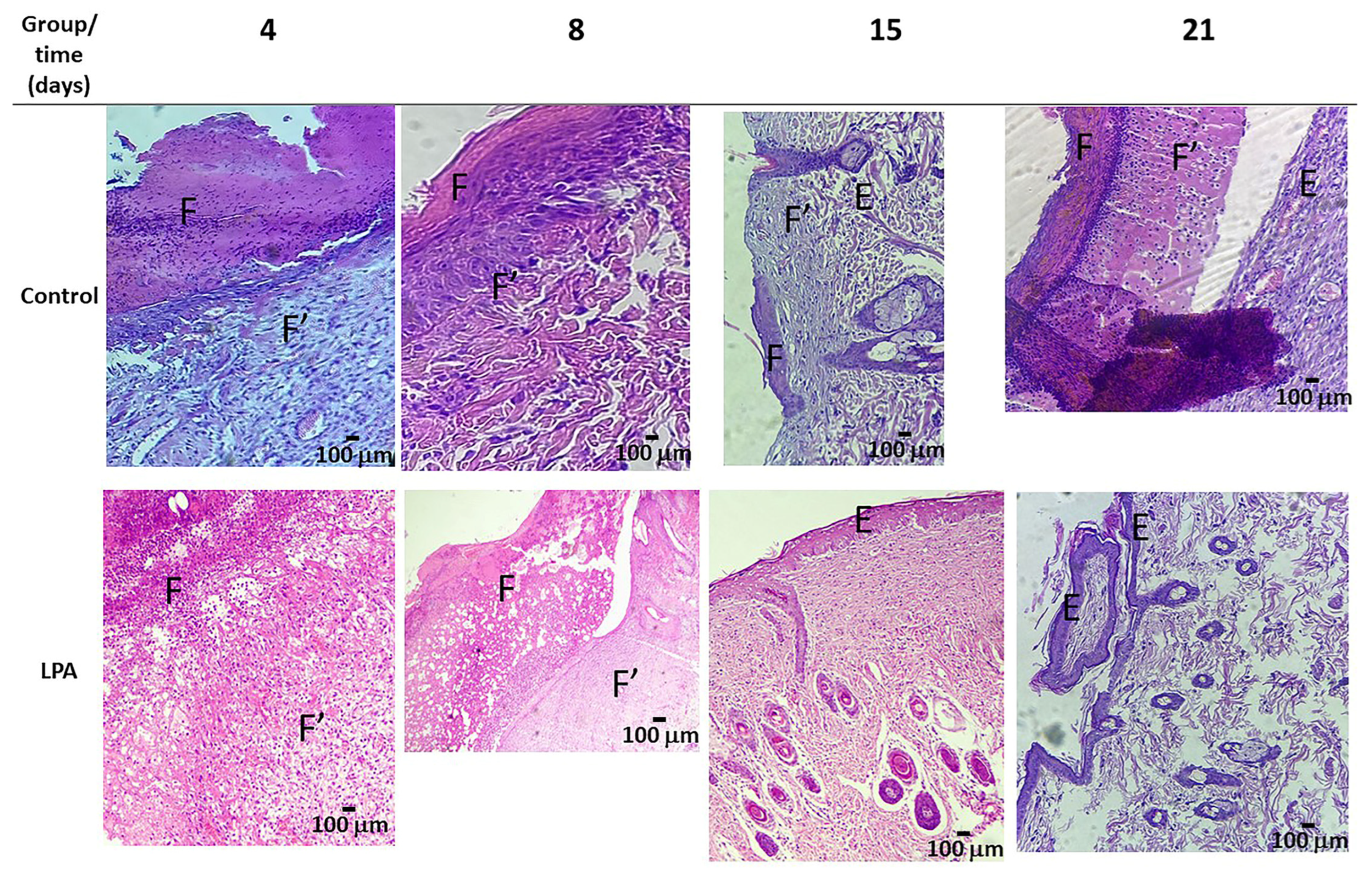
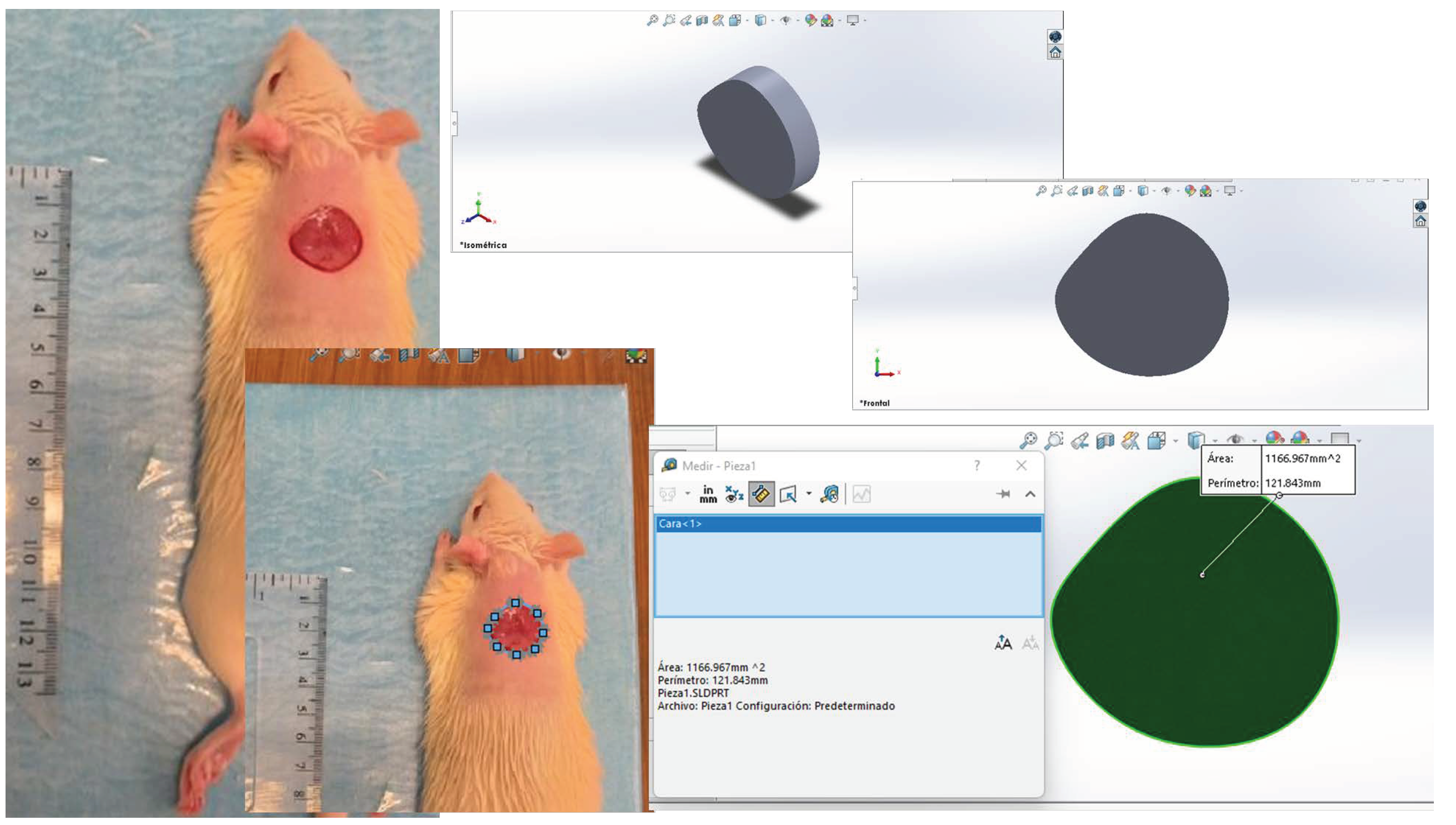
2.5. Healing Process
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of Pectin Hydrogel Enriched with Allantoin
4.3. Characterization of the Hydrogel
4.4. In Vivo Assay
4.5. Surgical Procedure
4.6. Macroscopic Analysis
4.7. Morphology Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Nanditha, C.K.; Vinod Kumar, G.S. Bioactive peptides laden nano and micro-sized particles enriched ECM inspired dressing for skin regeneration in diabetic wounds. Mater. Today Bio. 2022, 14, 100235. [Google Scholar] [CrossRef]
- Meza Valle, K.Z.; Saucedo Acuña, R.A.; Ríos Arana, J.V.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural Film Based on Pectin and Allantoin for Wound Healing: Obtaining, Characterization, and Rat Model. BioMed Res. Int. 2020, 2020, 6897497. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Noreen, A.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical aplications: A review. Int. J. Biol. Macromol. 2017, 101, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef] [PubMed]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Minocha, N. Natural polymers; their applications in food, cosmetic and pharmaceutical industries. Int. J. Adv. Res. 2020, 8, 1224–1238. [Google Scholar] [CrossRef]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Coma, V. Polysaccharide-based Biomaterials with Antimicrobial and Antioxidant Properties. Polímeros 2013, 23, 287–297. [Google Scholar] [CrossRef]
- Trupti, S.; Bangde, P.; Dandekar, P.; Adivarekar, R. Herbal hemostatic biopolymeric dressings of alginate/pectin coated with Croton oblongifolius extract. Carbohydr. Polym. Technol. Appl. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef]
- Han, Z.; Dong, L.; Li, A.; Li, Z.; Fu, L.; Zhang, Z.; Li, X.; Li, X. Efficient angiogenesis-based wound healing through hydrogel dressing with extracellular vesicles release. Mater. Today Bio. 2022, 16, 100427. [Google Scholar] [CrossRef]
- Marras-Marquez, T.; Peña, J.; Veiga-Ochoa, M.D. Robust and versatile pectin-based drug delivery systems. Int. J. Biol. Macromol. 2015, 479, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Busato, B.; de Almeida Abreu, E.C.; de Oliveira Petkowicz, C.L.; Martinez, G.R.; Noleto, G.R. Pectin from Brassica oleracea var. italica triggers immunomodulating effects in vivo. Int. J. Biol. Macromol. 2020, 161, 431–440. [Google Scholar] [CrossRef]
- Agarwal, T.; Costantini, M.; Kumar Maiti, T. Extrusion 3D printing with Pectin-based ink formulations: Recent trends in tissue engineering and food manufacturing. Biomed. Eng. Adv. 2021, 2, 100018. [Google Scholar] [CrossRef]
- Morello, G.; Quarta, A.; Gaballo, A.; Moroni, L.; Gigli, G.; Polini, A.; Gervaso, F. A thermo-sensitive chitosan/pectin hydrogel for long-term tumor spheroid culture. Carbohydr. Polym. 2021, 274, 118633. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy. Acta Biomater. 2021, 131, 149–161. [Google Scholar] [CrossRef]
- Ghorbania, M.; Roshangara, L.; Soleimani Rad, J. Development of reinforced chitosan/pectin scaffold by using the cellulose nanocrystals as nanofillers: An injectable hydrogel for tissue engineering. Eur. Polym. J. 2020, 130, 109697. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Inter. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef]
- Robert, B.; Chenthamara, D.; Subramaniam, S. Fabrication and biomedical applications of Arabinoxylan, Pectin, Chitosan, soy protein, and silk fibroin hydrogels via laccase—Ferulic acid redox chemistry. Inter. J. Biol. Macromol. 2022, 201, 539–556. [Google Scholar] [CrossRef]
- Goel, H.; Gupta, N.; Santhiya, D.; Dey, N.; Bohidar, H.B.; Bhattacharya, A. Bioactivity reinforced surface patch bound collagen-pectin hydrogel. Int. J. Biol. Macromol. 2021, 174, 240–253. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Alsakhawy, M.A.; Abdelmonsif, D.A.; Haroun, M.; Sabra, S.A. Naringin-loaded Arabic gum/pectin hydrogel as a potential wound healing material. Inter. J. Biol. Macromol. 2022, 222, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chang, R.; Liu, X.; Ma, X.; Chen, D.; Wang, Y.; Li, W.; Qin, J. Self-healing hydrogel based on polyphosphate-conjugated pectin with hemostatic property for wound healing applications. Biomater. Adv. 2022, 139, 212974. [Google Scholar] [CrossRef] [PubMed]
- Chetouani, A.; Elkolli, M.; Haffar, H.; Chader, H.; Riahi, F.; Varacavoudin, T.; Le Cerf, D. Multifunctional hydrogels based on oxidized pectin and gelatin for wound healing improvement. Int. J. Biol. Macromol. 2022, 212, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Mort, A. Structure of a Rhamnogalacturonan Fragment from Apple Pectin: Implications for Pectin Architecture. Int. J. Carbohydr. Chem. 2014, 2014, 347381. [Google Scholar] [CrossRef]
- Meza-Valle, K.Z.; Saucedo-Acuña, R.A.; Tovar-Carrillo, K.L.; Cuevas-González, J.C.; Zaragoza-Contreras, E.A.; Melgoza-Lozano, J. Characterization and Topical Study of Aloe Vera Hydrogel on Wound-Healing Process. Polymers 2021, 13, 3958. [Google Scholar] [CrossRef]
- Sari, M.; Tamrin; Kaban, J.; Alfian, Z. A novel composite membrane pectin from Cyclea Barbata Miers blend with chitosan for accelerated wound healing. Polym. Test. 2021, 99, 107207. [Google Scholar] [CrossRef]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio. 2022, 16, 100429. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Li, B.; Ji, Y.; Zeng, C. Multifunctional Coordination Polymer Nanoparticles Based on Allantoin: Single Peak Upconversion Emission, Drug Delivery and Cytotoxicity Study. J. Inorg. Organomet. Polym. 2016, 26, 527–535. [Google Scholar] [CrossRef]
- Menezes, J.E.S.A.; dos Santos, H.S.; Ferreira, M.K.A.; Magalhães, F.; da Silva, D.; Bandeira, P.; Saraiva, G.; Pessoa, O.; Ricardo, N.; Cruz, B.; et al. Preparation, structural and spectroscopic characterization of chitosan membranes containing allantoin. J. Mol. Struct. 2020, 1199, 126968. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V. Final Report of the Safety Assessment of Allantoin and Its Related Complexes. Int. J. Toxicol. 2010, 29 (Suppl. 2), 84S–97S. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.; Alam, M.; Gupta, B. Composite Wound Dressings of Pectin and Gelatin with Aloe vera and Curcumin as Bioactive Agents. Int. J. Biol. Macromol. 2015, 82, 104–113. [Google Scholar] [CrossRef]
- Florentino, I.F.; Silva, D.P.B.; Galdino, P.M.; Lino, R.C.; Martins, J.L.; Silva, D.M.; de Paula, J.R.; Tresvenzol, L.M.; Costa, E.A. Antinociceptive and anti-inflammatory effects of Memora nodosa and allantoin in mice. J. Ethnopharmacol. 2016, 186, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Alcaide, L.M.; Molinero-Mourelle, P.; González-Serrano, J.; Rubio-Alonso, L.; Bornstein, M.; López-Quiles, J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: A randomized and placebo-controlled clinical trial. Med. Oral Patol. Oral Cir. Bucal 2020, 5, e644–e651. [Google Scholar] [CrossRef] [PubMed]
- Moreira da Silvaa, D.; Rodrigues Martinsa, J.L.; Ramos de Oliveiraa, D.; Florentino, I.F.; da Silva, D.P.B.; dos Santos, F.C.A.; Costa, E.A. Effect of allantoin on experimentally induced gastric ulcers: Pathways of gastroprotection. Eur. J. Pharmacol. 2018, 82, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Delibas, B.; Kuruoglu, E.; Bereket, M.C.; Onger, M.E. Allantoin, a purine metabolite, enhances peripheral nerve regeneration following sciatic nerve injury in rats: A stereological and immunohistochemical study. J. Chem. Neuroanat. 2021, 117, 102002. [Google Scholar] [CrossRef]
- Xie, H.; Bai, Q.; Kong, F.; Li, J.; Zha, X.; Zhang, L.; Zhao, Y.; Gao, S.; Li, P.; Jiang, Q. Allantoin-functionalized silk fibroin/sodium alginate transparent scaffold for cutaneous wound healing. Int. J. Biol. Macromol. 2022, 207, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Svetlichny, G.; Külkamp-Guerreiro, I.; Lana, D.F.D.; Bianchin, M.; Pohlmann, A.; Fuentefria, A.; Guterres, S. Assessing the performance of copaiba oil and allantoin nanoparticles on multidrug-resistant Candida parapsilosis. J. Drug Deliv. Sci. Technol. 2017, 40, 59–65. [Google Scholar] [CrossRef]
- Ahmad, N. In Vitro and In Vivo Characterization Methods for Evaluation of Modern Wound Dressings. Pharmaceutics 2023, 15, 42. [Google Scholar] [CrossRef]
- Nokoorani, Y.D.; Shamloo, A.; Bahadoran, M.; Moravvej, H. Fabrication and characterization of scaffolds containing different amounts of allantoin for skin tissue engineering. Sci. Rep. 2011, 11, 16164. [Google Scholar] [CrossRef]
- Alam, M.J.; Ahmad, S. FTIR, FT-Raman, UV–Visible spectra and quantum chemical calculations of allantoin molecule and its hydrogen bonded dimers. Pectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 961–978. [Google Scholar] [CrossRef]
- Otálora, M.A.; Wilches-Torres, A.; Gómez Castaño, J.A. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Morrison, N.B. Química Orgánica, 5th ed.; Person Addison Wesley: Boston, MA, USA, 1999; p. 672. [Google Scholar]
- Al-Arjan, W.S.; Khan, M.U.A.; Nazir, S.; Razak, S.I.A.; Kadir, M.R.A. Development of Arabinoxylan-Reinforced Apple Pectin/Graphene Oxide/Nano-Hydroxyapatite Based Nanocomposite Scaffolds with Controlled Release of Drug for Bone Tissue Engineering: In-Vitro Evaluation of Biocompatibility and Cytotoxicity against MC3T3-E. Coatings 2020, 10, 1120. [Google Scholar] [CrossRef]
- Li, D.Q.; Wang, S.Y.; Meng, Y.J.; Guo, Z.W.; Cheng, M.M.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L.W. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Inutsuka, M.; Tanoue, H.; Yamada, N.L.; Ito, K.; Yokoyama, H. Dynamic contact angle on a reconstructive polymer surface by segregation. RSC Adv. 2017, 7, 17202–17207. [Google Scholar] [CrossRef]
- Charu Dwivedi, C.; Pandey, I.; Pandey, H. Chapter 9: Electrospun Nanofibrous Scaffold as a Potential Carrier of Antimicrobial Therapeutics for Diabetic Wound Healing and Tissue Regeneration. In Nano- and Microscale Drug Delivery Systems; Elsevier: New York, NY, USA, 2017; pp. 147–164. [Google Scholar] [CrossRef]
- Sierra-García, G.D.; Castro-Ríos, R.; González-Horta, A.; Lara-Arias, J.; Chávez-Montes, A. Acemannan, an extracted polysaccharide from Aloe vera: A literature review. Nat. Prod. Commun. 2014, 9, 1217–1221. [Google Scholar] [CrossRef]
- Pathalamuthu, P.; Siddharthan, A.; Giridev, V.R.; Victoria, V.; Thangam, R.; Sivasubramanian, S.; Savariar, V.; Hemamalini, T. Enhanced performance of Aloe vera incorporated chitosan-polyethylene oxide electrospun wound scaffold produced using novel Spirograph based collector assembly. Int. J. Biol. Macomol. 2019, 140, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xu, L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol. 2020, 160, 352–363. [Google Scholar] [CrossRef]
- Zeng, W.M.; Parus, A.; Barnes, C.W.; Hiro, M.E.; Robson, M.C.; Payne, W.G. Aloe vera- Mechanisms of Action, Uses, and Potential Uses in Plastic Surgery and Wound Healing. Surg. Sci. 2020, 11, 312–328. [Google Scholar] [CrossRef]
- Mustatab Wahedi, H.; Jeong, M.; Kyoung Chae, J.; Gil Do, S.; Yoon, H.; Kim, S.Y. Aloesin from Aloe vera accelerates skin wound healing by modulatingMAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine 2017, 28, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Eftimov, P.; Stefanova, N.; Lalchev, Z.; Georgiev, G.A. Effect of hydrophilic polymers on the wettability, static and dynamic, of solid substrate covered by confluent monolayer of air-damaged SIRC cells. Biotechnol. Biotechnol. Equip. 2015, 29, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Animal Ethics Infolink. A NSW Department of Primary Industries and Animal Research Review Panel initiative. ARRP Webinar Series 2022—Animal Research Statistics. Available online: https://www.animalethics.org.au/animal-use-statistics (accessed on 9 August 2022).
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Sala, R.L.; Venâncio, T.; Camargo, E.R. Probing the Structural Dynamics of the Coil-Globule Transition of Thermosensitive Nanocomposite Hydrogels. Langmuir 2021, 37, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Abbasi-Firouzjah, M.; Shokri, B. Investigation of antibacterial and wettability behaviours of plasma-modified PMMA films for application in ophthalmology. J. Phys. D Appl. Phys. 2014, 47, 085401. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef]
- Synytsya, A.; Poučková, P.; Zadinová, M.; Troshchynska, Y.; Štětina, J.; Synytsya, A.; Saloň, I.; Král, V. Hydrogels based on low-methoxyl amidated citrus pectin and flaxseed gum formulated with tripeptide glycyl-l-histidyl-l-lysine improve the healing of experimental cutting wounds in rats. Int. J. Biol. Macromol. 2018, 165, 3156–3168. [Google Scholar] [CrossRef]
- Basit, H.M.; Ali, M.; Shah, M.M.; Shah, S.U.; Wahab, A.; Albarqi, H.A.; Alqahtani, A.A.; Walbi, I.A.; Khan, N.R. Microwave Enabled Physically Cross Linked Sodium Alginate and Pectin Film and Their Application in Combination with Modified Chitosan-Curcumin Nanoparticles. A Novel Strategy for 2nd Degree Burns Wound Healing in Animals. Polymers 2021, 13, 2716. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-García, A.K.; Santos-Garfias, E.; Alonso-Martínez, D.I.; Garambullo-Peña, T.I.; Covián-Nares, J.F.; Gómez-Barroso, M.; Montoya-Pérez, R. Healing of Wounds Treated with Chitosan Hydrogels with Extracts from Aloe vera and Calendula officinalis. Rev. Mex. De Ing. Biomed. 2022, 43, 19–31. [Google Scholar] [CrossRef]
- Alsareii, S.A.; Ahmad, J.; Umar, A.; Ahmad, M.Z.; Shaikh, I.A. Enhanced In Vivo Wound Healing Efficacy of a Novel Piperine-Containing Bioactive Hydrogel in Excision Wound Rat Model. Molecules 2023, 28, 545. [Google Scholar] [CrossRef] [PubMed]
- Cândido Bernardes, M.J.; Caldeira Gonçalves, R.; de Sousa Carvalho, C.; Rosa, L.M.; Ferreira, A.P.; Vilela, M.S.; Vinaud, M.C.; Galdino Junior, H.; Lino Junior, R.D.S. Hydrogel-based dressings in the treatment of partial thickness experimentally induced burn wounds in rats. Acta Cir. Bras. 2022, 37, 1–20. [Google Scholar] [CrossRef]
- Chu, B.; He, J.-M.; Liu, L.-L.; Wu, C.-X.; You, L.-L.; Li, X.-L.; Wang, S.; Chen, C.-S.; Tu, M. Proangiogenic Peptide Nanofiber Hydrogels for Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- EU Regulations on Animal Research. European Animal Research Asociation (EARA). 2022. Available online: https://www.eara.eu/animal-research-law#:~:text=Animals%20can%20only%20be%20used,using%20non%2Danimal%20alternative%20methods (accessed on 23 September 2022).
- Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes. Web ISBN 9781474112406 ID 19111403 12/14 44389 Printed in the UK by the Williams Lea Group on behalf of the Controller of her Majesty’s Stationery Office. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/388535/CoPanimalsWeb.pdf (accessed on 23 September 2022).



| Day | C (%) | LPA (%) |
|---|---|---|
| 4 | 21.48 | 50.87 ± 10.63 |
| 8 | 45.60 | 70.92 ± 8.51 |
| 15 | 82.85 | 100 ± 0.00 |
| 21 | 92.82 | 100 ± 0.00 |
| Day | C2020 (%) | LPA (%) | Significance |
|---|---|---|---|
| 4 | 18.48 ± 3.12 | 50.87 ± 10.63 | 0.002 |
| 8 | 47.49 ± 3.89 | 70.92 ± 8.51 | 0.018 |
| 15 | 83.99 ± 2.15 | 100 ± 0.00 | 0.002 |
| 21 | 93.58 ± 3.70 | 100 ± 0.00 | 0.025 |
| LSD (least significance difference) test (n = 15) for each group test (p < 0.05) | |||
| Group | t (Days) | Parameter | |||
|---|---|---|---|---|---|
| Inflammation | Fibrin | Angiogenesis | Epithelialization | ||
| CT | 4 | +++ | ++ | + | − |
| 8 | +++ | +++ | ++ | + | |
| 15 | ++ | +++ | +++ | ++ | |
| 21 | + | ++ | ++ | +++ | |
| LPA | 4 | +++ | +++ | +++ | − |
| 8 | +++ | +++ | +++ | + | |
| 15 | − | + | +++ | +++ | |
| 21 | − | − | +++ | +++ | |
| Parameter | Scale | |||
|---|---|---|---|---|
| Inflammation | − | + | ++ | +++ |
| Angiogenesis | − | + | ++ | +++ |
| Fibrin | − | + | ++ | +++ |
| Epithelialization | − | + | ++ | +++ |
| (−) absent, (+) mild, (++) moderate, (+++) intense | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucedo-Acuña, R.A.; Meza-Valle, K.Z.; Cuevas-González, J.C.; Ordoñez-Casanova, E.G.; Castellanos-García, M.I.; Zaragoza-Contreras, E.A.; Tamayo-Pérez, G.F. Characterization and In Vivo Assay of Allantoin-Enriched Pectin Hydrogel for the Treatment of Skin Wounds. Int. J. Mol. Sci. 2023, 24, 7377. https://doi.org/10.3390/ijms24087377
Saucedo-Acuña RA, Meza-Valle KZ, Cuevas-González JC, Ordoñez-Casanova EG, Castellanos-García MI, Zaragoza-Contreras EA, Tamayo-Pérez GF. Characterization and In Vivo Assay of Allantoin-Enriched Pectin Hydrogel for the Treatment of Skin Wounds. International Journal of Molecular Sciences. 2023; 24(8):7377. https://doi.org/10.3390/ijms24087377
Chicago/Turabian StyleSaucedo-Acuña, Rosa Alicia, Karen Zulema Meza-Valle, Juan Carlos Cuevas-González, Elsa Gabriela Ordoñez-Casanova, Manuel Iván Castellanos-García, Erasto Armando Zaragoza-Contreras, and Genaro Federico Tamayo-Pérez. 2023. "Characterization and In Vivo Assay of Allantoin-Enriched Pectin Hydrogel for the Treatment of Skin Wounds" International Journal of Molecular Sciences 24, no. 8: 7377. https://doi.org/10.3390/ijms24087377
APA StyleSaucedo-Acuña, R. A., Meza-Valle, K. Z., Cuevas-González, J. C., Ordoñez-Casanova, E. G., Castellanos-García, M. I., Zaragoza-Contreras, E. A., & Tamayo-Pérez, G. F. (2023). Characterization and In Vivo Assay of Allantoin-Enriched Pectin Hydrogel for the Treatment of Skin Wounds. International Journal of Molecular Sciences, 24(8), 7377. https://doi.org/10.3390/ijms24087377