Persicae Semen Promotes Bone Union in Rat Fractures by Stimulating Osteoblastogenesis through BMP-2 and Wnt Signaling
Abstract
:1. Introduction
2. Results
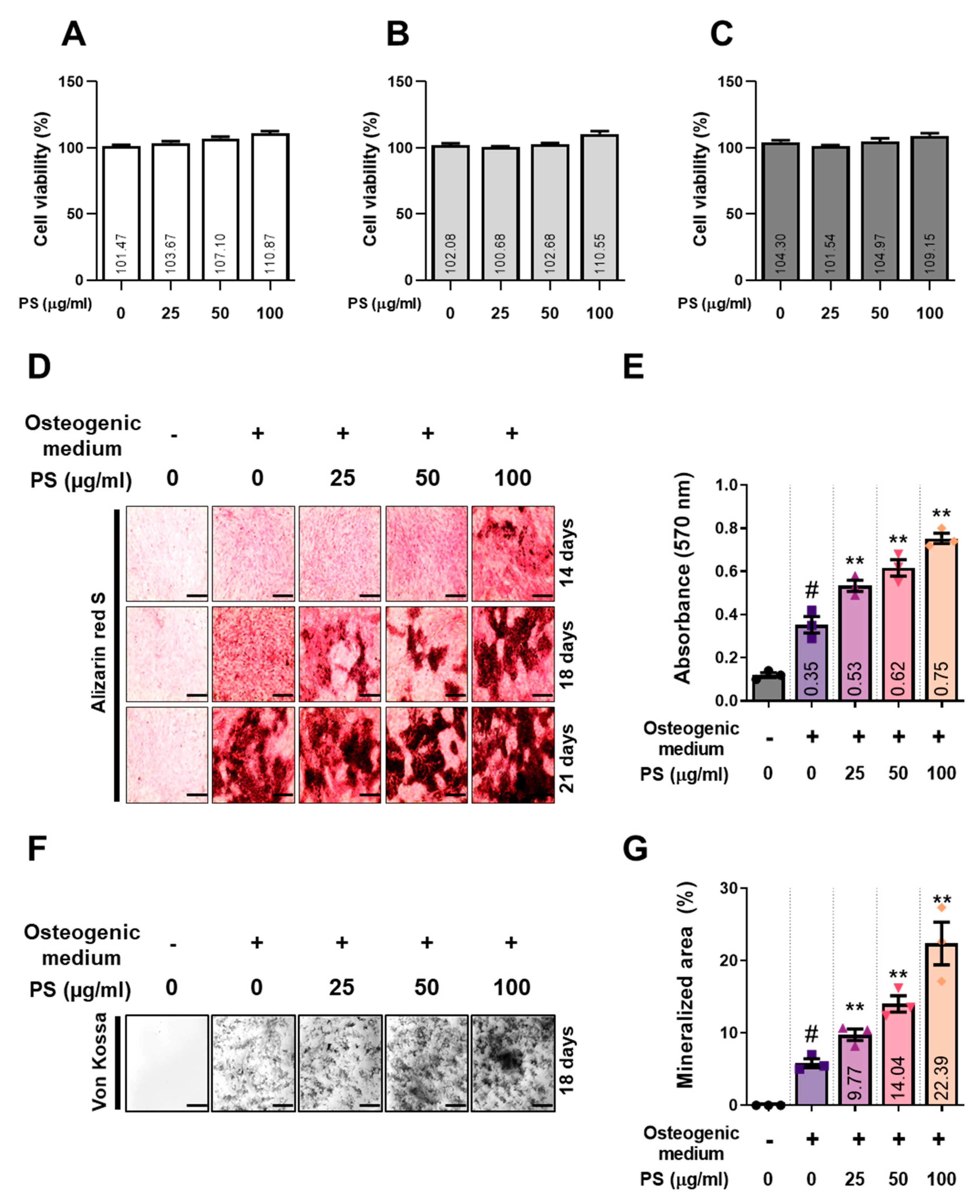
2.1. PS Enhances the Formation of Mineralized Nodules in MC3T3-E1 Cells
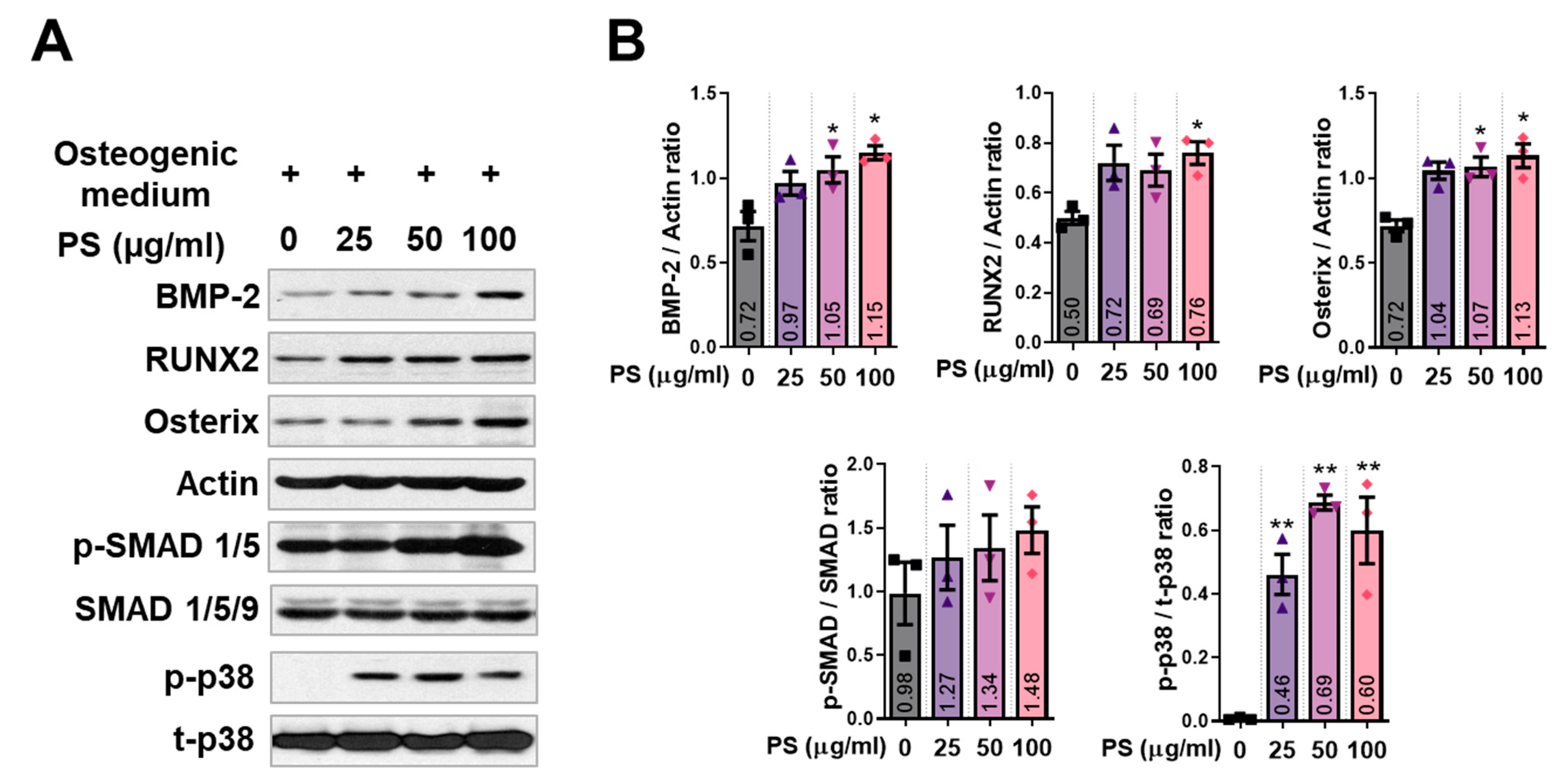
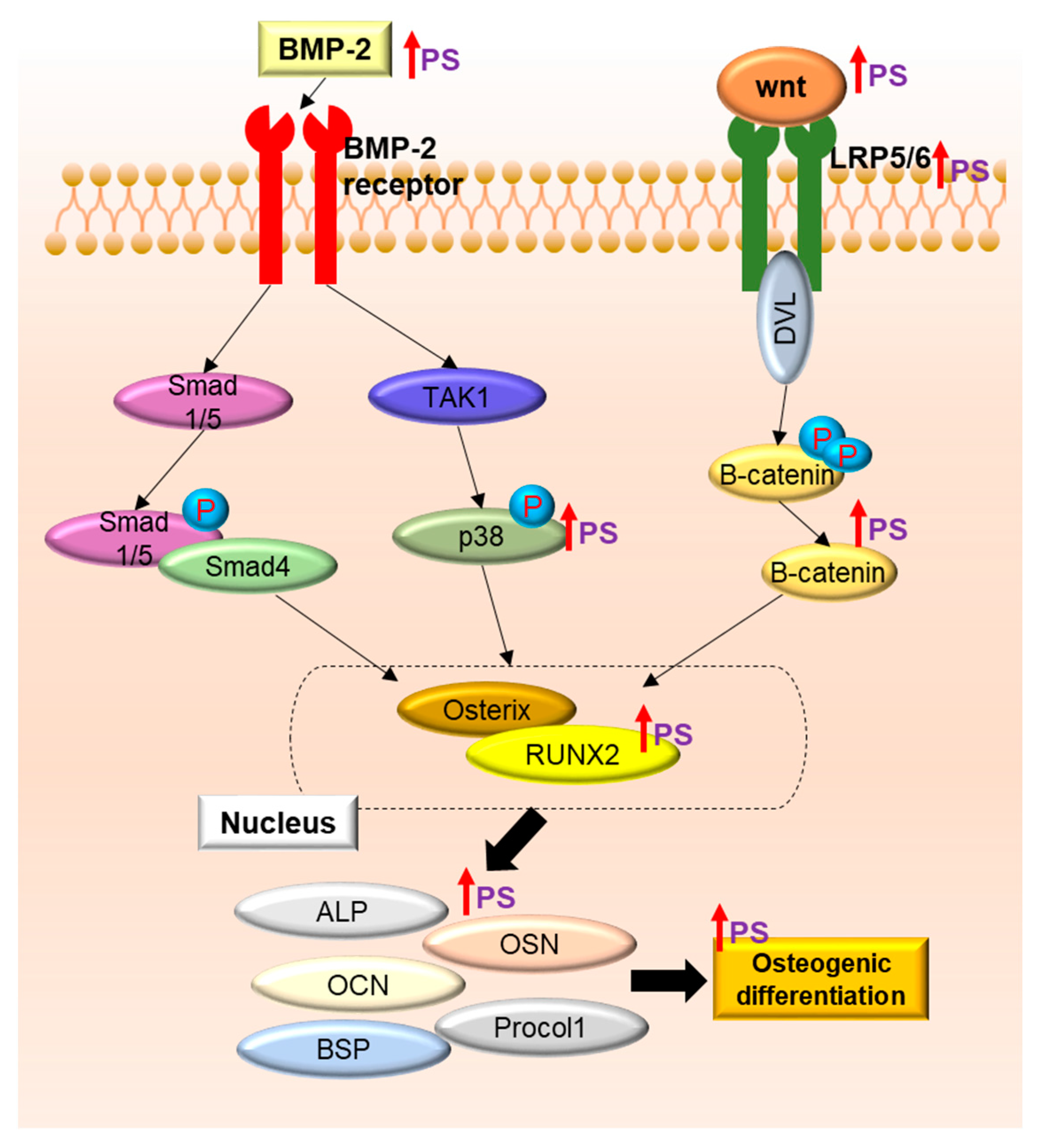
2.2. PS Increases the Expression of BMP-2 Signaling in MC3T3-E1 Cells
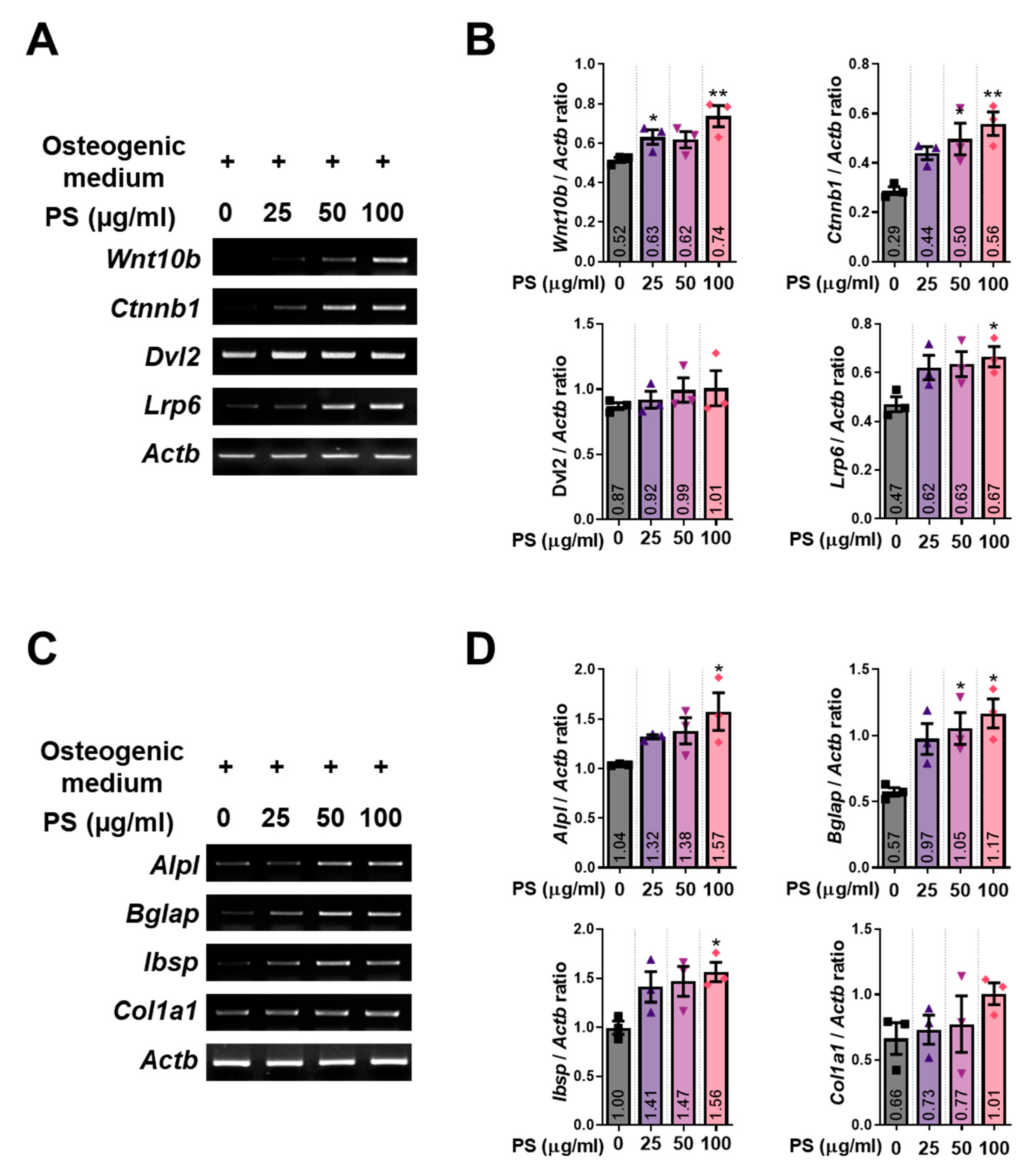
2.3. PS Increases the Expression of Wnt/β-Catenin Signaling and Osteoblast-Related Genes in MC3T3-E1 Cells
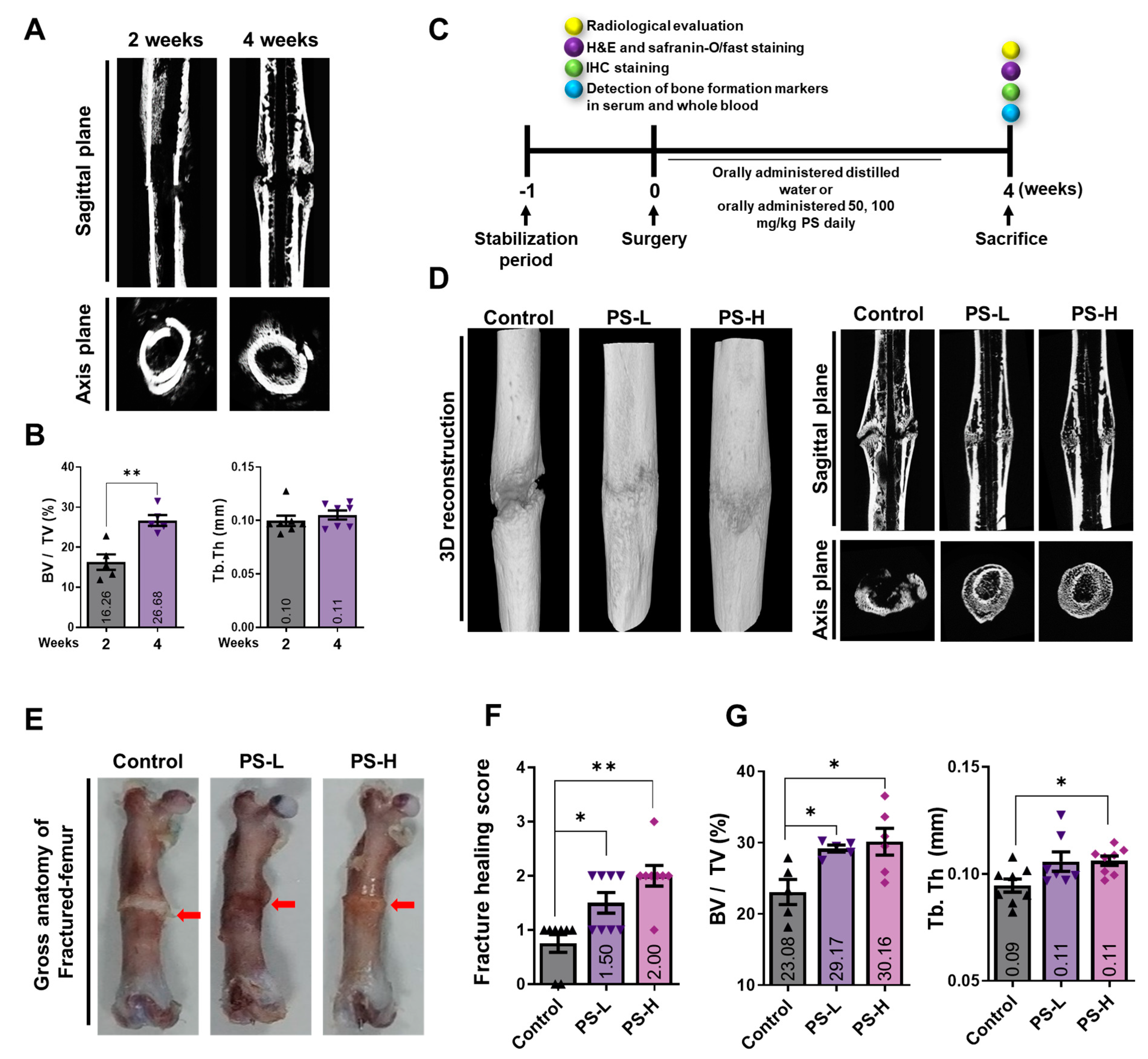
2.4. PS Promotes Bone Formation in Fracture-Induced Models
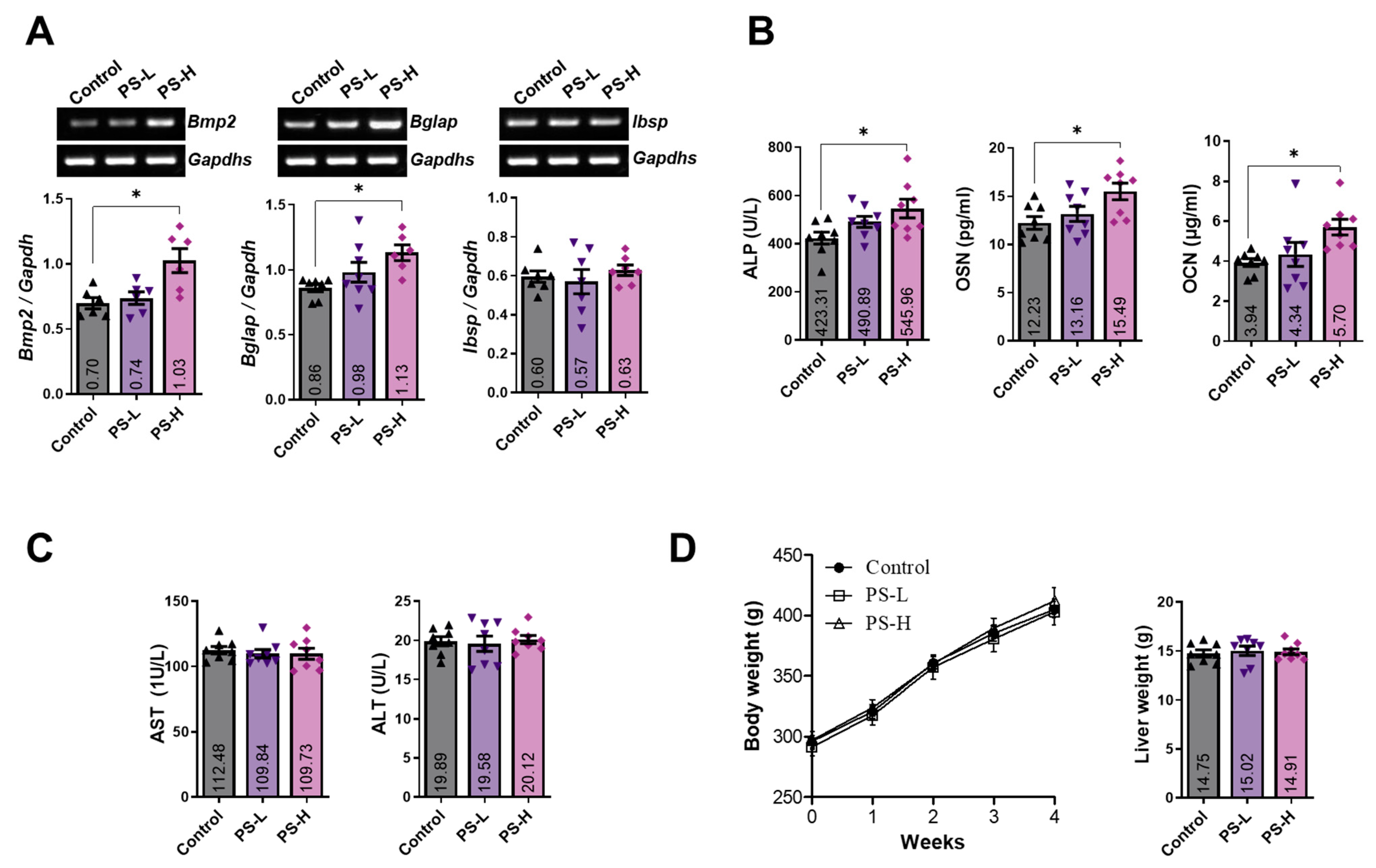
2.5. PS Promotes Osseous Callus Formation and Increases BMP-2, RUNX2, and OCN Expression in Femoral Tissue
2.6. PS Increases the Expression of BMP-2 and Osteoblast-Related Markers in Whole Blood and Serum
2.7. Standardization of PS and Determination of the Effect of Amygdalin on Osteoblasts
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of PS
4.3. Cell Culture and Cell Viability
4.4. Alizarin Red S and Von Kossa Staining
4.5. Western Blotting Analysis
4.6. RNA Isolation and RT-PCR Analysis for MC3T3-E1 Cells
4.7. Rat Femoral Fracture Model
4.8. Micro-CT and Evaluation of Fracture Recovery Ability
4.9. Whole Blood and Serum Analysis
4.10. Histopathological Examination
4.11. HPLC Analysis
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, 4–9. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M.; Amling, M.; Ignatius, A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cells Mater. 2018, 35, 365–385. [Google Scholar] [CrossRef]
- Adachi, J.D.; Adami, S.; Gehlbach, S.; Anderson, F.A., Jr.; Boonen, S.; Chapurlat, R.D.; Compston, J.E.; Cooper, C.; Delmas, P.; Diez-Perez, A.; et al. Impact of prevalent fractures on quality of life: Baseline results from the global longitudinal study of osteoporosis in women. Mayo Clin. Proc. 2010, 85, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef]
- Farhadieh, R.D.; Gianoutsos, M.P.; Yu, Y.; Walsh, W.R. The role of bone morphogenetic proteins BMP-2 and BMP-4 and their related postreceptor signaling system (Smads) in distraction osteogenesis of the mandible. J. Craniofac. Surg. 2004, 15, 714–718. [Google Scholar] [CrossRef]
- Noth, U.; Tuli, R.; Seghatoleslami, R.; Howard, M.; Shah, A.; Hall, D.J.; Hickok, N.J.; Tuan, R.S. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp. Cell Res. 2003, 291, 201–211. [Google Scholar] [CrossRef]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010, 658, 43–49. [Google Scholar] [CrossRef]
- Xi, S.; Qian, L.; Tong, H.; Yue, L.; Zhao, H.; Wang, D.; Lu, D.; Li, P.; Wang, X. Toxicity and clinical reasonable application of Taoren (Semen Persicae) based on ancient and modern literature research. J. Tradit. Chin. Med. 2013, 33, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Liu, Q.; Peng, D.; Wang, L.; Wang, S. Experimental study on anti-thrombus effect of different extracts from Semen Persicae. J. Chin. Med. Mater. 2002, 25, 414–415. [Google Scholar]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, 7–21. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Hong, S.P.; Hahn, D.H.; Kim, J.H. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch. Pharm. Res. 2003, 26, 157–161. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.M. Taepyeonghyeminhwajegukbang; Ren Min Wei Sheng Publishing Inc.: Beijing, China, 1985. [Google Scholar]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, M.; Hong, S.; Kim, E.Y.; Lee, H.; Jung, H.S.; Sohn, Y. Albiflorin Promotes Osteoblast Differentiation and Healing of Rat Femoral Fractures Through Enhancing BMP-2/Smad and Wnt/beta-Catenin Signaling. Front. Pharmacol. 2021, 12, 690113. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.W.; Horton, J.A. Variable osteogenic performance of MC3T3-E1 subclones impacts their utility as models of osteoblast biology. Sci. Rep. 2019, 9, 8299. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, A.; Komori, T.; Suda, T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr. Rev. 2000, 21, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takarada, T.; Hinoi, E.; Nakazato, R.; Ochi, H.; Xu, C.; Tsuchikane, A.; Takeda, S.; Karsenty, G.; Abe, T.; Kiyonari, H.; et al. An analysis of skeletal development in osteoblast-specific and chondrocyte-specific runt-related transcription factor-2 (Runx2) knockout mice. J. Bone Miner. Res. 2013, 28, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carballo, E.; Gamez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Tang, W.; Li, Y.; Yang, F.; Dowd, D.R.; MacDonald, P.N. Osteoblast-specific transcription factor Osterix increases vitamin D receptor gene expression in osteoblasts. PLoS ONE 2011, 6, e26504. [Google Scholar] [CrossRef]
- Baek, W.Y.; Lee, M.A.; Jung, J.W.; Kim, S.Y.; Akiyama, H.; de Crombrugghe, B.; Kim, J.E. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J. Bone Miner. Res. 2009, 24, 1055–1065. [Google Scholar] [CrossRef]
- Maupin, K.A.; Droscha, C.J.; Williams, B.O. A Comprehensive Overview of Skeletal Phenotypes Associated with Alterations in Wnt/beta-catenin Signaling in Humans and Mice. Bone Res. 2013, 1, 27–71. [Google Scholar] [CrossRef] [Green Version]
- Laine, C.M.; Joeng, K.S.; Campeau, P.M.; Kiviranta, R.; Tarkkonen, K.; Grover, M.; Lu, J.T.; Pekkinen, M.; Wessman, M.; Heino, T.J.; et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 2013, 368, 1809–1816. [Google Scholar] [CrossRef] [Green Version]
- Joeng, K.S.; Schumacher, C.A.; Zylstra-Diegel, C.R.; Long, F.; Williams, B.O. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev. Biol. 2011, 359, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Karner, C.M.; Long, F. Wnt signaling and cellular metabolism in osteoblasts. Cell. Mol. Life Sci. 2017, 74, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caetano-Lopes, J.; Canhao, H.; Fonseca, J.E. Osteoblasts and bone formation. Acta Reumatol. Port. 2007, 32, 103–110. [Google Scholar]
- Jo, S.; Han, J.; Lee, Y.L.; Yoon, S.; Lee, J.; Wang, S.E.; Kim, T.H. Regulation of osteoblasts by alkaline phosphatase in ankylosing spondylitis. Int. J. Rheum. Dis. 2019, 22, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Gordon, J.A.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Gu, Y.; Jiang, C.; Chen, L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J. Cell. Physiol. 2020, 235, 2220–2231. [Google Scholar] [CrossRef]
- Delany, A.M.; Kalajzic, I.; Bradshaw, A.D.; Sage, E.H.; Canalis, E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology 2003, 144, 2588–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Murao, H.; Yamamoto, K.; Matsuda, S.; Akiyama, H. Periosteal cells are a major source of soft callus in bone fracture. J. Bone Miner. Metab. 2013, 31, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Bukata, S.V.; Digiovanni, B.F.; Friedman, S.M.; Hoyen, H.; Kates, A.; Kates, S.L.; Mears, S.C.; Mendelson, D.A.; Serna, F.H., Jr.; Sieber, F.E.; et al. A guide to improving the care of patients with fragility fractures. Geriatr. Orthop. Surg. Rehabil. 2011, 2, 5–37. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets. Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Liu, L.; Xu, Q.; Shen, Q.; Li, W.; Zhao, J. Potential active compounds and molecular mechanism of Xuefu Zhuyu decoction for atherosclerosis, based on network pharmacology and molecular docking. Medicine 2022, 101, e29654. [Google Scholar] [CrossRef]
- Seo, K.H.; Choi, S.Y.; Jin, Y.; Son, H.; Kang, Y.S.; Jung, S.H.; Kim, Y.I.; Eum, S.; Bach, T.T.; Yoo, H.M.; et al. Anti-inflammatory role of Prunus persica L. Batsch methanol extract on lipopolysaccharide-stimulated glial cells. Mol. Med. Rep. 2020, 21, 2030–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.T.; Ngo, Q.V.; Le, D.T.T.; Nguyen, M.T.H.; Nguyen, P.T.M. beta-sitosterol from Clinacanthus nutans Lindau enhances osteoblastogenic activity via upregulation of differentiation related genes and proteins. Biosci. Biotechnol. Biochem. 2022, 86, 1615–1622. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomed. Rep. 2016, 5, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, I.D.; El-Sohemy, A. Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. J. Nutr. Biochem. 2009, 20, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Kim, M.B.; Song, Y.; Hwang, J.K. Kirenol stimulates osteoblast differentiation through activation of the BMP and Wnt/beta-catenin signaling pathways in MC3T3-E1 cells. Fitoterapia 2014, 98, 59–65. [Google Scholar] [CrossRef]
- Zhai, M.; Jing, D.; Tong, S.; Wu, Y.; Wang, P.; Zeng, Z.; Shen, G.; Wang, X.; Xu, Q.; Luo, E. Pulsed electromagnetic fields promote in vitro osteoblastogenesis through a Wnt/beta-catenin signaling-associated mechanism. Bioelectromagnetics 2016, 37, 152–162. [Google Scholar] [CrossRef] [PubMed]


| Source | Gene Name | Sequence (5′-3′) | Accession No. | Tm (°C) | Base Pair |
|---|---|---|---|---|---|
| Mouse | Alpl (ALP) | F: CGG GAC TGG TAC TCG GAT AA R: TGA GAT CCA GGC CAT CTA GC | NM_001287172.1 | 55 | 208 |
| Bglap (OCN) | F: GCA ATA AGG TAG TGA ACA GAC TCC R: GTT TGT AGG CGG TCT TCA AGC | NM_001032298.3 | 59 | 147 | |
| Ibsp (BSP) | F: AAA GTG AAG GAA AGC GAC GA R: GTT CCT TCT GCA CCT GCT TC | NM_008318.3 | 53 | 215 | |
| Col1a1 (COL1) | F: GCT CCT CTT AGG GGC CAC T R: CCA CGT CTC ACC ATT GGG G | NM_007742.4 | 60 | 103 | |
| Wnt10b (Wnt10b) | F: TTC TCT CGG GAT TTC TTG GAT TC R: TGC ACT TCC GCT TCA GGT TTT C | NM_011718.2 | 59 | 118 | |
| Ctnnb1 (β-catenin) | F: TGC TGA AGG TGC TGT CTG TC R: CTG CTT AGT CGC TGC ATC TG | NM_001165902.1 | 59 | 158 | |
| Dvl2 (DVL2) | F: GCT TCC ACA TGG CCA TGG GC R: TGG CAC TGC TGG TGA GAG TCA CAG | [51] | 64 | 195 | |
| Lrp6 (LRP6) | F: CAG CAC CAC AGG CCA CCA A R: TCG AGA CAT TCC TGG AAG AG | [52] | 58 | 220 | |
| Actb (β-actin) | F: TTC TAC AAT GAG CTG CGT GT R: CTC ATA GCT CTT CTC CAG GG | NM_007393 | 58 | 456 |
| Source | Gene Name | Sequence (5′-3′) | Accession No. | Tm (°C) | Base Pair |
|---|---|---|---|---|---|
| Rat | Bmp2 (BMP-2) | F: GAA GCC AGG TGT CTC CAA GAG R: GTG GAT GTC CTT TAC CGT CGT | NM_017178.1 | 58 | 122 |
| Bglap (OCN) | F: CAG TAA GGT GGT GAA TAG ACT CCG R: GGT GCC ATA GAT GCG CTT G | NM_013414.1 | 58 | 172 | |
| Ibsp (BSP) | F: AGA AAG AGC AGC ACG GTT GAG T R: GAC CCT CGT AGC CTT CAT AGC C | NM_012587.2 | 58 | 175 | |
| Gapdhs (GAPDH) | F: CCT GCA CCA CCA ACT GCT TA R: GGC CAT CCA CAG TCT TCT GAG | NM_017008.4 | 58 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, J.-Y.; Kim, J.-H.; Kim, M.; Hong, S.; Kim, M.; Ryu, G.-H.; Park, J.H.; Jung, H.-S.; Sohn, Y. Persicae Semen Promotes Bone Union in Rat Fractures by Stimulating Osteoblastogenesis through BMP-2 and Wnt Signaling. Int. J. Mol. Sci. 2023, 24, 7388. https://doi.org/10.3390/ijms24087388
Jun J-Y, Kim J-H, Kim M, Hong S, Kim M, Ryu G-H, Park JH, Jung H-S, Sohn Y. Persicae Semen Promotes Bone Union in Rat Fractures by Stimulating Osteoblastogenesis through BMP-2 and Wnt Signaling. International Journal of Molecular Sciences. 2023; 24(8):7388. https://doi.org/10.3390/ijms24087388
Chicago/Turabian StyleJun, Jae-Yun, Jae-Hyun Kim, Minsun Kim, Sooyeon Hong, Myunghyun Kim, Gwang-Hyun Ryu, Jae Ho Park, Hyuk-Sang Jung, and Youngjoo Sohn. 2023. "Persicae Semen Promotes Bone Union in Rat Fractures by Stimulating Osteoblastogenesis through BMP-2 and Wnt Signaling" International Journal of Molecular Sciences 24, no. 8: 7388. https://doi.org/10.3390/ijms24087388
APA StyleJun, J.-Y., Kim, J.-H., Kim, M., Hong, S., Kim, M., Ryu, G.-H., Park, J. H., Jung, H.-S., & Sohn, Y. (2023). Persicae Semen Promotes Bone Union in Rat Fractures by Stimulating Osteoblastogenesis through BMP-2 and Wnt Signaling. International Journal of Molecular Sciences, 24(8), 7388. https://doi.org/10.3390/ijms24087388








