Systematic Analysis of NRAMP Family Genes in Areca catechu and Its Response to Zn/Fe Deficiency Stress
Abstract
:1. Introduction
2. Results
2.1. Identification and Characterization of AcNRAMPs
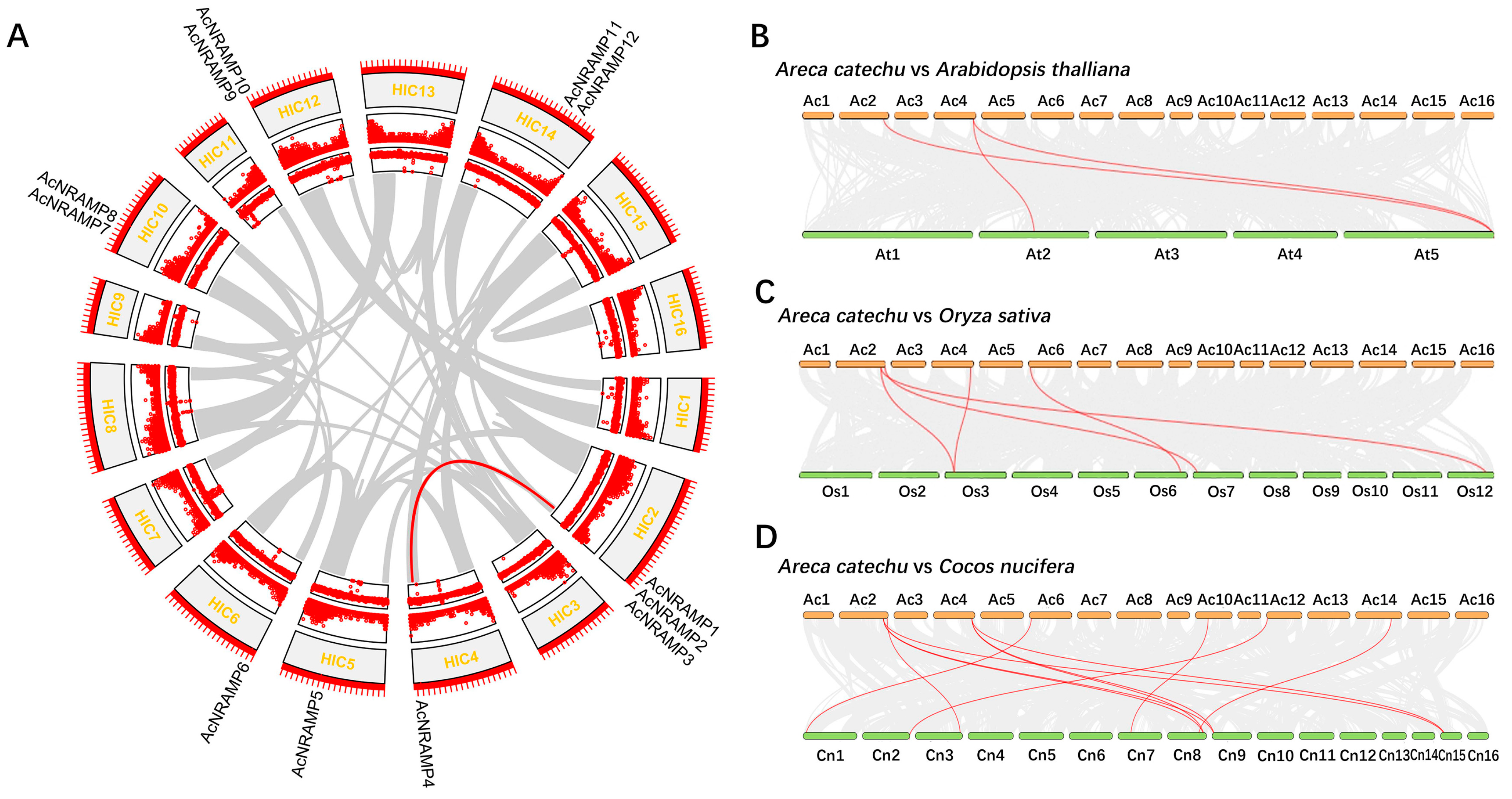
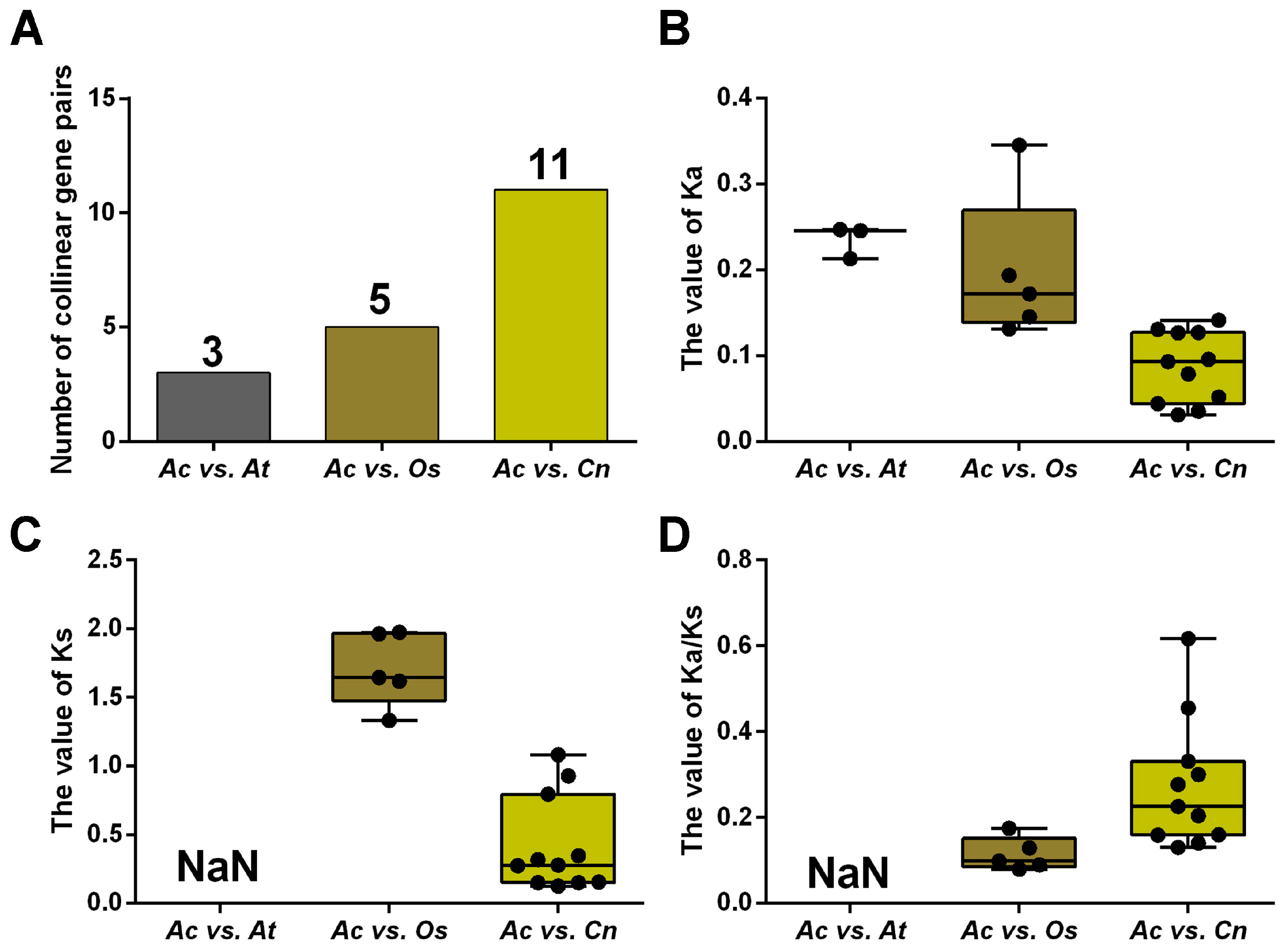
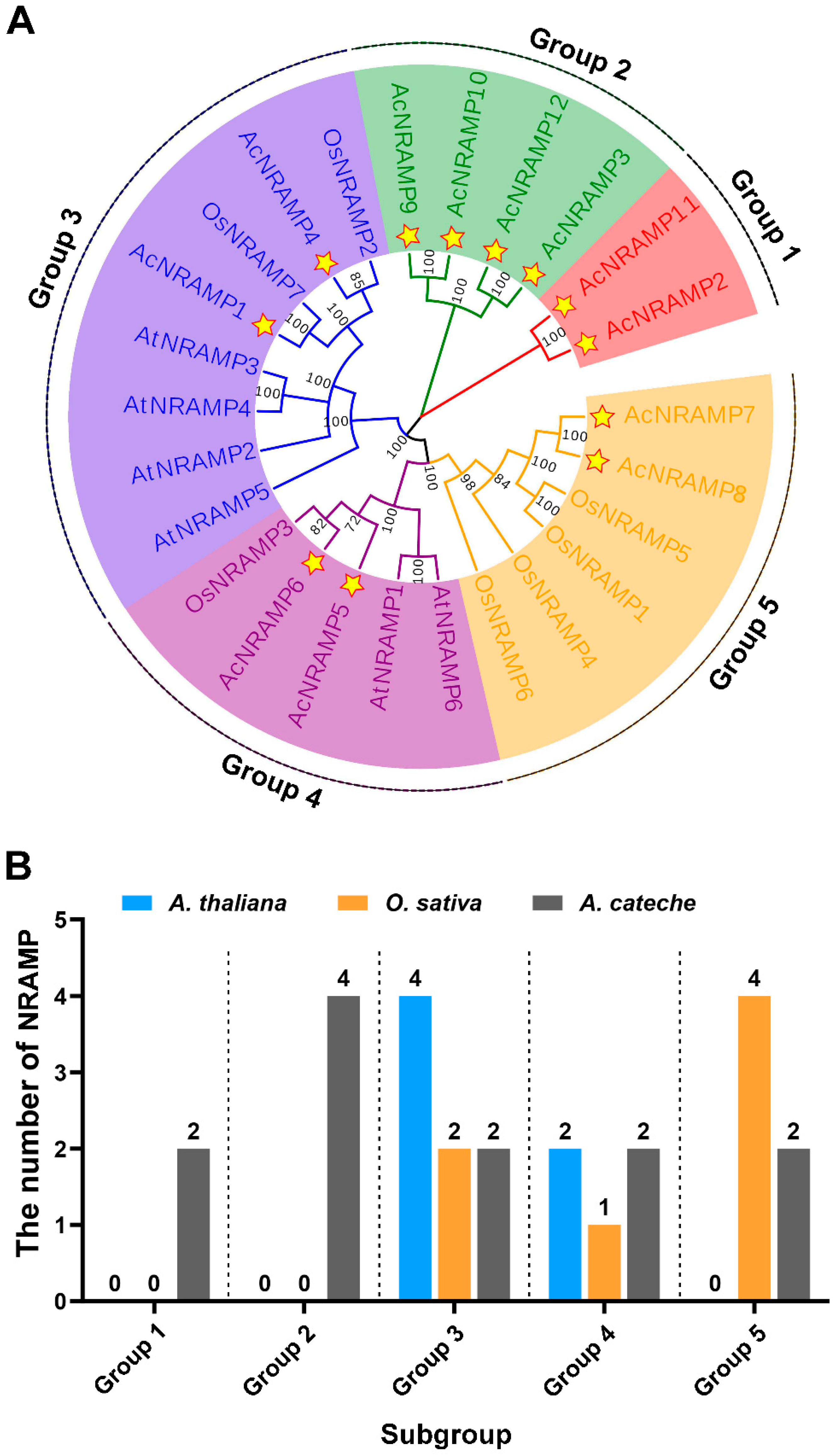
2.2. Duplication Analysis and Phylogenetic Analysis of AcNRAMPs
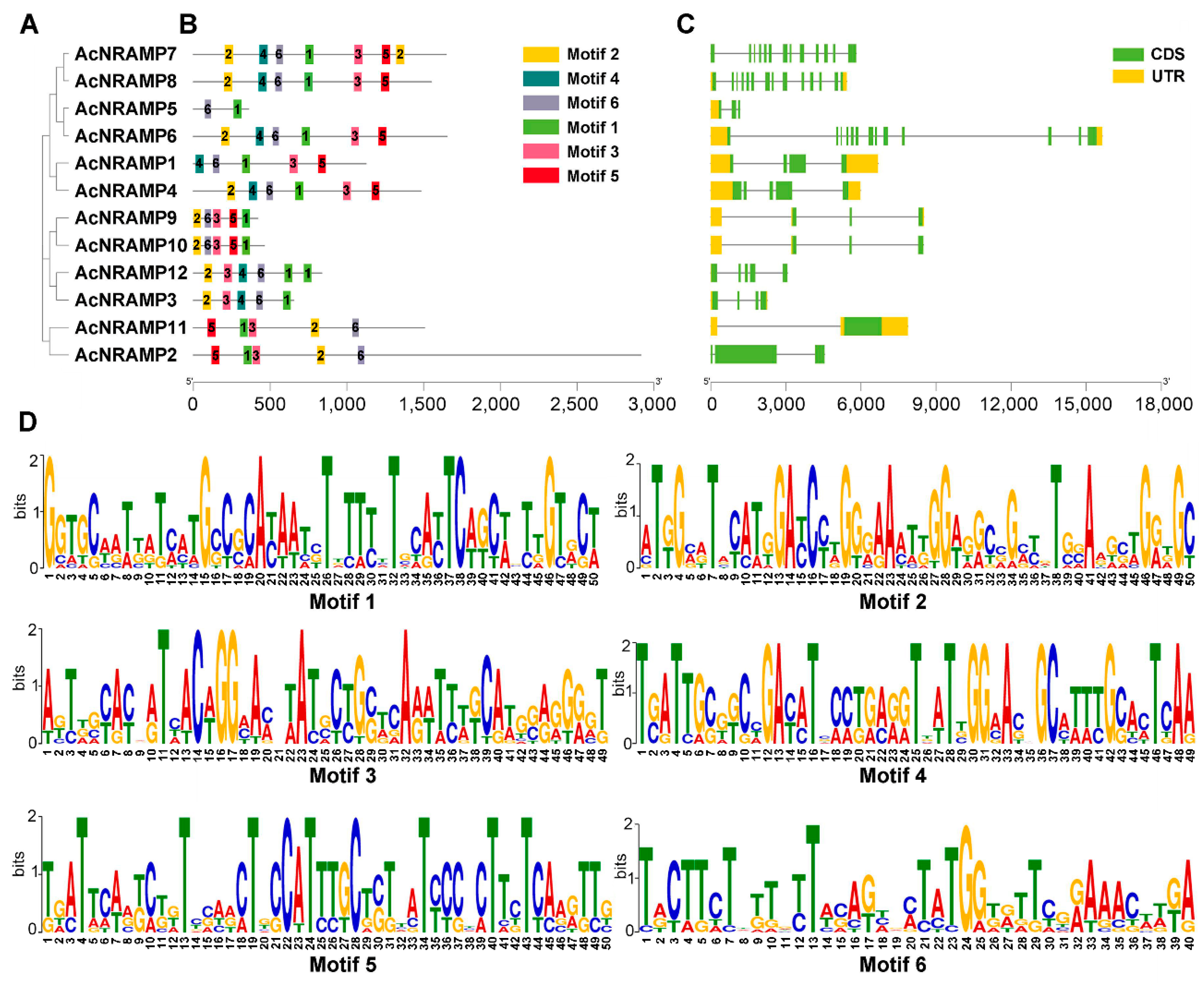
2.3. Sequence Features of AcNRAMPs
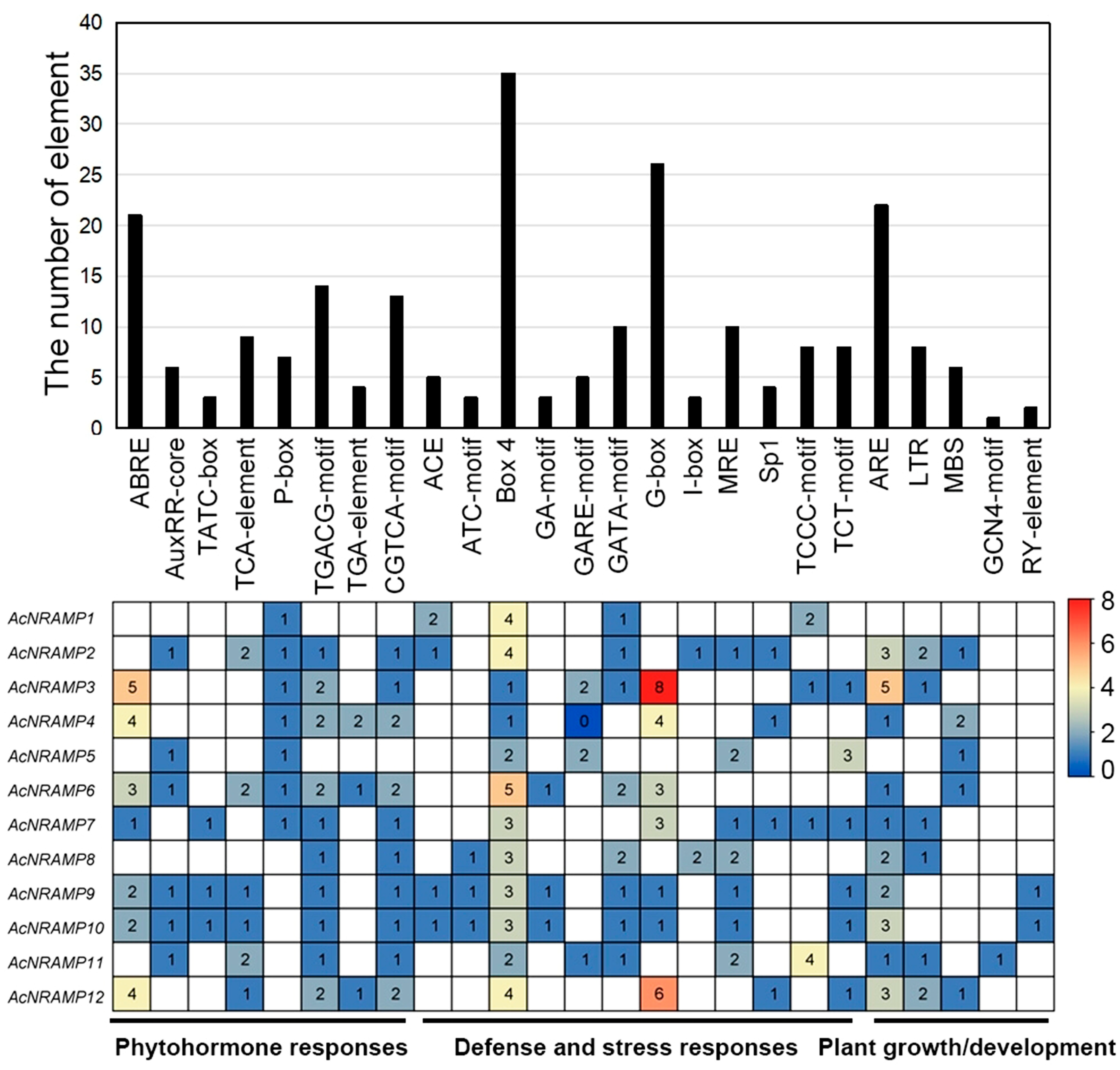
2.4. Cis-Acting Elements of AcNRAMPs
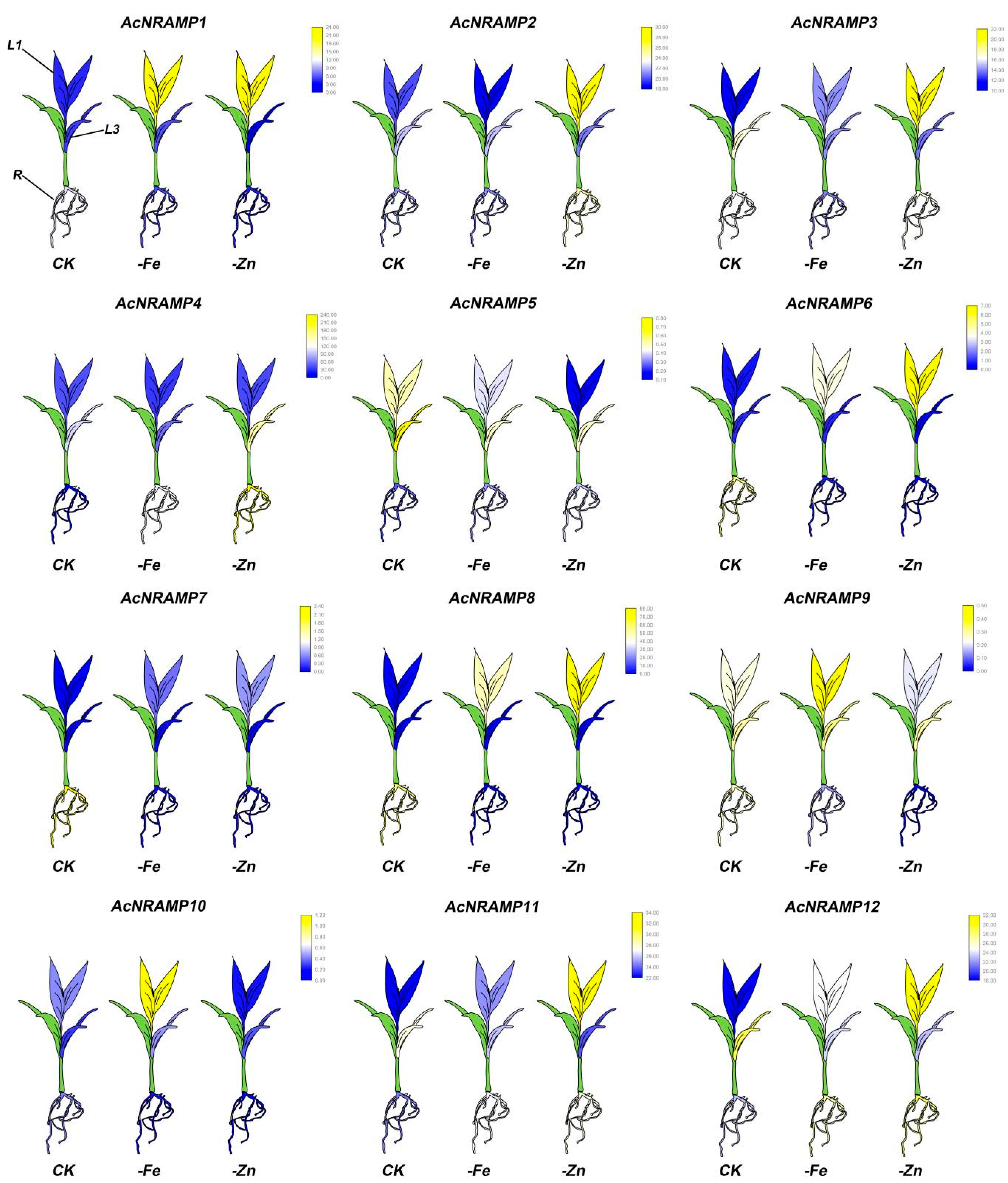
2.5. Expression Analysis of AcNRAMPs in Different Tissues and under Fe and Zn Deficiency
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Identification of AcNRAMP Genes in A. catechu
4.3. Analysis of Physicochemical Properties of AcNRAMPs
4.4. Analysis of Evolutional Relationships of NRAMP Genes in A. catechu, O. sativa, and A. thaliana
4.5. Analysis of Sequence Features, Chromosome Distribution, and Syntenic Relationships of AcNRAMPs
4.6. Analysis of Cis-Acting Elements
4.7. Transcriptomic Data Analysis of AcNRAMPs
4.8. qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of zinc nutrition for increasing zinc availability, uptake, yield, and quality of maize (Zea mays L.) grains: An overview. Commun. Soil Sci. Plant 2020, 51, 2001–2021. [Google Scholar]
- Eroglu, S.; Meier, B.; Wirén, N.V.; Peiter, E. The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiol. 2016, 170, 1030–1045. [Google Scholar] [CrossRef]
- Briat, J.F.; Lebrun, M. Plant responses to metal toxicity. Comptes Rendus Acad. Sci. III 1999, 322, 43–54. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.M.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, E.P.; Guerinot, M.L. Put the metal to the petal: Metal uptake and transport throughout plants. Curr. Opin. Plant Biol. 2006, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, R.; Filiz, E.; Ozyigit, I.I. In silico analysis of Mn transporters (NRAMP1) in various plant species. Mol. Biol. Rep. 2016, 43, 151–163. [Google Scholar] [CrossRef]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Bannona, D. Divalent Metal Transporter 1 in lead and cadmium transport. Ann. N. Y. Acad. Sci. USA 2004, 1012, 142–152. [Google Scholar] [CrossRef]
- Pinner, E.; Gruenheid, S.; Raymond, M.; Gros, P. Functional complementation of the yeast divalent cation transporter family SMF by NRAMP2, a member of the mammalian natural resistance-associated macrophage protein family. J. Biol. Chem. 1997, 272, 28933–28938. [Google Scholar] [CrossRef]
- Gunshin, H.; MacKenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nat. Cell Biol. 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Chang, J.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef]
- Chen, H.M.; Wang, Y.M.; Yang, H.L.; Zeng, Q.Y.; Liu, Y.J. NRAMP1 promotes iron uptake at the late stage of iron deficiency in poplars. Tree Physiol. 2019, 39, 1235–1250. [Google Scholar] [CrossRef]
- Ishida, J.K.; Caldas, D.G.; Oliveira, L.R.; Frederici, G.C.; Leite, L.M.P.; Mui, T.S. Genome-wide characterization of the NRAMP gene family in Phaseolus vulgaris provides insights into functional implications during common bean development. Genet. Mol. Biol. 2018, 41, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Wang, Y.; Eide, D.J.; Dunwell, J.M. Evolution, and functional analysis of natural resistance-associated macrophage proteins (NRAMPs) from Theobroma cacao and their role in cadmium accumulation. Sci. Rep. 2018, 8, 14412. [Google Scholar] [CrossRef]
- Zhang, X.D.; Meng, J.G.; Zhao, K.X.; Chen, X.; Yang, Z.M. Annotation and characterization of Cd-responsive metal transporter genes in rapeseed (Brassica napus). BioMetals 2017, 31, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Cailliatte, R.; Lapeyre, B.; Briat, J.; Mari, S.; Curie, C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem. J. 2009, 422, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, W.; Dong, H.X.; Zhang, Y.Y.; Lv, K.; Wang, D.J.; Lian, X.M. OsNRAMP3 Is a vascular bundles-specific manganese transporter that is responsible for manganese distribution in rice. PLoS ONE 2013, 8, e83990. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaj, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cdmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.P.; Dong, D.K.; Jia, X.M.; McCouch, S.R.; Kochian, L.V. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. 2014, 111, 6503–6508. [Google Scholar] [CrossRef]
- An, Q.Y.; Cui, C.; Muhammad, K.N.; Zhou, G.Z.; Wan, Y.L. Genome-wide investigation of ZINC-IRON PERMEASE (ZIP) genes in Areca catechu and potential roles of ZIPs in Fe and Zn uptake and transport. Plant Signal. Behav. 2021, 16, 1995647. [Google Scholar] [CrossRef]
- Pottier, M.; Oomen, R.; Picco, C.; Giraudat, J.; Scholz-starke, J.; Richaud, P.; Carpaneto, A.; Thomine, S. Identification of mutations allowing natural resistance associated macrophage proteins (NRAMP) to discriminate against cadmium. Plant J. 2015, 83, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Ihnatowicz, A.; Siwinska, J.; Meharg, A.A.; Carey, M.; Koorneef, M.; Reymond, M. Conserved histidine of metal transporter AtNRAMP1 is crucial for optimal plant growth under manganese deficiency at chilling temperatures. New Phytol. 2014, 202, 1173–1183. [Google Scholar] [CrossRef]
- Halligan, B.D. ProMoST: A tool for calculating the pI and molecular mass of phosphorylated and modified proteins on two-dimensional gels. Methods Mol. Biol. 2009, 527, 283–298. [Google Scholar] [PubMed]
- Mohanta, T.K.; Khan, A.; Hashem, A.; Abd-Allah, E.F.; Al-Harrasi, A. The molecular mass and isoelectric point of plant proteomes. BMC Genom. 2019, 20, 631. [Google Scholar] [CrossRef]
- Qing, J.; Dawei, W.; Zhou, J.; Yulan, X.; Bingqi, S.; Fan, Z. Genomewide characterization and expression analyses of the MYB superfamily genes during developmental stages in Chinese jujube. PeerJ 2019, 7, e6353. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zheng, L.; Li, Y.; Zhou, X.; Li, J.; Gu, D.; Xu, E.; Lu, Y.; Chen, X.; et al. The Intracellular Transporter AtNRAMP6 Is Involved in Fe Homeostasis in Arabidopsis. Front. Plant Sci. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high-affinity metal transporters NRAMP1 and IRT1 team up to take up iron under sufficient metal provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Sasaki, A.; Xia, J.X.; Yokosho, K.; Ma, J.F. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.; Xiao, Z.; Zhang, X.; Han, Z.; Wang, Y. Cloning and functional analysis of MxNRAMP1 and MxNRAMP3, two gens related to high metal tolerance of Malus xiaojinensis. S Afr. J. Bot. 2016, 102, 75–80. [Google Scholar] [CrossRef]
- Alejandro, S.; Cailliatte, R.; Alcon, C.; Dirick, L.; Domergue, F.; Correia, D.; Castaings, L.; Briat, J.F.; Mari, S.; Curie, C. Intracellular distribution of manganese by the Trans-Golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. Plant Cell 2017, 29, 3068–3084. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef] [PubMed]
- Mary, V.; Ramos, M.S.; Gillet, C.; Socha, A.L.; Giraudat, J.; Agorio, A.; Merlot, S.; Clairet, C.; Kim, S.A.; Punshon, T.; et al. Bypassing iron storage in endodermal vacuoles rescues the iron mobilization defect in the natural resistance associated-macrophage protein3natural resistance associated-macrophage protein4 double mutant. Plant Physiol. 2015, 169, 748–759. [Google Scholar] [CrossRef]
- Mani, A.; Sankaranarayanan, K. In silico analysis of natural resistance-associated macrophage protein1 (NRAMP) family of transporters in rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Song, H.; Zhao, J.; Shabala, S.; Tian, S.; Yang, X. A novel plasma membrane-based NRAMP transporter contributes to Cd and Zn hyperaccumulation in Sedum alfredii Hance. Environ. Exp. Bot. 2020, 176, 104121. [Google Scholar] [CrossRef]
- Qin, L.; Han, P.P.; Chen, L.Y.; Walk, T.C.; Li, Y.S.; Hu, X.J.; Xie, L.H.; Liao, H.; Liao, X. Genome-Wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal. Behav. 2011, 6, 1813–1816. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Bashir, K.; Nakanishi, H.; Nishizawa, N.K. OsNRAMP5, a major player for constitutive iron and manganese uptake in rice. Plant Signal. Behav. 2012, 7, 763–766. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.Y.; Zhang, L.J.; Hu, J.T.; Zhang, X.; Lu, K.; Dong, H.X.; Wang, D.J.; Zhao, F.J.; Huang, C.F.; et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Xiong, E.H.; Zheng, C.Y.; Wu, X.L.; Wang, W. Protein subcellular location: The gap between prediction and experimentation. Plant Mol. Biol. Rep. 2016, 34, 52–61. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Mega, X. Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.H.; Hu, S. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Busker, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.Y.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lu, S.N.; Wang, J.Y.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Geadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; An, Q.; Liu, Z.; Wan, Y.; Bao, W. Systematic Analysis of NRAMP Family Genes in Areca catechu and Its Response to Zn/Fe Deficiency Stress. Int. J. Mol. Sci. 2023, 24, 7383. https://doi.org/10.3390/ijms24087383
Zhou G, An Q, Liu Z, Wan Y, Bao W. Systematic Analysis of NRAMP Family Genes in Areca catechu and Its Response to Zn/Fe Deficiency Stress. International Journal of Molecular Sciences. 2023; 24(8):7383. https://doi.org/10.3390/ijms24087383
Chicago/Turabian StyleZhou, Guangzhen, Qiyuan An, Zheng Liu, Yinglang Wan, and Wenlong Bao. 2023. "Systematic Analysis of NRAMP Family Genes in Areca catechu and Its Response to Zn/Fe Deficiency Stress" International Journal of Molecular Sciences 24, no. 8: 7383. https://doi.org/10.3390/ijms24087383
APA StyleZhou, G., An, Q., Liu, Z., Wan, Y., & Bao, W. (2023). Systematic Analysis of NRAMP Family Genes in Areca catechu and Its Response to Zn/Fe Deficiency Stress. International Journal of Molecular Sciences, 24(8), 7383. https://doi.org/10.3390/ijms24087383






