Anti-Neuroinflammatory and Neuroprotective Effect of Intermedin B Isolated from the Curcuma longa L. via NF-κB and ROS Inhibition in BV2 Microglia and HT22 Hippocampal Cells
Abstract
:1. Introduction
2. Results
2.1. Chemical Structures of 17 Compounds Isolated from C. longa
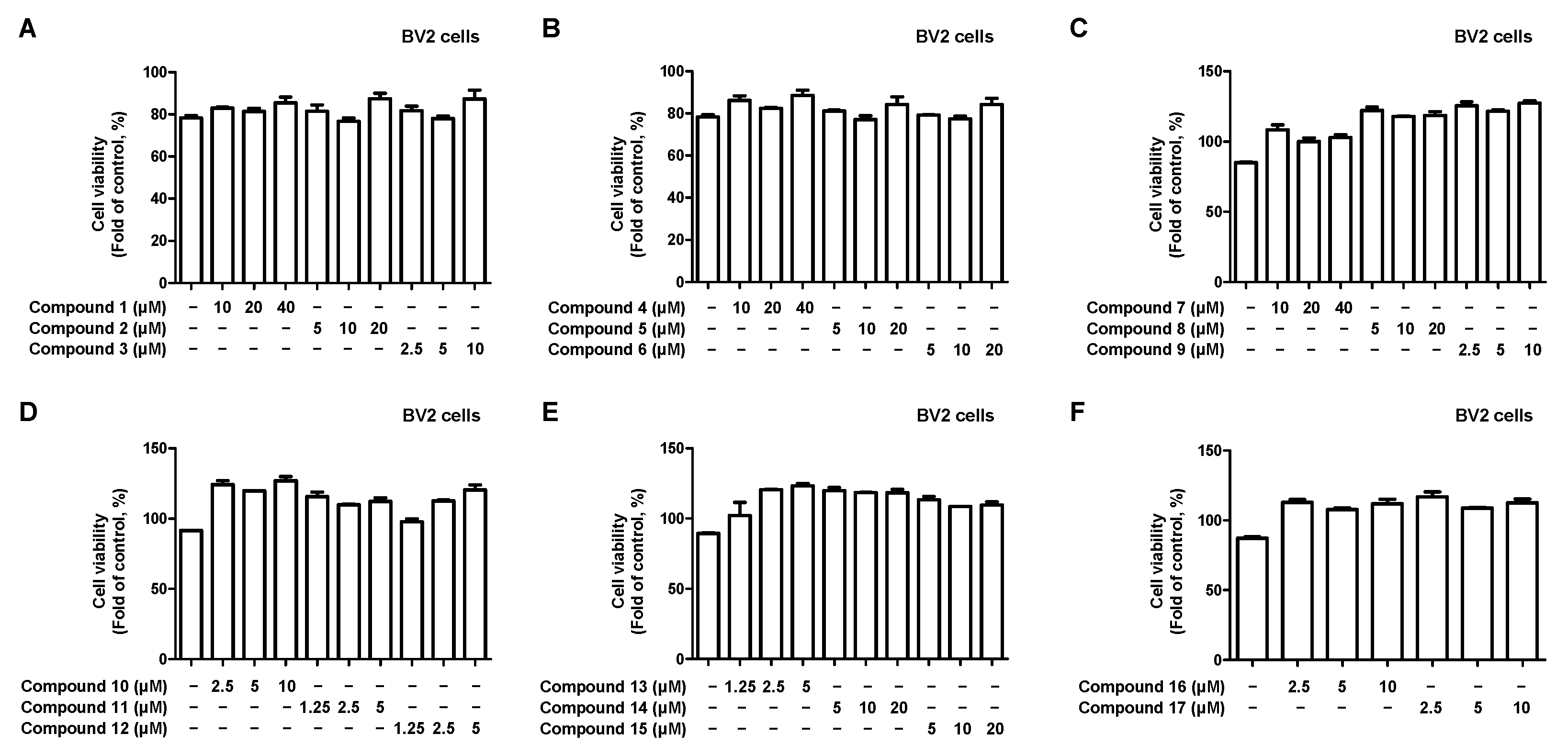
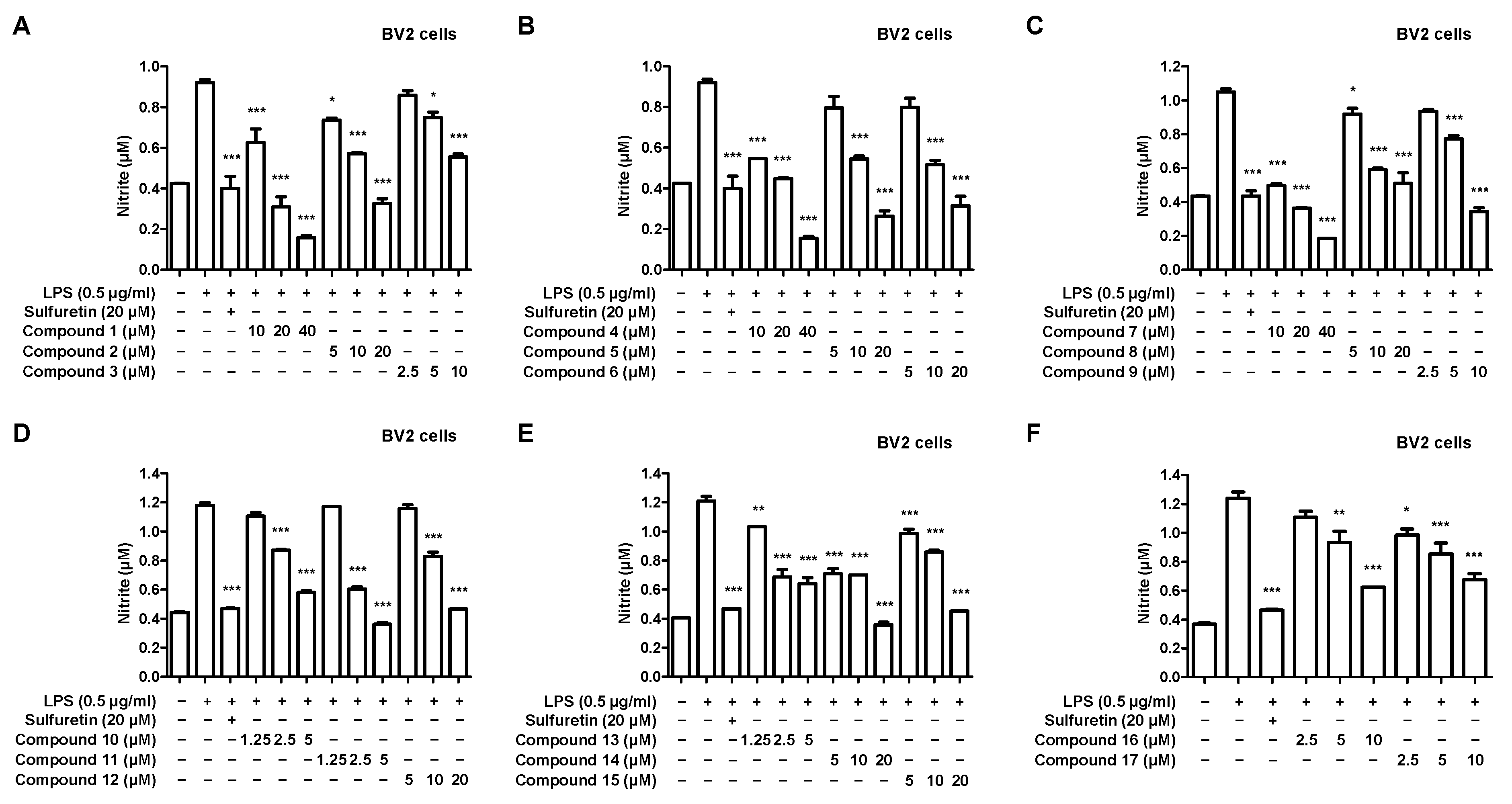
2.2. Effects of 17 Compounds from C. longa on Nitrite Inhibition in LPS-Induced BV2 Microglia
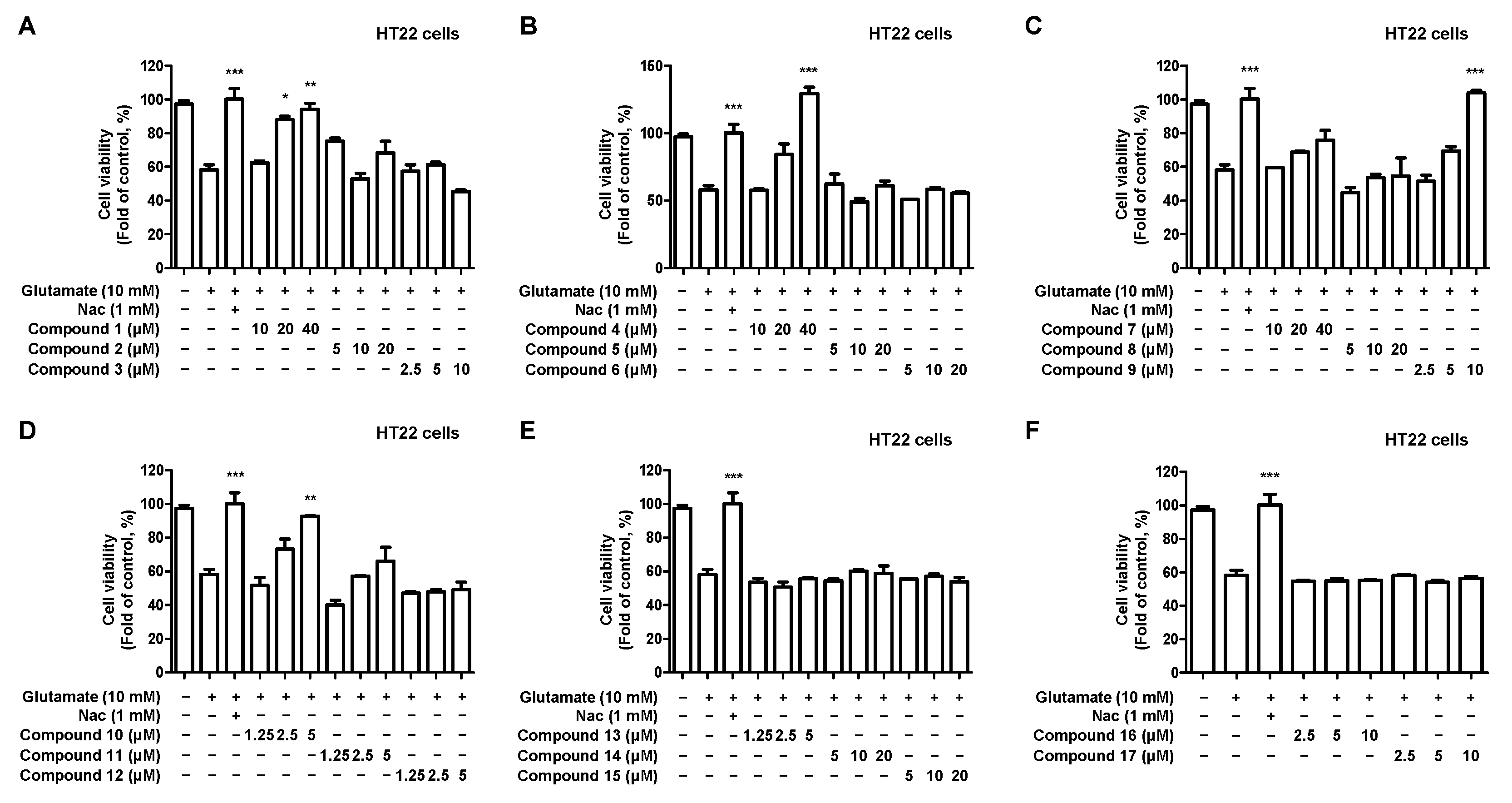
2.3. Effects of 17 Compounds from C. longa on Oxidative Stress in Glutamate-Induced HT22 Hippocampal Cells
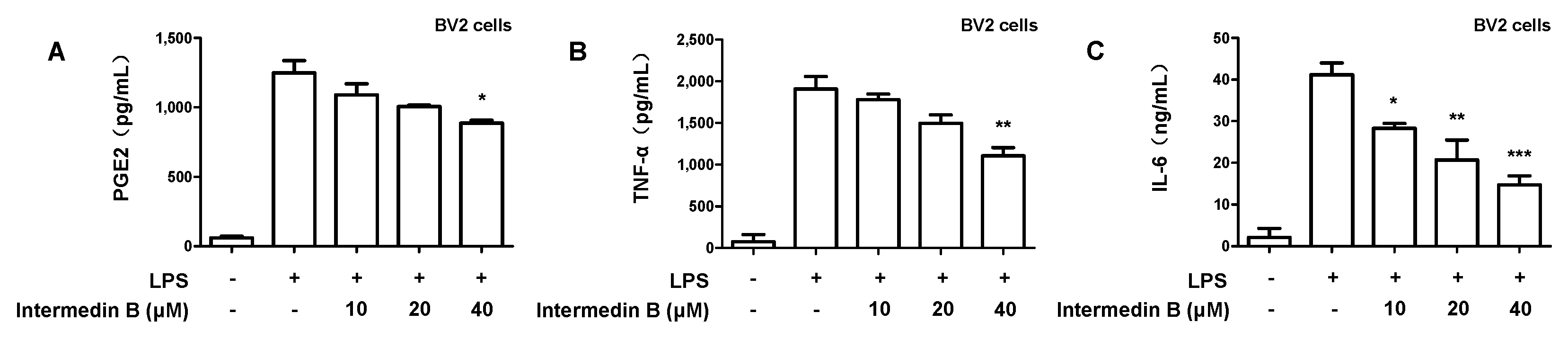
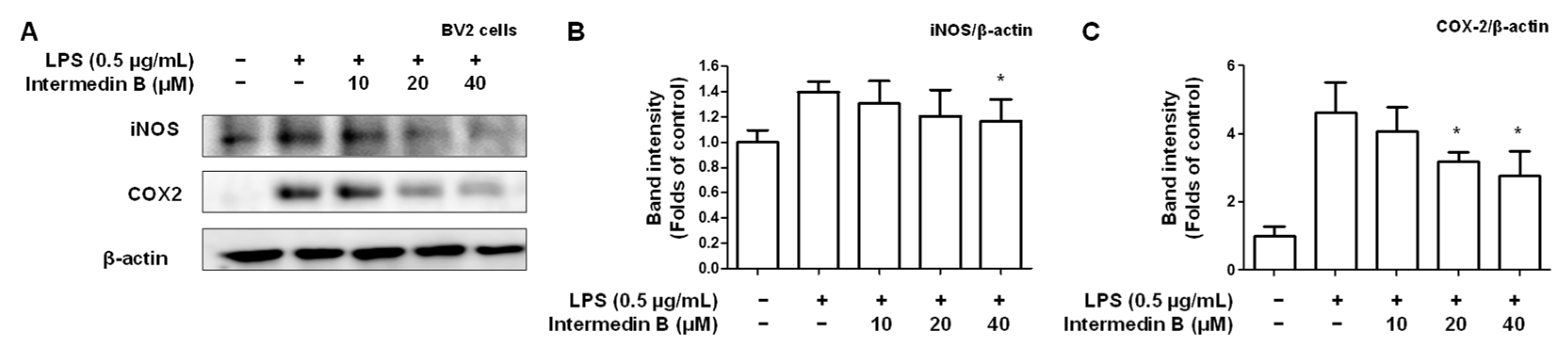
2.4. Effects of Intermedin B on Levels of Pro-Inflammatory Mediators and Cytokines in LPS-Induced BV2 Microglia
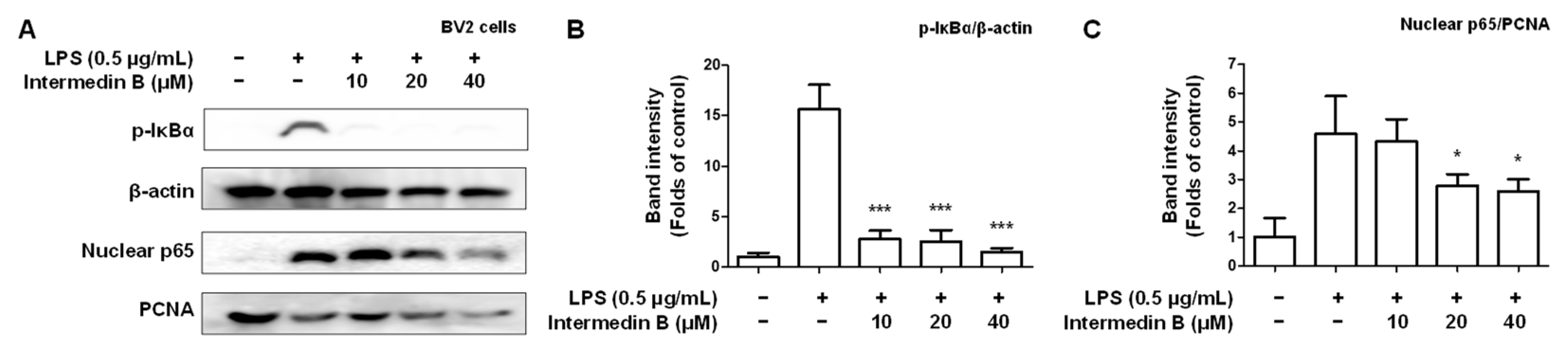
2.5. Effects of Intermedin B on NF-κB Activation in LPS-Induced BV2 Microglia
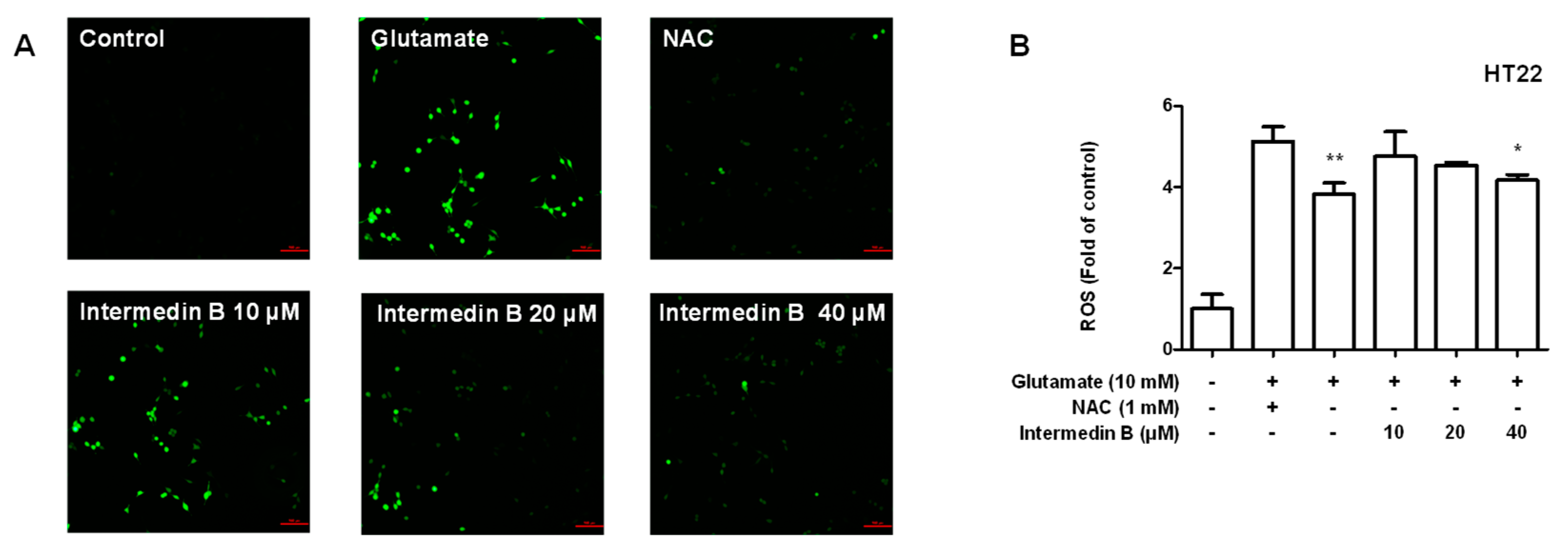
2.6. Effect of Intermedin B on Glutamate-Induced ROS Generation in HT22 Hippocampal Cells
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation Materials
4.2. Plant Material and Extraction
4.3. Cell Culture
4.4. MTT Assay
4.5. Measurement of NO Generation
4.6. Measurement of Neuroprotective Effects
4.7. PGE2 Assay
4.8. IL-6 and TNF-α Assay
4.9. Western Blot Analysis
4.10. Preparation of Nucleus and Cytosolic Fraction
4.11. ROS Assays
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, C. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine. Netw. 2006, 17, 4–12. [Google Scholar] [PubMed]
- Gonzalez-Scarano, F.; Baltuch, G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999, 22, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baud, O.; Vartanian, T.; Volpe, J.J.; Rosenberg, P.A. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 9936–9941. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Kawamata, T.; Walker, D.G.; Akiyama, G.; Tooyama, I.; McGeer, E.G. Microglia in degenerative neurological disease. Glia 1993, 7, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends. Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Koppula, S.; Hong, S.S.; Jeon, S.B.; Kwon, J.H.; Hwang, B.Y.; Park, E.J.; Choi, D.K. Regulation of microglia activity by glaucocalyxin-A: Attenuation of lipopolysaccharide-stimulated neuroinflammation through NF-κB and p38 MAPK signaling pathways. PLoS ONE 2013, 8, e55792. [Google Scholar] [CrossRef]
- Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Landum, R.W.; Cheng, M.S.; Wu, J.F.; Floyd, R.A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc. Natl. Acad. Sci. USA 1991, 88, 3633–3636. [Google Scholar] [CrossRef]
- Hu, D.; Serrano, F.; Oury, T.D.; Klann, E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2006, 26, 3933–3941. [Google Scholar] [CrossRef]
- Davis, J.B.; Maher, P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994, 652, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Zhu, B.T. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic. Biol. Med. 2010, 48, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Rssler, O.G.; Bauer, I.; Chung, H.Y.; Thiel, G. Glutamate-induced cell death of immortalized murine hippocampal neurons: Neuroprotective activity of heme oxygenase-1, heat shock protein 70, and sodium selenite. Neurosci. Lett. 2004, 362, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, N.M.; Toscano, R.; Villanueva, A.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S.; Millan-Linares, M. Neuroprotective protein hydrolysates from hemp (Cannabis sativa L.) seeds. Food. Funct. 2019, 10, 6732–6739. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R.J. Copper, iron and zinc in Alzheimer’s disease senile plaques. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Ling, E.A.; Kaur, C. Consequences of iron accumulation in microglia and its implications in neuropathological conditions. CNS Neurol. Disord. Drug Targets 2013, 12, 785–798. [Google Scholar] [CrossRef]
- James, S.A.; Volitakis, I.; Adlard, P.A.; Duce, J.A.; Masters, C.L.; Cherny, R.A.; Bush, A.I. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 298–302. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of C. longa (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the Active Substance of C. longa: Its Effects on Health and Ways to Improve its Bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Yagi, N.; Miyazawa, M. Acetylcholinesterase Inhibitory Activity of Volatile Oil from Peltophorum dasyrachis Kurz ex Bakar (Yellow Batai) and Bisabolane-Type Sesquiterpenoids. J. Agric. Food Chem. 2010, 58, 2824–2829. [Google Scholar] [CrossRef]
- Shi, H.M.; Long, B.S.; Cui, X.M.; Min, Z.D. A new bisabolane sesquiterpenoid from Euphorbia chrysocoma. J. Asian Nat. Prod. Res. 2005, 7, 857–860. [Google Scholar] [CrossRef]
- Letourneux, Y.; Brunel, J.M.; Fernandez, R.; Dherbomez, M.; Debitus, C. Isolation and characterization of new tetrahydropyranyl substituted sesquiterpene and Myrmekiodermin glycolipid ether isolated from the marine Sponge Myrmekioderma. Heterocycl. Comm. 2005, 11, 291–298. [Google Scholar] [CrossRef]
- Imai, S.; Morikiyo, M.; Furihata, K.; Hayakawa, Y.; Seto, H. Turmeronol A and turmeronol B, new inhibitors of soybean lipoxygenase. Agric. Biol. Chem. 1990, 54, 2367–2371. [Google Scholar]
- Li, H.M.; Lei, C.; Luo, Y.M.; Li, X.N.; Li, X.L.; Pu, J.X.; Zhou, S.Y.; Li, R.T.; Sun, H.D. Intermedins A and B; New metabolites from Schisandra propinqua var. intermedia. Arch. Pharm. Res. 2008, 31, 684–687. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, J.; Qu, G.; Qiu, F. Chemical constituents of Curcuma longa I: Bisabolane sesquiterpenes. Zhongguo Yaowu Huaxue Zazhi 2007, 17, 238–241. [Google Scholar]
- Liu, X.; Wu, Q.X.; Shi, Y.P. Terpenoids from the flower of Cacalia tangutica. J. Chin. Chem. Soc. 2005, 52, 369–374. [Google Scholar] [CrossRef]
- Gu, H.; Shi, B.; Jiang, H.; Zhang, W. Chemical compositions of Senecio filiferus. Nanjing Zhongyiyao Daxue Xuebao 2011, 27, 375–377. [Google Scholar]
- Kim, H.; Ralph, J.; Lu, F.; Ralph, S.A.; Boudet, A.M.; MacKay, J.J.; Sederoff, R.R.; Ito, T.; Kawai, S.; Ohashi, H.; et al. NMR analysis of lignins in CAD-deficient plants. Part 1. Incorporation of hydroxycinnamaldehydes and hydroxybenzaldehydes into lignins. Org. Biomol. Chem. 2003, 1, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Chang, F.R.; Wang, H.K.; Park, Y.K.; Ikegaki, M.; Kilgore, N.; Lee, K.H. Anti-AIDS Agents. 48. Anti-HIV Activity of Moronic Acid Derivatives and the New Melliferone-Related Triterpenoid Isolated from Brazilian Propolis. J. Nat. Prod. 2001, 64, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Improved HPLC Method for the Determination of Curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, F.; Liu, Y.; Qu, G.; Yao, X. Isolation and identification of phase 1 metabolites of demethoxycurcumin in rats. Drug Metab. Dispos. 2007, 35, 1564–1573. [Google Scholar] [CrossRef]
- Manville, J.F.; Fraser, T.; Tracey, A.S. Characterization of lasiocarpenonol and conformation of four sesquiterpenoids from alpine fir. Phytochemistry 1989, 28, 3073–3080. [Google Scholar] [CrossRef]
- Joseph-Nathan, P.; Tovar-Miranda, R.; Martinez, E.; Santillan, R.L. Carbon-13 NMR studies of curcumenes. J. Nat. Prod. 1988, 51, 1116–1128. [Google Scholar] [CrossRef]
- Lu, J.; Xie, X.; Chen, B.; She, X.; Pan, X. An enantioselective synthesis of (S)- and (R)-curcuphenol. Tetrahedron Asymmetry 2005, 16, 1435–1438. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Yeh, H.C.; Song, P.L.; Li, H.T.; Li, W.J. A New Norsesquiterpenoid from the Rhizomes of Curcuma longa. Chem. Nat. Compd. 2020, 56, 75–77. [Google Scholar] [CrossRef]
- Menelaou, M.A.; Marcias, F.A.; Weidenhamer, J.D.; Williamson, G.B.; Fischer, N.H. Sesquiterpenes from Chrysoma pauciflosculosa. Spectrosc. Lett. 1995, 28, 1061–1074. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Robinson, H.; King, R.M. Naturally occurring terpene derivatives. Part 397. Germacranolides from Lychnophora species. Phytochemistry 1982, 21, 1087–1091. [Google Scholar] [CrossRef]
- Uehara, S.; Yasuda, I.; Takeya, K.; Itokawa, H. New bisabolane sesquiterpenoids from the rhizomes of Curcuma xanthorrhiza (Zingiberaceae). Chem. Pharm. Bull. 1989, 37, 237–240. [Google Scholar] [CrossRef]
- Kreiser, W.; Korner, F. Stereospecific synthesis of (−)-β-turmerone and (−)-bisacurol. Helv. Chim. Acta 1999, 82, 1610–1629. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Xie, P.; Deng, M.; Sun, Q.; Jiang, B.; Xu, H.; Liu, J.; Zhou, Y.; Ma, Y.; Chen, Z. Curcumin protects BV2 cells against lipopolysaccharide-induced injury via adjusting the miR-362-3p/TLR4 axis. Mol. Biol. Rep. 2020, 47, 4199–4208. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug. Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Rinwa, P.; Kumar, A. Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res. 2013, 1513, 63–73. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart. J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Tracey, K.J.; Fong, Y.; Hesse, D.G.; Manogue, K.R.; Lee, A.T.; Kuo, G.C.; Lowry, S.F.; Cerami, A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987, 330, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis. Res. Ther. 2006, 8 (Suppl. 2), S3. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Jeong, G.S.; Li, B.; Park, H.; Kim, Y.C. Anti-inflammatory effects of sulfuretin from Rhus verniciflua Stokes via the induction of heme oxygenase-1 expression in murine macrophages. Int. Immunopharmacol. 2010, 10, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Liu, Z.; Yoon, C.S.; Dong, L.; Ko, W.; Woo, E.R.; Lee, D.S. Anti-Neuroinflammatory and Anti-Inflammatory Activities of Phenylheptatriyne Isolated from the Flowers of Coreopsis lanceolata L. via NF-κB Inhibition and HO-1 Expression in BV2 and RAW264.7 Cells. Int. J. Mol. Sci. 2021, 22, 7482. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Liu, Z.; Dong, L.; Lee, D.Y.; Yoon, D.; Oh, H.; Kim, Y.-C.; An, R.-B.; Lee, D.-S. Anti-Neuroinflammatory and Neuroprotective Effect of Intermedin B Isolated from the Curcuma longa L. via NF-κB and ROS Inhibition in BV2 Microglia and HT22 Hippocampal Cells. Int. J. Mol. Sci. 2023, 24, 7390. https://doi.org/10.3390/ijms24087390
Lee H, Liu Z, Dong L, Lee DY, Yoon D, Oh H, Kim Y-C, An R-B, Lee D-S. Anti-Neuroinflammatory and Neuroprotective Effect of Intermedin B Isolated from the Curcuma longa L. via NF-κB and ROS Inhibition in BV2 Microglia and HT22 Hippocampal Cells. International Journal of Molecular Sciences. 2023; 24(8):7390. https://doi.org/10.3390/ijms24087390
Chicago/Turabian StyleLee, Hwan, Zhiming Liu, Linsha Dong, Dae Young Lee, Dahye Yoon, Hyuncheol Oh, Youn-Chul Kim, Ren-Bo An, and Dong-Sung Lee. 2023. "Anti-Neuroinflammatory and Neuroprotective Effect of Intermedin B Isolated from the Curcuma longa L. via NF-κB and ROS Inhibition in BV2 Microglia and HT22 Hippocampal Cells" International Journal of Molecular Sciences 24, no. 8: 7390. https://doi.org/10.3390/ijms24087390
APA StyleLee, H., Liu, Z., Dong, L., Lee, D. Y., Yoon, D., Oh, H., Kim, Y.-C., An, R.-B., & Lee, D.-S. (2023). Anti-Neuroinflammatory and Neuroprotective Effect of Intermedin B Isolated from the Curcuma longa L. via NF-κB and ROS Inhibition in BV2 Microglia and HT22 Hippocampal Cells. International Journal of Molecular Sciences, 24(8), 7390. https://doi.org/10.3390/ijms24087390





