Intra-Specific Variation in Desiccation Tolerance of Citrus sinensis ‘bingtangcheng’ (L.) Seeds under Different Environmental Conditions in China
Abstract
1. Introduction
2. Results
2.1. Desiccation Tolerance (DT) of Citrus sinensis ‘bingtangcheng’ Seed Lots from Different Locations
2.2. Correlation Studies between DT of Citrus sinensis ‘bingtangcheng’ Seed Lots and Other Seed Traits
2.3. Correlation Studies between Seed DT and Meteorological Data
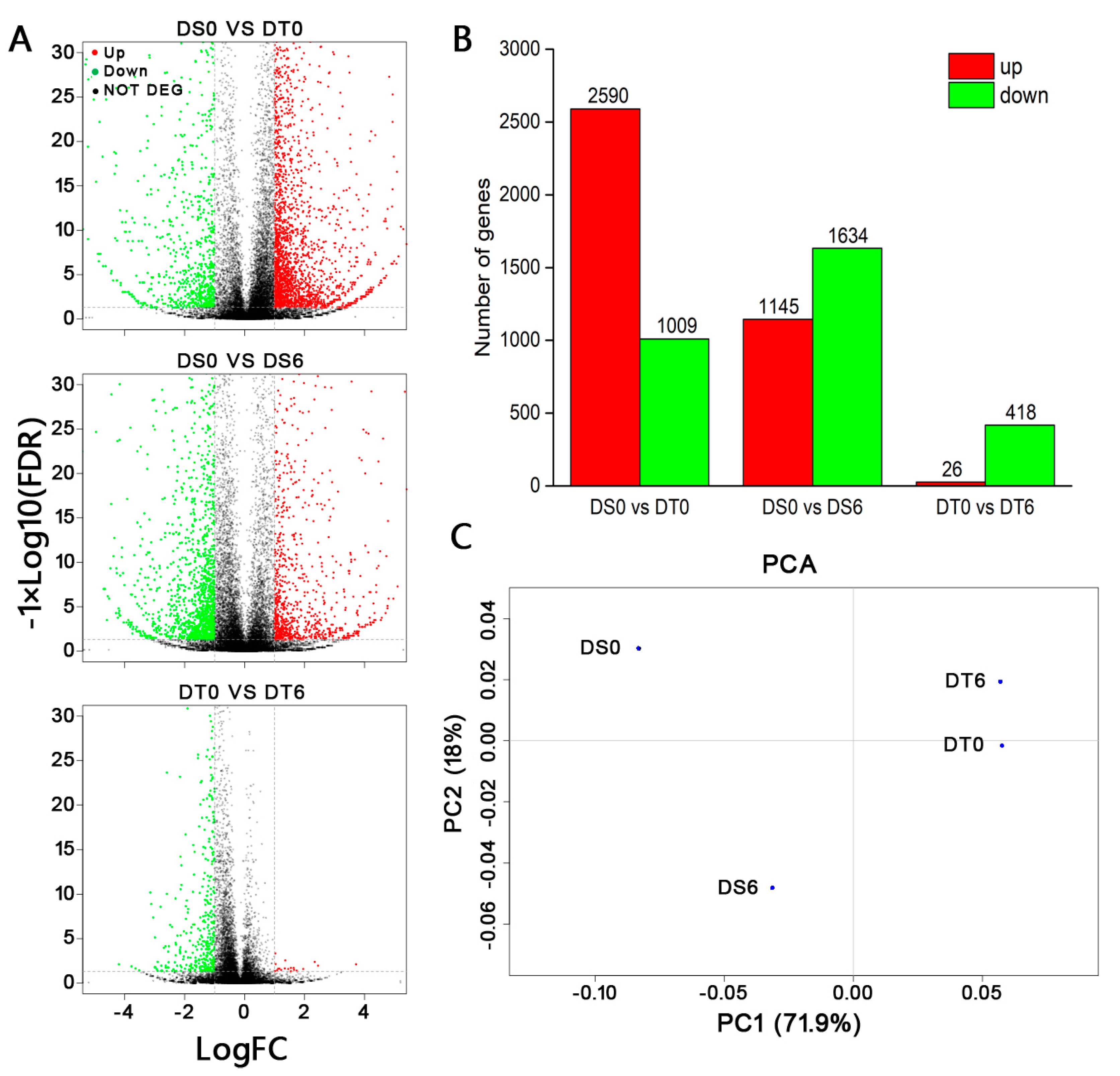
2.4. Transcriptome Analysis of DS and DT Seed Lots from Different Locations
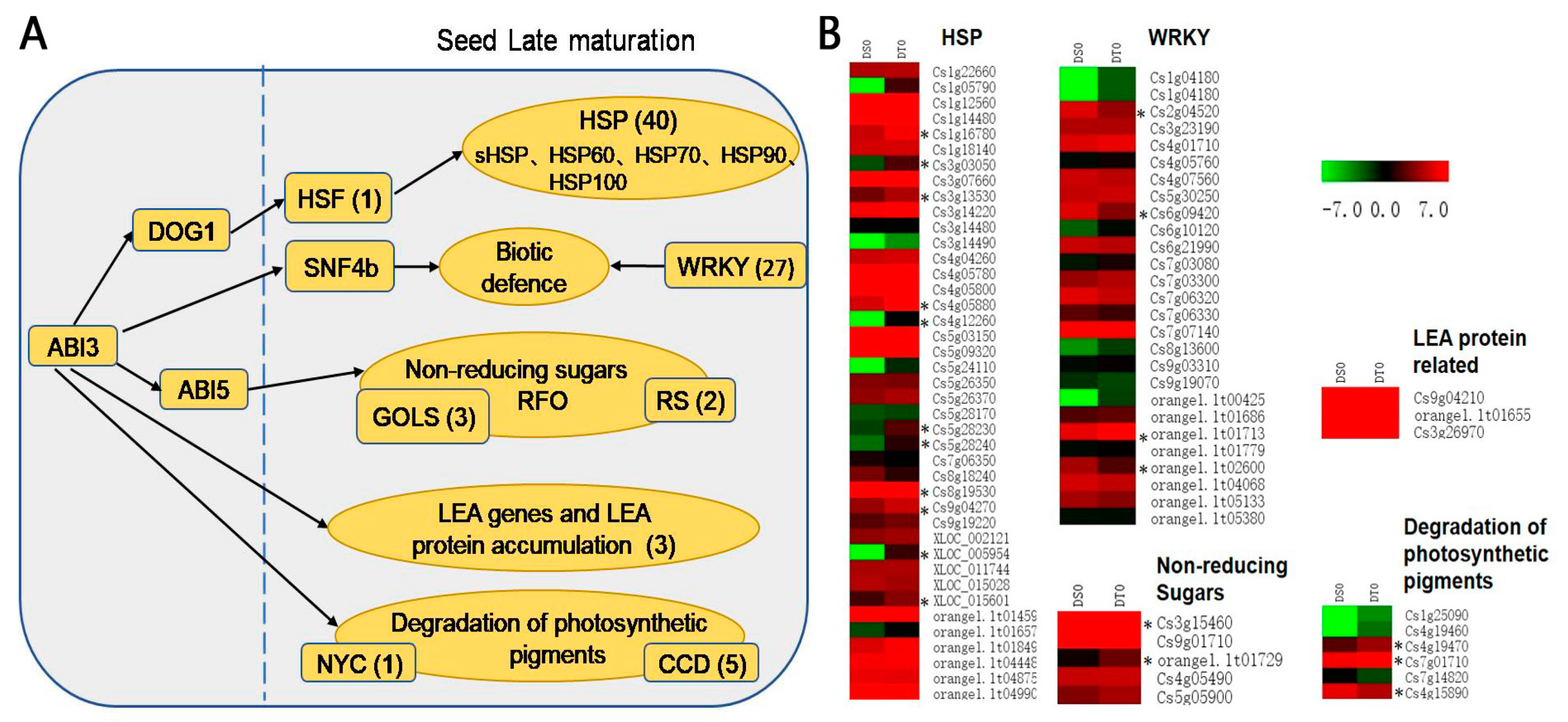
2.5. Gene Expression Patterns of DT Citrus sinensis ‘bingtangcheng’ Seeds Are Characteristic of a Late Seed Maturation Stage
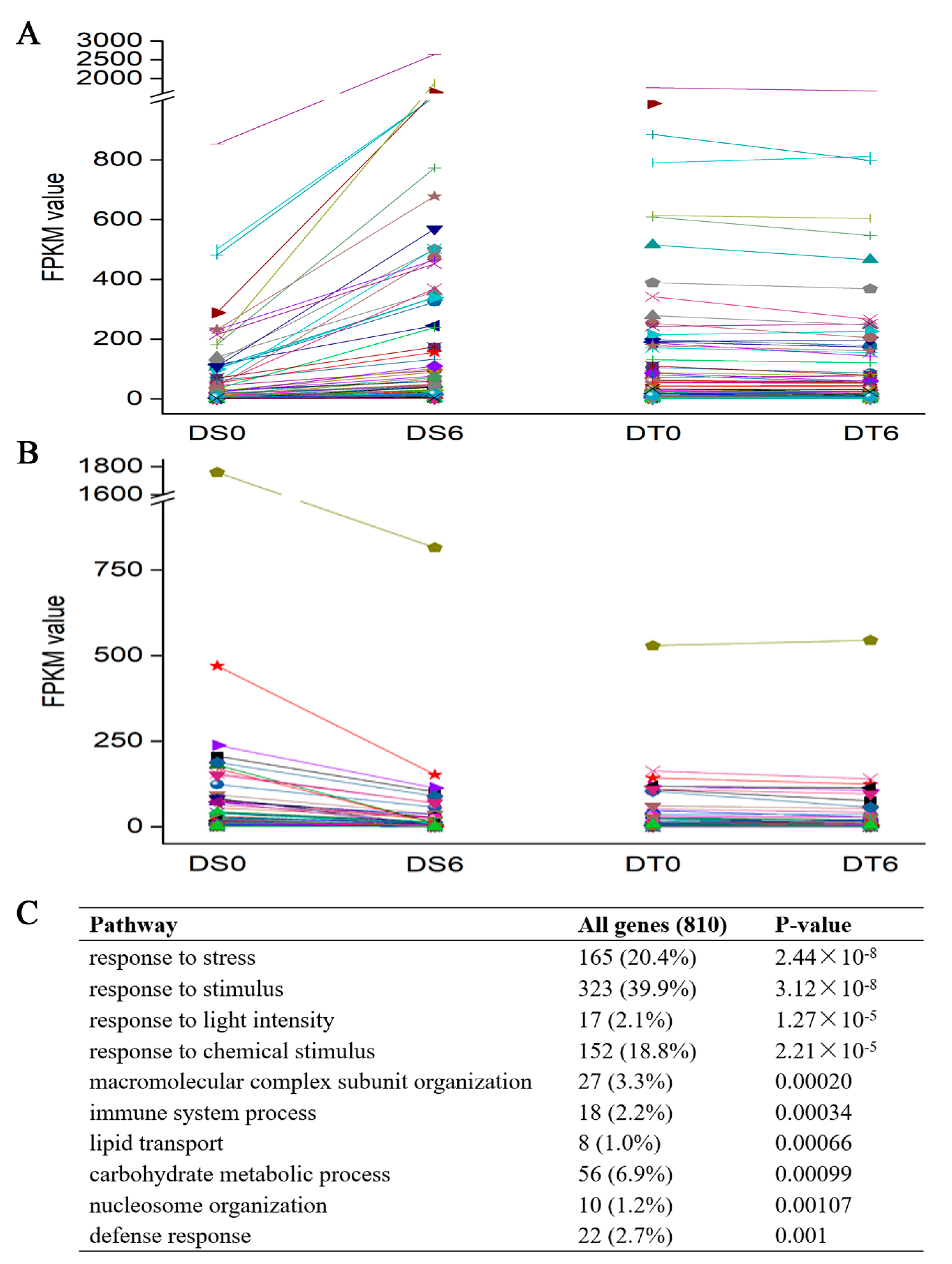
2.6. DT Citrus sinensis ‘bingtangcheng’ Seeds Show Stable Levels of Stress Responsive Genes during Drying
3. Discussion
3.1. Desiccation Tolerance of Seeds from Mature Citrus sinensis ‘bingtangcheng’ Fruits and Relationships with Other Seed Traits and Environmental Conditions
3.2. Transcriptome Analysis before and after Drying of Two C. sinensis ‘bingtangcheng’ Seed Lots Differing in DT
4. Materials and Methods
4.1. Seed Material
4.2. Desiccation Treatments and Germination Test
4.3. Environmental Conditions at the Sampling Sites
4.4. Isolation of RNA, Library Construction and Sequencing
4.5. Gene Expression and GO Enrichment Analyses
4.6. Data Analysis and Presentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dussert, S.; Chabrillange, N.; Engelmann, F.; Anthony, F.; Louarn, J.; Hamon, S. Relationship between seed desiccation sensitivity, seed water content at maturity and climatic characteristics of native environments of nine Coffea L. species. Seed Sci. Res. 2000, 10, 293–300. [Google Scholar] [CrossRef]
- Daws, M.I.; Cleland, H.; Chmielarz, P.; Gorian, F.; Leprince, O.; Mullins, C.E.; Thanos, C.A.; Vandvik, V.; Pritchard, H.W. Variable desiccation tolerance in Acer pseudoplatanus seeds in relation to developmental conditions: A case of phenotypic recalcitrance? Funct. Plant Biol. 2006, 33, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pritchard, H.W.; Seal, C.E.; Nadarajan, J.; Li, W.; Yang, S.; Kranner, I. Post desiccation germination of mature seeds of tea (Camellia sinensis L.) can be enhanced by pro-oxidant treatment, but partial desiccation tolerance does not ensure survival at −20 °C. Plant Sci. 2012, 184, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ganatsas, P.; Tsakaldimi, M.; Zarkadi, P.; Stergiou, D. Intraspecific differences in the response to drying of Quercus ithaburensis acorns. Plant Biosyst. 2017, 151, 878–886. [Google Scholar] [CrossRef]
- Daws, M.I.; Lydall, E.; Chmielarz, P.; Leprince, O.; Matthews, S.; Thanos, C.A.; Pritchard, H.W. Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytol. 2004, 162, 157–166. [Google Scholar] [CrossRef]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late seed maturation: Drying without dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef]
- Fait, A.; Angelovici, R.; Less, H.; Ohad, I.; Urbanczyk-Wochniak, E.; Fernie, A.R.; Galili, G. Arabidopsis seed development and germination is associated with temporally distinct metabolic switches. Plant Phys. 2006, 142, 839–854. [Google Scholar] [CrossRef]
- Righetti, K.; Vu, J.L.; Pelletier, S.; Vu, B.L.; Glaab, E.; Lalanne, D.; Pasha, A.; Patel, R.V.; Provart, N.J.; Verdier, J.; et al. Inference of longevity-related genes from a robust coexpression network of seed maturation identifies regulators linking seed storability to biotic defense-related pathways. Plant Cell 2015, 27, 2692–2708. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Sershen Tsan, F.Y.; Wen, B.; Jaganathan, G.K.; Calvi, G.; Pence, V.C.; Mattana, E.; Ferraz, I.D.K.; Seal, C.E. Chapter 19—Regeneration in recalcitrant-seeded species and risks from climate change. In Plant Regeneration from Seeds: A Global Warming Perspective; Baskin, C.C., Baskin, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 259–273. [Google Scholar]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Swingle, W.T.; Reece, P.C. The botany of Citrus and its wild relatives. In The Citrus Industry; Reuther, W., Ed.; University of California Press: Berkeley, CA, USA, 1967. [Google Scholar]
- Wu, G.A.; Terol, J.; Ibanez, V.; Lopez-Garcia, A.; Perez-Roman, E.; Borreda, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Webber, H.J. History and development of the Citrus industry. In The Citrus Industry; Reuther, W., Ed.; University of California Press: Berkeley, CA, USA, 1967; pp. 1–39. [Google Scholar]
- Gmitter, F.G.; Hu, X. The possible role of Yunnan province, China, in the origin of contemporary Citrus species (Rutaceae). Econ. Bot. 1990, 44, 267–277. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Anton, M.T.; et al. Tomato fruit photosynthesis is seemingly unimportant in primary metabolism and ripening but plays a considerable role in seed development. Plant Physiol. 2011, 157, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science 2002, 296, 343–346. [Google Scholar] [CrossRef]
- de Carvalho, D.U.; Boakye, D.A.; Gast, T.; Leite Junior, R.P.; Alferez, F. Determining seed viability during fruit maturation to improve seed production and availability of new Citrus rootstocks. Front. Plant Sci. 2021, 12, 777078. [Google Scholar] [CrossRef]
- Hong, T.D.; Ahmad, N.B.; Murdoch, A.J. Optimum air-dry storage conditions for sweet orange (Citrus sinensis (L.) Osbeck) and lemon (Citrus limon (L.) Burm. f.) seeds. Seed Sci. Technol. 2001, 29, 183–192. [Google Scholar]
- Pritchard, H.W.; Daws, M.I.; Fletcher, B.J.; Gamene, C.S.; Msanga, H.P.; Omondi, W. Ecological correlates of seed desiccation tolerance in tropical African dryland trees. Am. J. Bot. 2004, 91, 863–870. [Google Scholar] [CrossRef]
- Seymour, G.; Poole, M.; Manning, K.; King, G.J. Genetics and epigenetics of fruit development and ripening. Curr. Opin. Plant Biol. 2008, 11, 58–63. [Google Scholar] [CrossRef]
- Le, B.H.; Cheng, C.; Bui, A.Q.; Wagmaister, J.A.; Henry, K.F.; Pelletier, J.; Kwong, L.; Belmonte, M.; Kirkbride, R.; Horvath, S.; et al. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 8063–8070. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.L.; Saqib, H.S.; Yuan, W.; Xu, W.F.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Nakajima, S.; Ito, H.; Tanaka, R.; Tanaka, A. Chlorophyll b reductase plays an essential role in maturation and storability of Arabidopsis seeds. Plant Physiol. 2012, 160, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Delmas, F.; Sankaranarayanan, S.; Deb, S.; Widdup, E.; Bournonville, C.; Bollier, N.; Northey, J.G.; McCourt, P.; Samuel, M.A. ABI3 controls embryo degreening through Mendel’s I locus. Proc. Natl. Acad. Sci. USA 2013, 110, E3888–E3894. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.N.; Ligterink, W.; Franca-Neto Jde, B.; Hilhorst, H.W.; da Silva, E.A. Gene expression profiling of the green seed problem in soybean. BMC Plant Biol. 2016, 16, 37. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry architecture-towards an understanding of the variation of longevity in desiccation-tolerance germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Cocotl-Yanez, M.; Moreno, S.; Encarnacion, S.; Lopez-Pliego, L.; Castaneda, M.; Espin, G. A small heat-shock protein (Hsp20) regulated by RpoS is essential for cyst desiccation resistance in Azotobacter vinelandii. Microbiology 2014, 160, 479–487. [Google Scholar] [CrossRef]
- Shu, K.; Zhang, H.; Wang, S.; Chen, M.; Wu, Y.; Tang, S.; Liu, C.; Feng, Y.; Cao, X.; Xie, Q. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in arabidopsis. PLoS Genet. 2013, 9, e1003577. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyo, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Qin, F.; Yu, B.Z.; Li, W.Q. Heat shock protein 101 (HSP101) promotes flowering under nonstress conditions. Plant Physiol. 2021, 186, 407–419. [Google Scholar] [CrossRef]
- Wehmeyer, N.; Vierling, E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol. 2000, 122, 1099–1108.33. [Google Scholar] [CrossRef]
- Kotak, S.; Vierling, E.; Baumlein, H.; von Koskull-Doring, P. A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell 2007, 19, 182–195. [Google Scholar] [CrossRef]
- Malik, S.K.; Chaudhury, R.; Pritchard, H.W. Long-term, large scale banking of citrus species embryos: Comparisons between cryopreservation and other seed banking temperatures. CryoLetters 2012, 33, 453–464. [Google Scholar] [PubMed]
- Mizrahi, T.; Goldenberg, S.; Heller, J.; Arad, Z. Natural variation in resistance to desiccation and heat shock protein expression in the land snail Theba pisana along a climatic gradient. Physiol. Biochem. Zool. 2015, 88, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef]
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of Medicago truncatula seeds. Plant Physiol. 2013, 163, 757–774. [Google Scholar] [CrossRef]
- Tsou, P.L.; Zhu, H.J.; Godfrey, T.; Blackman, S. Post-excision drying of immature Phalaenopsis seeds improves germination and desiccation tolerance. S. Afr. J. Bot. 2022, 150, 1184–1191. [Google Scholar] [CrossRef]
- Peng, L.; Huang, X.; Qi, M.; Prtichard, H.W.; Xue, H. Mechanistic insights derived from re-establishment of desiccation tolerance in germination xerophytic seeds: Caragana korshinskii as an example. Front. Plant Sci. 2022, 13, 1029997. [Google Scholar] [CrossRef] [PubMed]
- Salonia, F.; Ciacciulli, A.; Poles, A.; Pappalardo, D.H.; La Malfa, S.; Licciardello, C. New plant breeding techniques in citrus for the improvement of important agronomic traits. A Review. Front. Plant Sci. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Jia, H.; Orbović, V.; Wang, N. CRISPR-LbCas12a-mediated modification of citrus. Plant Biotechnol. J. 2019, 17, 1928–1937. [Google Scholar] [CrossRef]
- Griffith, M.; Walker, K.R.; Spies, N.C.; Ainscough, B.J.; Griffith, O.L. Informatics for RNA sequencing, a web resource for analysis on the cloud. PLoS Comput. Biol. 2015, 11, e1004393. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lin, W.; Luo, J. Promotion of microalgal growth by co-culturing with Cellvibrio pealriver using xylan as feedstock. Bioresour. Technol. 2016, 200, 1050–1054. [Google Scholar] [CrossRef] [PubMed]


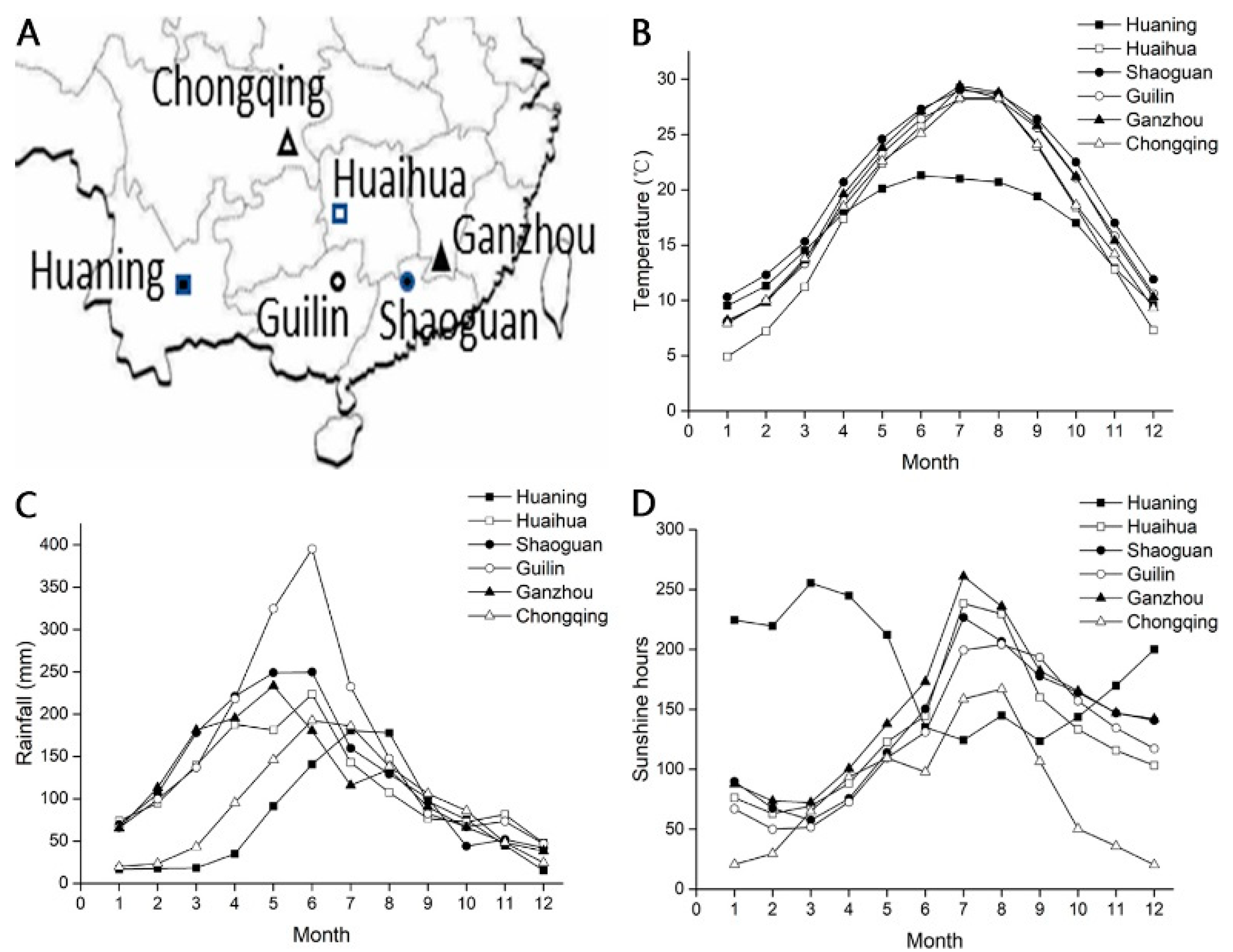

| Seed Lot | Location | Altitude (m) | Collection Date | MC (%) at TG Probit 5.5 | Whole Seed Dry Mass (mg) | Initial Moisture Content (%) |
|---|---|---|---|---|---|---|
| Huaning | 24°12′ N; 103°07′ E | 1133 | 5 December 2014 | 9.2 | 77.5 ± 2.5 d | 48.7 ± 1.4 b |
| Huaihua | 27°47′ N; 109°48′ E | 244 | 9 December 2014 | 22.3 | 91.7 ± 3.5 b,c | 49.4 ± 2.6 b |
| Shaoguan | 24°41′ N; 113°49′ E | 373 | 26 December 2014 | 4.5 | 100.2 ± 2.8 b | 45.4 ± 1.4 b,c |
| Guilin | 25°24′ N; 110°19′ E | 164 | 31 December 2014 | 7.9 | 87.7 ± 3.1 c | 48.4 ± 2.1 b |
| Ganzhou | 25°70′ N; 115°20′ E | 319 | 15 December 2014 | 13.6 | 120 ± 3.5 a | 41.8 ± 1.4 c |
| Chongqing | 29°45′ N; 106°22′ E | 245 | 28 December 2014 | 25.5 | 63.6 ± 2.6 e | 57.0 ± 1.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Visscher, A.M.; Ai, Q.; Yang, L.; Pritchard, H.W.; Li, W. Intra-Specific Variation in Desiccation Tolerance of Citrus sinensis ‘bingtangcheng’ (L.) Seeds under Different Environmental Conditions in China. Int. J. Mol. Sci. 2023, 24, 7393. https://doi.org/10.3390/ijms24087393
Chen H, Visscher AM, Ai Q, Yang L, Pritchard HW, Li W. Intra-Specific Variation in Desiccation Tolerance of Citrus sinensis ‘bingtangcheng’ (L.) Seeds under Different Environmental Conditions in China. International Journal of Molecular Sciences. 2023; 24(8):7393. https://doi.org/10.3390/ijms24087393
Chicago/Turabian StyleChen, Hongying, Anne M. Visscher, Qin Ai, Lan Yang, Hugh W. Pritchard, and Weiqi Li. 2023. "Intra-Specific Variation in Desiccation Tolerance of Citrus sinensis ‘bingtangcheng’ (L.) Seeds under Different Environmental Conditions in China" International Journal of Molecular Sciences 24, no. 8: 7393. https://doi.org/10.3390/ijms24087393
APA StyleChen, H., Visscher, A. M., Ai, Q., Yang, L., Pritchard, H. W., & Li, W. (2023). Intra-Specific Variation in Desiccation Tolerance of Citrus sinensis ‘bingtangcheng’ (L.) Seeds under Different Environmental Conditions in China. International Journal of Molecular Sciences, 24(8), 7393. https://doi.org/10.3390/ijms24087393






